| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 1, Number 4-5, October 2012, pages 108-111
Extremely Late Extramedullary Relapse of Acute Promyelocytic Leukemia, Case Report and Review of the Literature
Joshua Kraa, d, Ilan Shapiraa, c, Michael L. Grossbardb, c
aBeth Israel Medical Center, Albert Einstein College of Medicine, Department of Internal Medicine, Division of Hematology-Oncology, New York,USA
bSt.Lukes-Roosevelt Hospital Center, Columbia University College of Physicians and Surgeons, Department of Internal Medicine, Division of hematology-Oncology, New York, USA
cContinuum Cancer Centers of New York, New York, USA
dCorresponding author: Joshua Kra, Beth Israel Medical Center, Department of Internal Medicine, 16th Street at 1st Avenue, New York, NY 10003, USA
Manuscript accepted for publication August 17, 2012
Short title: Extremely Late Extramedullary Relapse of APL
doi: https://doi.org/10.4021/jh41e
| Abstract | ▴Top |
With the use of all-trans retinoic acid (ATRA) as frontline treatment for acute promyelocytic leukemia (APL), there has been an increase in overall remission rates and extended survival, but relapses still occur. While most cases of relapse are limited to the bone marrow and/or blood, APL can relapse in extramedullary sites as well. These relapses may develop several years after remission. While different reasons for extramedullary relapse have been presented in the literature, the exact mechanism and reason for late relapse still needs to be defined. Our case documents the longest interval from diagnosis to relapse of APL described in the literature to date.
Keywords: Acute promyelocytic leukemia; Atra; Extramedullary relapse
| Introduction | ▴Top |
Myeloid leukemias are caused by neoplastic hematopoietic cells that have already committed to the myeloid lineage. Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML), with a translocation involving the PML-RARA fusion gene t(15:17). APL accounts for between 5-20% of all AML cases, with approximately 600-800 new cases of APL diagnosed yearly in the United States [1]. The incidence of APL is unique in that APL occurs across most age groups, with a decreased incidence seen in early childhood and in people over 70 years old [2].
Due to the presence of an abundance of immature promyelocytes, patients who present with APL are at high risk of developing coagulopathies [3]. Hemorrhage and infection are the main causes of early mortality in APL. Primary drug-resistance occurs only rarely in APL. Currently, over 90% of patients will obtain a complete remission with primary treatment [4]. However, the relapse rate for APL is 10-15%, with a 20-30% relapse rate for high-risk forms of the disease. Late recurrence, defined as a recurrence after 4 years or more, occurs in approximately 3% of all cases [5].
While most cases of relapse are limited to the bone marrow and/or blood, APL can relapse in extramedullary sites as well. Such presentation is often referred to as a chloroma or myeloid sarcoma. While such relapse might occur as frequently as 3-5% of the time, there is little information about the pathogenesis and treatment of such disease [6]. Myeloid sarcomas often occur in the central nervous system, but can be found in any organ. Once diagnosed with myeloid sarcoma, and according to the newest version of the WHO classification of myeloid neoplasm and acute leukemia, a patient is considered to have had a full relapse, and is treated systemically, even if bone marrow biopsy is negative for malignancy [7-9]. Radiation therapy is used occasionally for localized disease [10].
| Case Report | ▴Top |
In 1994, a 23-year-old female was diagnosed with APL. She received induction therapy with cytarabine, daunorubicin, and all-trans retinoic acid (ATRA). Consolidation was done with two cycles of daunorubicin and all-trans retinoid acid, followed by ATRA maintenance for one year. Repeat bone marrow biopsy showed complete remission, and she was followed for several years without evidence of relapse.
In May 2009, 180 months after the patient’s first presentation, she began to develop upper respiratory symptoms, including sinus congestion, ear fullness, and left sided hearing loss. She completed a course of ciprofloxacin, but failed to show improvement. The patient was seen by an otolaryngologist who found a mass in her left ear canal; CT imaging revealed a soft tissue mass in the left external auditory canal abutting the tympanic membrane. The mass was biopsied and pathology was consistent with a granulocytic sarcoma, consisting of immature myeloid cells that stained positive for both anti CD-33 and myeloperoxidase (Fig. 1). Florescence in-situ hybridization (FISH) analysis of the tumor was positive for t(15;17) in 91.5% of the cells (Fig. 2).
