| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 9, Number 4, December 2020, pages 132-136
Unraveling the Mystery of Gray Zone Lymphoma in Human Immunodeficiency Virus-Seropositive Patients: Two Cases
Amith Ahluwaliaa, b, Leena Bondilia, Julius Salameraa, Michelle Cholankerila, b
aRutgers New Jersey Medical School, Trinitas Regional Medical Center, Elizabeth, NJ 07202, USA
bCorresponding Authors: Amith Ahluwalia and Michelle Cholankeril, Rutgers New Jersey Medical School, Trinitas Regional Medical Center, 225 Williamson St, Elizabeth, NJ 07202, USA. Emails:and
Manuscript submitted March 4, 2020, accepted July 10, 2020, published online October 1, 2020
Short title: GZL in HIV Patients
doi: https://doi.org/10.14740/jh554
| Abstract | ▴Top |
Gray zone lymphoma (GZL) is an uncommon neoplasm with intermediate features of both classic Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL). It was identified in the World Health Organization (WHO) classification as its own neoplasm in 2008. Patients infected with human immunodeficiency virus (HIV) have been rarely diagnosed with this type of lymphoma and treatment strategies for this subset of patients is not well described. Here we present two cases of patients with HIV that were diagnosed with GZL in a single community-based institution. A 68-year-old male with HIV/acquired immunodeficiency syndrome (AIDS) on highly active antiretroviral therapy (HAART) presented with 6-month history of dyspnea and weight loss. Computed tomography (CT) of the chest revealed multiple lung and mediastinal lesions, the largest measuring 9.4 × 5.5 cm lesion in the right perihilar region. Lymph node biopsy revealed abnormal lymphocytes with immunohistochemistry (IHC) positive for cluster of differentiation 30 (CD30), CD20 and Epstein-Barr virus (EBV), consistent with a diagnosis of GZL. The patient received dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and rituximab (DA-EPOCH-R) and attained a complete response. He since completed maintenance rituximab therapy and remains disease-free at 33 months. A 40-year-old female with HIV/AIDS on HAART presented with high-grade fever, dyspnea, and weight loss. CT imaging revealed multiple lung lesions, hepatosplenomegaly and diffuse lymphadenopathy in the chest and abdomen. Lymph node and bone marrow biopsy revealed cells positive for CD20, CD30, and EBV within atypical lymphoid cells. With this, a diagnosis of GZL was made and she was treated with DA-EPOCH-R. She attained a complete response and was on maintenance rituximab therapy. At 9 months she relapsed, she has now received a bone marrow transplant. GZL is a rarely described neoplasm within the HIV population. Here we describe two HIV patients diagnosed with GZL that were successfully treated at our institution. DA-EPOCH-R was able to induce durable remission with limited side effects and it represents a viable strategy for treating patients in this population. Further studies need to be performed to better characterize this lymphoma, especially in HIV patients. Treatment strategies for this select group of patients also need to be better defined.
Keywords: Gray zone lymphoma; Classic Hodgkin lymphoma; Diffuse large B-cell lymphoma; Human immunodeficiency virus; Acquired immunodeficiency syndrome
| Introduction | ▴Top |
Gray zone lymphoma (GZL) is a rare lymphoma with histological features of both non-Hodgkin lymphoma (NHL), and classic Hodgkin lymphoma (cHL). This uncertain biology has left many unanswered questions regarding treatment algorithms for these patients. Various treatment algorithms that have been attempted in this patient population, but GZL continues to have a dismal prognosis.
