| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 4, December 2019, pages 165-167
The Use of R-Hyper-CVAD in a Rare Case of Primary Bone Marrow Diffuse Large B-Cell Lymphoma
Allison Reeda, David Sommerhalderb, c
aDepartment of Pharmacy, Ochsner Louisiana State University Health Shreveport, 1541 Kings Hwy, Shreveport, LA 71103, USA
bDepartment of Medicine, Division of Hematology and Oncology, Louisiana State University Health Science Center Shreveport, 1501 Kings Hwy, Shreveport, LA 71103, USA
cCorresponding Author: David Sommerhalder, Department of Medicine, Division of Hematology and Oncology, Louisiana State University Health Science Center Shreveport, 1501 Kings Hwy, Shreveport, LA 71103, USA
Manuscript submitted August 27, 2019, accepted October 30, 2019
Short title: R-Hyper-CVAD in PBM DLBCL
doi: https://doi.org/10.14740/jh559
| Abstract | ▴Top |
Primary bone marrow lymphoma (PBML) is a rare clinical entity. Because of its rarity, there is no standard therapy defined. Prognosis in this disease is poor, and further studies for effective treatments are needed. In this report, we will discuss a patient with PBML who was treated with a hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine (hyper-CVAD) plus rituximab regimen with a favorable outcome. We believe this is the first reported use of this regimen in this type of lymphoma.
Keywords: Primary bone marrow lymphoma; R-Hyper-CVAD; B-cell lymphoma
| Introduction | ▴Top |
Here we describe a patient who presented with primary bone marrow (PBM) diffuse large B-cell lymphoma (DLBCL) who was treated with rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine (R-Hyper-CVAD) with a complete response (CR). Primary bone marrow lymphoma (PBML) is a rare malignancy compromising less than 1% of all B-cell lymphoma cases with a poor prognosis and no current standard of care exists [1]. Because of this, it is often treated with the same standard of care as typical DLBCL with mixed results.
| Case Report | ▴Top |
An 18-year-old African American female with no past medical history and no known drug allergies presented initially to an outside hospital with complaints of fatigue and generalized weakness. She was found to have a hemoglobin of 4.5 g/dL at that time. Testing revealed signs of hemolytic anemia, and she was treated with steroids, intravenous immunoglobulin (IVIG), and transfusions. Once her anemia stabilized, she was discharged home on steroids. She returned to the same hospital a few weeks later again with fatigue and now with new symptoms of severe back pain. Testing again showed anemia. Magnetic resonance imaging (MRI) of her spine was done for the back pain, and the results showed marrow abnormalities and a possible infarct of the L4 vertebrae. These abnormal findings directed physicians to perform a bone marrow biopsy which resulted several days later as DLBCL, activated B-cell type. The bone marrow morphology showed that the bone marrow spaces were almost entirely replaced by diffuse intermediate/large atypical appearing cells with a marked decrease of normal trilineage hematopoiesis. Immunohistochemical (IHC) stains on the biopsy demonstrated positivity for cluster of differentiation 20 (CD20), CD79a, PAX5, B-cell lymphoma 2 (BCL2) (weakly), BCL6, MUM1 (30%), Ki67 (65%). IHC testing resulted as negative for CD3, CD10, CD34, terminal deoxynucleotidyl transferase (TdT), myeloperoxidase (MPO), CD30, CD117, and cyclin D1. Fluorescence in situ hybridization (FISH) testing for BCL2, BCL6, and MYC was attempted on the sample but was unsuccessful due to inability to hybridize. Positron emission tomography (PET) scan (Fig. 1a) had been done at the outside hospital, and results had shown diffuse osseous uptake in the axial and appendicular skeleton with no other sites of uptake. Based on these results, it was determined that she had PBML. At that time, she was transferred to our facility for evaluation by a bone marrow transplant oncologist.