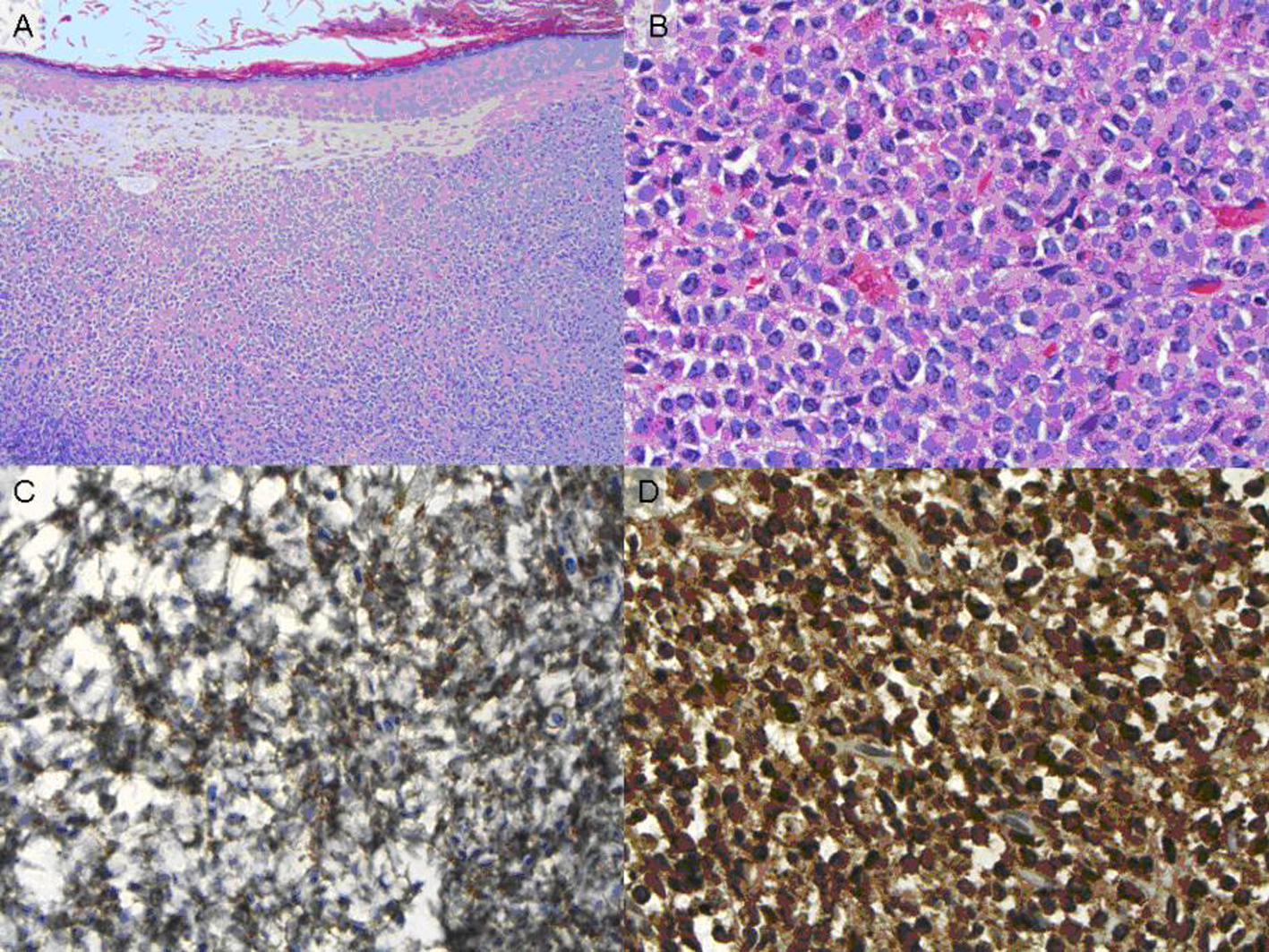 Click for large image | Figure 1. (A) H&E stain, low power (B) H&E stain, high power (C) CD-33 staining (D) Myeloperoxidase staining. |
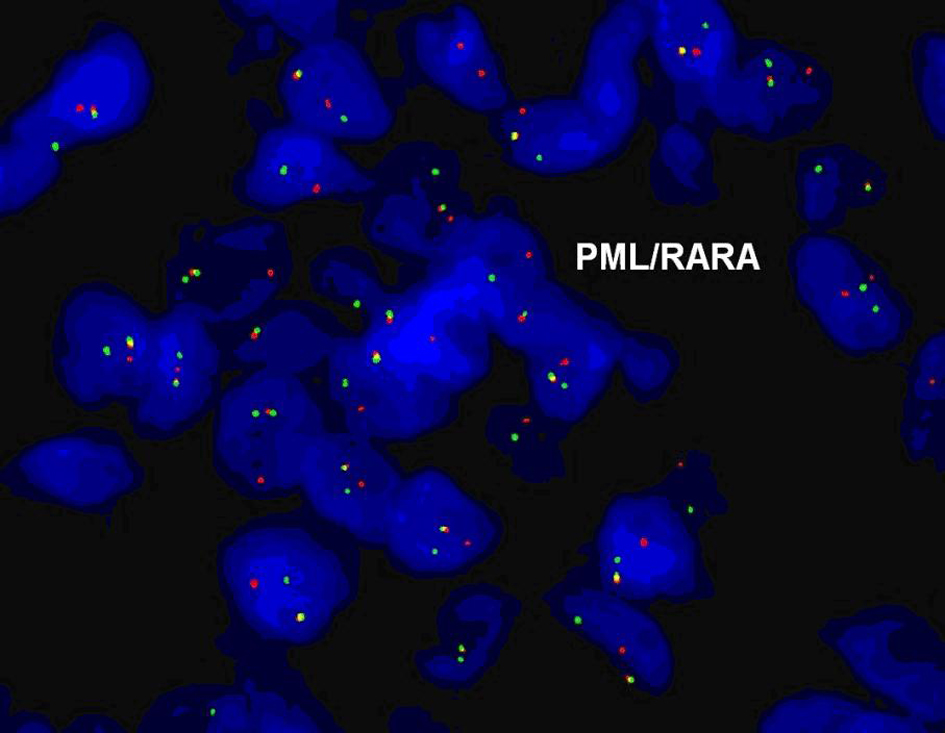 Click for large image | Figure 2. FISH analysis for PML/RARA t(15;17). |
Complete blood count at the time was unremarkable, with a white blood cell count of 8.3 × 109 cells per liter, normal white blood cell differential, hemoglobin 13.3 grams per liter, and platelet count of 399 × 109 per liter. A bone marrow biopsy at the time was normocellular and showed no increase in myeloblasts or promyelocytes. Chromosomal analysis revealed a normal female karyotype (46XX). FISH of the bone marrow was borderline positive, with 0.4% of cells showing a PML/RARA fusion signal. Polymerase chain reaction (PCR) for PML/RARA transcripts was positive at 0.074. PCR from peripheral blood for PML/RARA was negative.