Lymphomas in human immunodeficiency virus (HIV) patients have been historically noted as acquired immunodeficiency syndrome (AIDS)-defining malignancies. This subset of patients has a 60 - 200-fold and 8 - 10-fold higher relative risk of developing NHL and HL, respectively [1]. However, GZL has rarely been reported in this patient population [2]. Furthermore, Epstein-Barr virus (EBV) has been known to have a role in the pathogenesis of lymphomas and has been identified in 40% of all HIV-associated lymphomas [2]. HIV infection and its immunomodulatory effects have synergistic role in the pathogenesis of EBV-associated cancers [1]. We report two rare cases at our single institution of GZL in HIV-infected patients that were found to have EBV positivity.
| Case Reports | ▴Top |
Case 1
RS is a 70-year-old Hispanic man with past medical history of HIV. He presented with a 1-month history of fever, chills, anorexia, axial and inguinal lymphadenopathy, and 20-pound weight loss. His most recent HIV parameters showed undetectable viral polymerase chain reaction (PCR), and a cluster of differentiation 4 (CD4) count of 221 cells/µL, while on combination antiretroviral therapy (ART) consisting of efavirenz/emtricitabine/tenofovir disoproxil fumarate. Blood work revealed leukocytosis, microcytic anemia, and thrombocytosis. Alkaline phosphatase, gamma-glutamyl transferase, and lactate dehydrogenase were elevated. Computed tomography (CT) imaging showed a large heterogeneous, irregular lung mass in the right middle lobe measuring 9.4 × 5.5 cm, closely associated with the pericardium and the right hilar structures. Ill-defined scattered parenchymal nodules were seen in the right lower lobe along with masses in the liver, and spleen. Bronchoscopy confirmed a right mainstream bronchus lesion extending to the right middle lobe and lower lobe. Positron emission tomography (PET) scan found fluorodeoxyglucose (FDG)-avid lesions in the mediastinum, right hilar region, left axillary, liver, spleen, bowel, retroperitoneum, pelvis, and groin. Biopsies from the bronchial mass, inguinal and axillary lymph nodes revealed abnormal EBV+, CD20+, CD30+, and CD15-lymphocytes with intermediate features between diffuse large B-cell lymphoma (DLBCL) and cHL (Fig. 1). On flow cytometry cells were multiple myeloma oncogene 1 (MUM1)+, organic cation transporter 2 (OCT2)+, and B-cell-specific octamer-binding protein-1 (BOB1)-. He was diagnosed with Ann Arbor stage IV GZL with an International Prognostic Score (IPS) of 5. Due to extensive disease and high IPS, he was started on dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and rituximab (DA-EPOCH-R) concurrently with chest radiation for bulky disease. His CD4 count nadir was 32 cells/µL by the end of his chemotherapy course; however, his viral load remained undetectable. His course was complicated by discovery of central nervous system (CNS) disease for which he received intrathecal methotrexate. He was administered seven cycles of treatment and achieved complete remission. He continued receiving rituximab every 8 weeks for 2 years following remission. He remains disease-free at 53 months, with immunologic recovery.
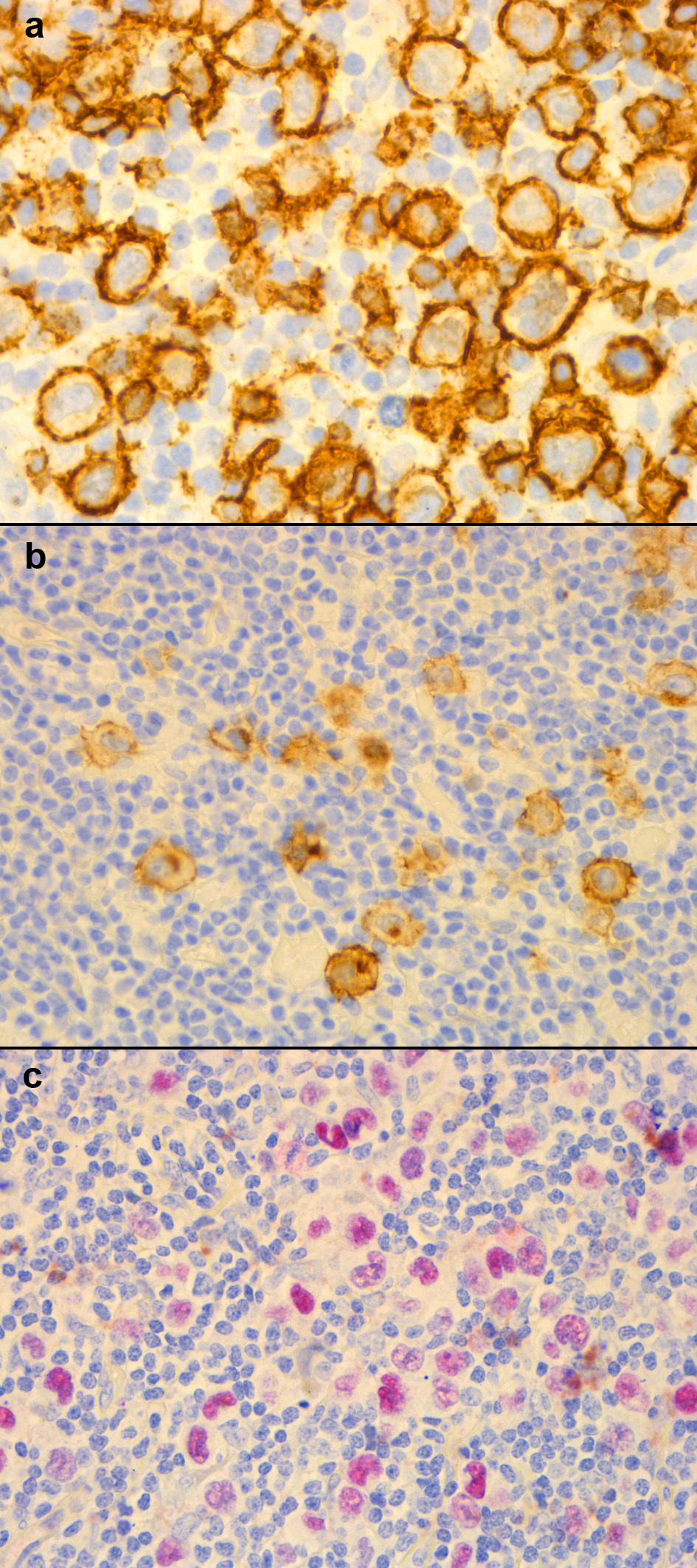 Click for large image | Figure 1. Case 1 immunohistochemistry of biopsy samples taken from the left axillary lymph node: (a) CD20+; (b) CD30+; (c) EBVR+. CD: cluster of differentiation; EBVR: Epstein-Barr virus receptor. |
Case 2
DL is a 40-year-old Brazilian female with a past medical history of HIV infection. She was maintained on elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide and achieved complete virologic suppression with a peak CD4 count of 166 cells/µL. On presentation, she reported severe headache, high-grade fevers, sinusitis, dyspnea, vomiting, and diarrhea. Blood work showed pancytopenia with significant lymphopenia. Cerebrospinal fluid (CSF) analysis was normal however CT imaging showed hepatosplenomegaly, with extensive lymphadenopathy in the cervical, mandibular, axillary, mesenteric, retroperitoneum, pelvic, and inguinal areas. Biopsies were obtained from bone marrow, as well as cervical, submandibular and supraclavicular lymph nodes. These biopsies revealed EBV+, CD20+, CD30+, and CD15-lymphoid cells (Fig. 2). Flow cytometry showed MUM1+, OCT2-, and BOB1+cells. DL was diagnosed with Ann Arbor Stage IV non-mediastinal GZL with an IPS of 3. She was initiated on DA-EPOCH-R regimen and received prophylactic intrathecal methotrexate. During treatment, elvitegravir/cobicistat had to be substituted with dolutegravir in light of potential interactions between vincristine and cobicistat. Her CD4 count nadir was 44 cells/µL but she improved progressively while on chemotherapy. She successfully achieved remission after six cycles with minimal side effects and was started on a maintenance course of rituximab every 8 weeks for 2 years. Unfortunately, 9 months into maintenance therapy, on PET scan, an FDG-avid lesion was found on the left adrenal gland. Laparoscopic adrenalectomy confirmed recurrent GZL. She is currently alive at 31 months, and a donor has been found for a hematopoietic stem cell transplant (SCT).
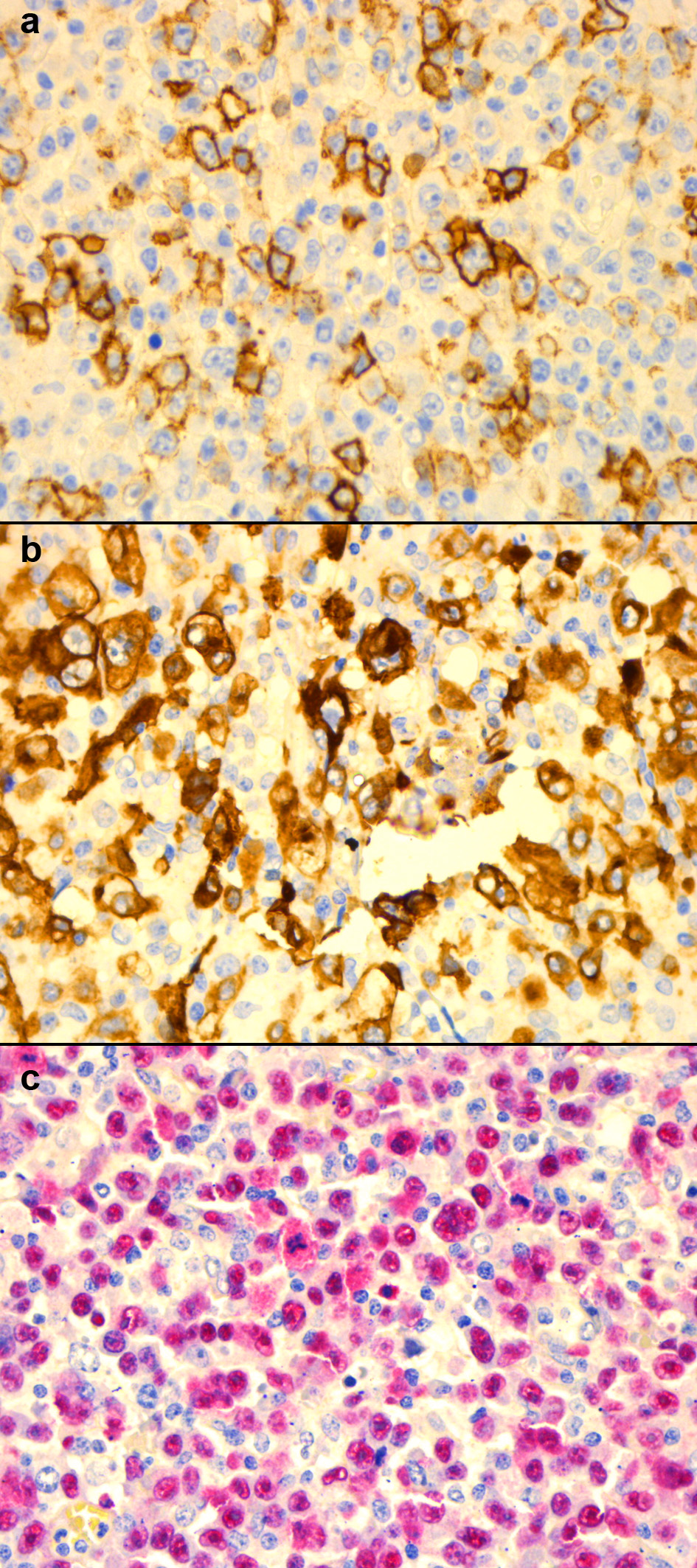 Click for large image | Figure 2. Case 2 immunohistochemistry of samples taken from cervical lymph node biopsy: (a) CD20+; (b) CD30+; (c) EBVR+. CD: cluster of differentiation; EBVR: Epstein-Barr virus receptor. |
| Discussion | ▴Top |
HIV has long been associated with the development of lymphomas [3]. Despite widespread use of effective antiretroviral therapy, persistent immune activation in HIV-latent infected cells has contributed to impaired cellular immunity, and in turn, predisposes to the development of cancer [4]. Unfortunately, despite the increased use of ART therapy, nearly one quarter of lymphoma is diagnosed in a controlled population with virologic suppression and immune recovery [5]. The combination of chronic B-cell stimulation and T-cell immunodeficiency is thought to contribute to loss of control of transforming viruses resulting in the potentiation of tumor generating effects of viruses like EBV and may be the basis of these lymphomas.