The patient underwent radiation therapy of the ear mass in July 2009, with an initial treatment of 1800 cGy at 6 mV followed by a cone-down of 600 cGy at 6mV. She also underwent systemic therapy with arsenic trioxide for 14 weeks, starting in August 2009. Bone marrow biopsy in December 2009 confirmed that she was in a second complete molecular remission. She subsequently underwent successful peripheral stem cell mobilization and received an autologous transplant in August 2010. She tolerated the transplant well, fully recovered her counts and remained in complete molecular remission.
| Discussion | ▴Top |
This case is an example of an extremely late relapse of APL with extramedullary involvement. At 180 months, it is the longest interval from diagnosis to relapse of APL described in the literature to date. In general, with current treatments of APL, relapse occurs in 10-15% of all patients [11]. Late relapse in APL (> 4 years) is a rare event, occurring in approximately 3% of all cases, and 12% of all relapse cases [5]. In the literature, late relapses have been well described for 28 cases (Fig. 3) [12, 13] Most cases of late relapsing disease occur within the first 7 years, with the previously longest recorded time to relapse occurring at 155 months. In two cases, the immunophenotypic, cytogenetic, and molecular pattern at relapse were the same as those at diagnosis, which confirmed that the late relapse was indeed a relapse of the original disease, not a new malignancy [14]. In almost all cases of late relapse described, patients were initially treated with ATRA and chemotherapy, but the treatment given after relapse differed. Many patients either received a second round of ATRA and chemotherapy, while others received arsenic trioxide. There were also several cases where patients received a transplant post-treatment, either allogenic or autologous. All patients who survived the initiation of treatment entered a second complete remission.
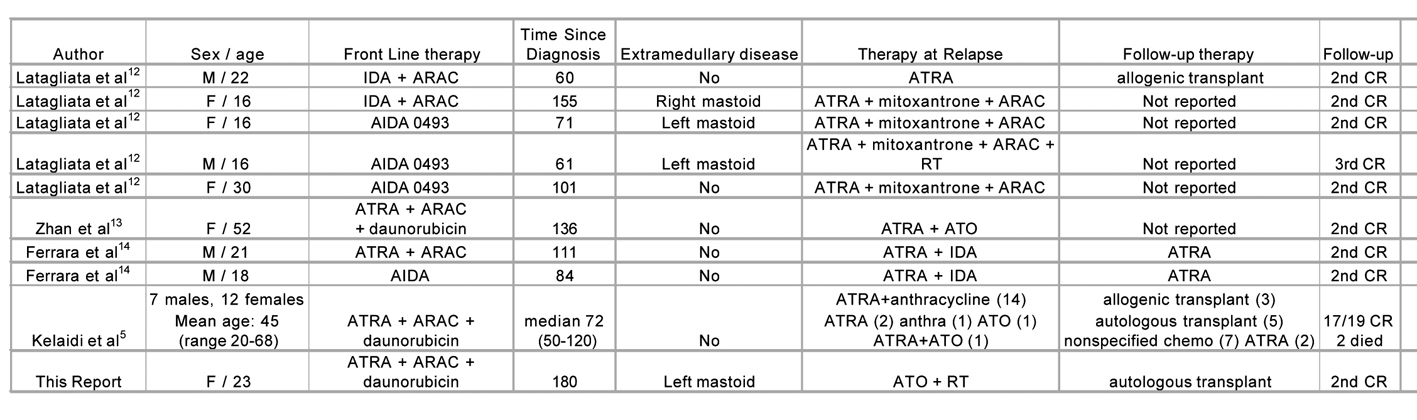 Click for large image | Figure 3. Late relapse of acute promyelocytic leukemia. ARAC, cytarabine; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; CR, complete remission; IDA, idarubicin; RT, radiotherapy. |
Our patient is also unique in that her relapse presented as extramedullary involvement of the ear canal. Extramedullay relapse can occur in various parts of the body, including (but not limited to) skin, central nervous system, testes, and ear [15]. While some studies have found a correlation between increased incidence of CNS disease and the use of ATRA [16], other studies have not found an increased incidence of extramedullary disease with ATRA administration [15]. Of note is that all reported cases of extramedually relapse in late-recurring disease occurred in the head and neck region (Fig. 3). A recent review of external auditory canal and middle ear relapse found 23 similar cases previously described, with most cases occurring within the first 2 years after diagnosis [17]. While the reason for extramedullary relapse is not certain, several theories exist. One theory is that certain adhesion molecules are disturbed with ATRA administration, due to ATRA’s differentiating activity. This disturbance permits leukemia cells to migrate into sanctuary sites and survive for a long period of time. Potential molecules include CD56 [18], intercellular adhesion molecule-1 and CD18 [19]. Treatment of extramedullary relapse has not been thoroughly studied, but it appears that arsenic trioxide is useful not only in medullary relapse, but in extramedullary relapse as well. This may be due to arsenic’s ability to deposit well in epidermal tissues [17].