GZL is an uncommon form of lymphoma and literature on its occurrence in HIV patients is scarce. It is currently not considered an HIV-associated lymphoma and in a 2015 study it represented about 1% of lymphomas found in patients with AIDS [3]. A search of the literature found one case report of an HIV-seropositive patient with GZL in Uganda [6]. As with our cases, the patient in Uganda was both EBV+ and CD20+ [6]. EBV-infected cells are known to play a role in the pathogenesis of HL. Furthermore, co-infection with HIV, is thought to contribute to high B-cell turnover, and cytokine deregulation resulting in an increase the risk of developing lymphoma [7]. EBV receptor positivity represented a fascinating connection between our reported cases and the case in Uganda since EBV is only found in 16-28% of GZLs and could possibly play a role in HIV-associated GZL [8, 9]. Further research investigating EBV+ GZL in HIV+ could result in classification of GZL as a HIV-associated lymphoma. GZL is very rare in the setting of HIV, and thus it is difficult to draw any definitive conclusions on this correlation; more research and greater awareness is needed to help understand this connection.
Distinguishing between cHL, GZL, and Non-Hodgkin’s DLBCL remains a challenge and expert hematopathologists should be consulted to aid in the diagnosis. GZL was first described in 2005 as an entity with intermediate features between cHL and DLBCL [10]. In 2008, histological and immune-phenotypic criteria were officially defined by the World Health Organization (WHO) classification [11]. Since that time, diagnosis has become increasingly complex with the sub-classification of cHL-like and DLBCL-like disease as well as mediastinal disease and non-mediastinal disease [8, 12]. In their 2015 study, Evens et al found CD20+, CD30+, CD79a+, paired box 5 (PAX5)+, OCT2+, and MUM1+to be the most common positive markers in GZL [8]. Despite extensive characterization, GZL remains a challenging disease to diagnose. A 2017 expert panel investigating 68 cases of GZL from 15 large academic centers found that 62% of cases were incorrectly diagnosed [13]. To aid in diagnosis, a scoring system to assist in the diagnosis of GZL has been developed by O’Malley et al [14]. His team investigated the expression of CD15, CD45, CD20, PAX5, CD79a, MUM1, EBV-encoded small ribonucleic acid (EBER), and OCT2/BOB1. In this system, the samples were rated +1, 0, or -1 for each marker. Negative ratings would more likely indicate a DLBCL diagnosis, while more positive ratings would indicate a cHL diagnosis. An intermediate score ranging between +2 to -2 would represent a diagnosis of GZL [14]. Using this system, both DL and SR had scores of -1, corroborating the diagnosis respectively. This scoring system represents a potentially useful tool for diagnosing GZL in the future; however, further validation is required.
Multiple treatment strategies have been used since GZL was first described in 2008. These protocols have ranged from cHL-like regimens, doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazine, and prednisone (escBEACOPP), to DLBCL-like regimens, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP), doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACBVP), cyclophosphamide, etoposide, vincristine and prednisone (CEOP), and DA-EPOCH, and have had varying degree of success. Additionally, depending on the CD20 status of the lymphoma, treatment protocols have included rituximab. The first prospective study done with GZL was in 2014 by Wilson et al [12]. His group used DA-EPOCH with rituximab on 24 patients with mediastinal GZL (MGZL). All 24 patients responded, with 19 complete remissions and five partial remissions. At the median follow-up of 5 years, progression-free survival (PFS) was 62% with an overall survival (OS) of 74% [12]. Since that time, three retrospective studies have been done investigating other treatment protocols. All of these studies aided in the treatment strategies used in the cases reported here.
A 2015 study retrospectively looked at 112 patients from 19 north American centers. In this study, patients were treated with ABVD ± R, CHOP ± R, or DA-EPOCH + R. Of the three protocols, DA-EPOCH-R was found to have the best response rate of 82% with 73% complete remissions. This was markedly superior to the cHL treatment of ABVD ± R with overall and complete response rates of 61% and 55%, respectively [8]. Interestingly, patients treated with a DLCBL-like regimen had an improved 2-year PFS of 52% compared to 23% for patients cHL-like regimen [8]. This study concluded a DLCBL-like regimen was superior in the treatment of GZL. This study along with data that has shown DA-EPOCH to be an effective treatment regimen in HIV-associated lymphomas aided in our decision to treat our patients with this regimen [15].
In 2017, a small report highlighted the significance of dose intensity in addition to “lymphoma-like” in the treatment of GZL [16]. A retrospective study by Chihara et al [17] investigated the difference between dose-intensive (DA-EPOCH + R, cyclophosphamide, doxorubicin vincristine, and dexamethasone/methotrexate and cytarabine (hyper-CVAD/MA) + R) and less intense regimens (CHOP + R, ABVD). Among the 16 patients investigated, a 100% complete response was observed in patients that received the more intensive regimens, while CHOP + R and ABDV had overall and complete response rates of 50% and 33%, respectively [17]. Another larger retrospective study by Sarkozy et al was conducted in 2017, again the dose-intensive regimens were found to be superior to standard-dose regimens with a (3-year; event-free survival (EFS) of 74% versus 48%; OS of 90% versus 67%) [9]. The best strategy when approaching GZL appears to involve the use of dose-intensive DLBCL-like regimens such as R-EPOCH as was used our patients. However, this must be done with caution especially in HIV patients as the risk of side effects and drug-drug interactions is much greater.
GZL is known to be a more aggressive and a more challenging cancer to treat and given the CD20+ featured we found in both our patients Rituximab was used as part of both induction and maintenance therapy. This is not something that had not been done previously in GZL. However, it was something that was highlighted in the 2011 primary rituximab and maintenance (PRIMA) trial conducted on patients with follicular lymphoma. In that trial, which was done in follicular lymphoma, PFS was significantly improved when patients were treated with maintenance rituximab therapy for 2 years following chemotherapy [18]. Using this as a template we found mixed success in our patients. While both our patients are alive, one relapsed as noted above. It is important to note that our n = 2, and thus no definitive conclusion can be drawn from our case reports. However, this represents an intriguing treatment strategy for use in the future.
Conclusions
We described two cases at our institution that involved the rare combination of EBV+ GZL in HIV-infected patients. The oncogenesis of EBV has been well-described and it commonly seen in HIV-related lymphomas [19]. Although not currently considered an HIV-related lymphoma enhanced diagnosis aided with validated diagnostic criteria of GZL may help reveal a more definitive correlation between HIV and EBV+ GZL, Further EBV may play a more important role in the pathogenesis of GZL in HIV positive patients than in non-HIV-associated GZL.
We treated our patients with DA-EPOCH + R and attempted to maintain remission using rituximab every 8 weeks over 2 years. This treatment protocol has not been attempted previously in GZL. We found success both our patients achieved a complete response, and both patients are alive today. The use of DA-EPOCH + R followed by maintenance rituximab appears to be a viable treatment regimen for HIV-associated GZL. However, it requires further validation in a larger randomized prospective trial.