Declaration
Each author certifies that he has participated sufficiently in the intellectual content and the analysis of data. Each author has reviewed the final version of the manuscript and approves it for publication. Each author denies any conflict of interest related to this manuscript.
| References | ▴Top |
- Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control. 2008;19(4):379-390.
pubmed doi - Vickers M, Jackson G, Taylor P. The incidence of acute promyelocytic leukemia appears constant over most of a human lifespan, implying only one rate limiting mutation. Leukemia. 2000;14(4):722-726.
pubmed doi - Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol. 2009;22(1):153-163.
pubmed doi - Liu YJ, Wu DP, Liang JY, Qiu HY, Jin ZM, Tang XW, Fu CC, et al. Long-term survey of outcome in acute promyelocytic leukemia: a single center experience in 340 patients. Med Oncol. 2011;28(Suppl 1):S513-521.
pubmed - Kelaidi C, Ades L, Chevret S, Sanz M, Guerci A, Thomas X, de Botton S, et al. Late first relapses in APL treated with all-trans-retinoic acid- and anthracycline- based chemotherapy: the European APL group experience (APL 91 and APL 93 trials). Leukemia. 2006;20(5):905-907.
pubmed doi - Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20(1):57-65.
pubmed doi - Byrd JC, Weiss RB. Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8;21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer. 1994;73(8):2107-2112.
pubmed doi - Struhal W, Oberndorfer S, Lahrmann H, Lindeck-Pozza E, Hess B, Nussgruber V, Pohnl R, et al. Myeloid sarcoma in the central nervous system: case report and review of the literature. Acta Clin Croat. 2008;47(1):19-24.
pubmed - Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951.
pubmed doi - Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122(4):238-246.
pubmed doi - Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105(8):3019-3025.
pubmed doi - Latagliata R, Carmosino I, Breccia M, Minni A, Testi A, Iorio N, Lo-Coco F, et al. Late relapses in acute promyelocytic leukaemia. Acta Haematol. 2007;117(2):106-108.
pubmed doi - Zhan H, Rajasree R, Russo L, Patel D. Late relapse of acute promyelocytic leukemia in a patient with no maintenance therapy. Am J Hematol. 2007;82(3):248.
pubmed doi - Ferrara F, Selleri C, Mele G, Serio B, Palmieri S, Pocali B, Pane F, et al. Late relapse of acute promyelocytic leukemia treated with all- trans retinoic acid and chemotherapy: report of two cases. Ann Hematol. 2004;83(7):484-486.
pubmed doi - Tallman MS, Rowe JM. Long-term follow-up and potential for cure in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16(3):535-543.
pubmed doi - Specchia G, Lo Coco F, Vignetti M, Avvisati G, Fazi P, Albano F, Di Raimondo F, et al. Extramedullary involvement at relapse in acute promyelocytic leukemia patients treated or not with all-trans retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche dell'Adulto. J Clin Oncol. 2001;19(20):4023-4028.
pubmed - Lafayette TC, Coser VM, Brule AO, Coser PL, Pereira WV. External auditory canal and middle ear relapse of acute promyelocytic leukemia treated with arsenic trioxide: case report and review of the literature. J Pediatr Hematol Oncol. 2010;32(3):229-232.
pubmed doi - Ferrara F, Morabito F, Martino B, Specchia G, Liso V, Nobile F, Boccuni P, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000;18(6):1295-1300.
pubmed - Weiss MA, Warrell RP, Jr. Two cases of extramedullary acute promyelocytic leukemia. Cytogenetics, molecular biology, and phenotypic and clinical studies. Cancer. 1994;74(7):1882-1886.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