Acknowledgments
The authors would like to acknowledge Nisha Sookram at Pathology Department of Trinitas Regional Medical Center for helping provide records for case reports, and Heidi Fish for providing tumor histology images.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Amith Ahluwalia drafted the abstract, case 2, discussion, and edited the manuscript. Leena Bondili contributed to case 1. Julius Salamera edited the manuscript and contributed to HIV aspects of discussion. Michelle Cholankeril is the principle investigator, drafted the introduction, and edited the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- da Silva SR, de Oliveira DE. HIV, EBV and KSHV: viral cooperation in the pathogenesis of human malignancies. Cancer Lett. 2011;305(2):175-185.
doi pubmed - Gloghini A, Dolcetti R, Carbone A. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol. 2013;23(6):457-467.
doi pubmed - Schommers P, Hentrich M, Hoffmann C, Gillor D, Zoufaly A, Jensen B, Bogner JR, et al. Survival of AIDS-related diffuse large B-cell lymphoma, Burkitt lymphoma, and plasmablastic lymphoma in the German HIV lymphoma cohort. Br J Haematol. 2015;168(6):806-810.
doi pubmed - Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
doi pubmed - Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S, Olshan A, Dittmer DP, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis. 2013;57(5):756-764.
doi pubmed - Tumwine LK, Orem J, Ayers LW. EBV-positive grey zone lymphoma in an HIV infected man from Kampala, Uganda: case report. Int J Med Pharm Case Reports. 2014;2(5):110-116.
doi pubmed - Grewal R, Irimie A, Naidoo N, Mohamed N, Petrushev B, Chetty M, Tomuleasa C, et al. Hodgkin's lymphoma and its association with EBV and HIV infection. Crit Rev Clin Lab Sci. 2018;55(2):102-114.
doi pubmed - Evens AM, Kanakry JA, Sehn LH, Kritharis A, Feldman T, Kroll A, Gascoyne RD, et al. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. Am J Hematol. 2015;90(9):778-783.
doi pubmed - Sarkozy C, Molina T, Ghesquieres H, Michallet AS, Dupuis J, Damotte D, Morsschauser F, et al. Mediastinal gray zone lymphoma: clinico-pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica. 2017;102(1):150-159.
doi pubmed - Traverse-Glehen A, Pittaluga S, Gaulard P, Sorbara L, Alonso MA, Raffeld M, Jaffe ES. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol. 2005;29(11):1411-1421.
doi pubmed - Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019-5032.
doi pubmed - Wilson WH, Pittaluga S, Nicolae A, Camphausen K, Shovlin M, Steinberg SM, Roschewski M, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014;124(10):1563-1569.
doi pubmed - Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, Sehn LH, et al. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017;1(26):2600-2609.
doi pubmed - O'Malley DP, Fedoriw Y, Weiss LM. Distinguishing classical Hodgkin lymphoma, gray zone lymphoma, and large B-cell lymphoma: a proposed scoring system. Appl Immunohistochem Mol Morphol. 2016;24(8):535-540.
doi pubmed - Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245-3255.
doi pubmed - Yang XY, Jiang L, Jia RF, Hou AJ. Treatment of grey zone lymphoma using the R-CODOX-M/R-IVAC protocol: Two case reports. Medicine (Baltimore). 2017;96(39):e8121.
doi pubmed - Chihara D, Westin JR, Miranda RN, Cheah CY, Oki Y, Turturro F, Romaguera JE, et al. Dose adjusted-EPOCH-R and mediastinal disease may improve outcomes for patients with gray-zone lymphoma. Br J Haematol. 2017;179(3):503-506.
doi pubmed - Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Feugier P, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42-51.
doi - Bajaj BG, Murakami M, Robertson ES. Molecular biology of EBV in relationship to AIDS-associated oncogenesis. Cancer Treat Res. 2007;133:141-162.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