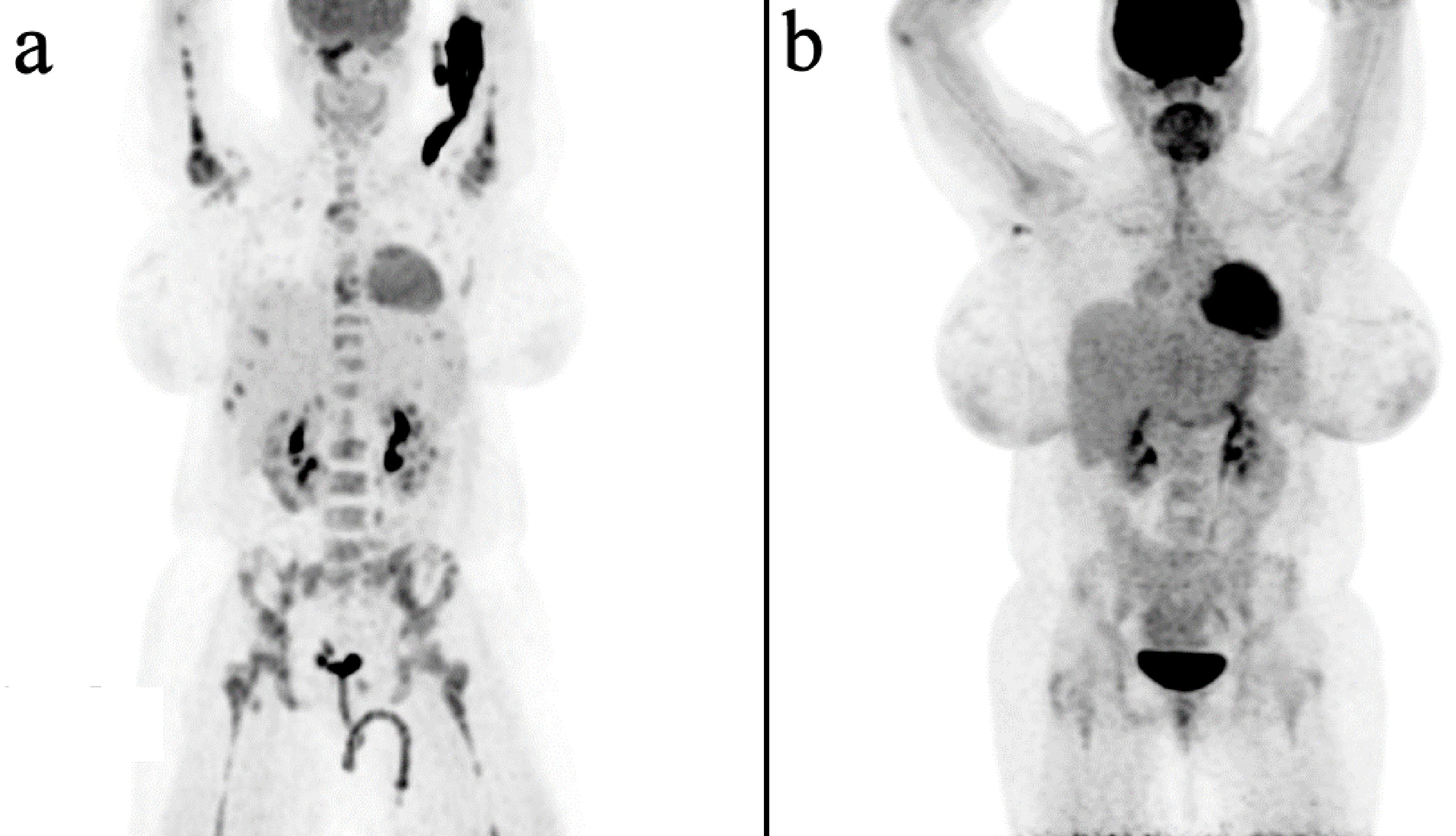 Click for large image | Figure 1. PET/CT images. (a) Prior to treatments at time of diagnosis with relative hyperintensity only noted within the bone marrow. (b) One month after completion of treatment with no evidence of disease. PET/CT: positron emission tomography/computed tomography. |
On arrival, patient’s labs showed: white blood cell (WBC) of 12.8 × 109/L, hemoglobin of 9.6 g/dL, hematocrit of 27.9%, platelets of 209 × 109/L, sodium of 137 mmol/L, potassium of 4.3 mmol/L, chloride of 107 mmol/L, bicarbonate of 25 mmol/L, glucose of 148 mg/dL, blood urea nitrogen of 16 mg/dL, creatinine of 0.64 mg/dL, phosphorus of 4.4 mg/dL, calcium of 8.4 mg/dL, albumin of 2.3 g/dL, total bilirubin of 1.2 mg/dL, aspartate aminotransferase (AST) of 32 U/L, alanine aminotransferase (ALT) of 38 U/L, uric acid of 1.8 mg/dL, lactate dehydrogenase (LDH) of 496 U/L, prothrombin time (PT) of 15.4 s (international normalized ratio (INR) of 1.25), activated partial thromboplastin time (aPTT) of 32 s, fibrinogen of 677 mg/dL, and D-dimer of 3.59 µg/mL. Infectious workup was negative except for a positive hepatitis B core Immunoglobulin G (IgG) antibody. Hepatitis B virus deoxyribonucleic acid (DNA) polymerase chain reaction (PCR) testing was then performed and resulted as undetectable. Entecavir 0.5 mg daily was used throughout and after treatment as hepatitis B reactivation prophylaxis. Baseline cerebrospinal fluid (CSF) testing showed normal cell counts and negative cytology. At this time, the decision was made to treat the patient with rituximab, cyclophosphamide, doxorubicin, vincristine, dexamethasone, and alternating with methotrexate and cytarabine as per the R-Hyper-CVAD protocol. She underwent four cycles of this regimen, with several complications. Complications included anemia requiring frequent transfusions, vertebral compression fracture of T9 with significant pain and debility, several episodes of neutropenic fever, pericardial effusion without tamponade, and a superior sagittal thrombus treated with 6 months of anticoagulation with apixaban. Some of these complications did result in delays in her treatment. The patient also completed six treatments of prophylactic intrathecal chemotherapy with methotrexate throughout the course of therapy.
After completion of all her treatments, the patient underwent another bone marrow biopsy as well as another PET scan (Fig. 1b). Both showed evidence of a CR. At the time of this writing, the patient is 1 year out from the time of her diagnosis. She is continuing to do well except for a stable mild leukopenia (WBC of 3.93 × 109/L). She has not shown any signs of relapse.
| Discussion | ▴Top |
This case demonstrates that R-Hyper-CVAD chemotherapy appears safe and effective in PBM DLBCL.
PBML, as described by Nishida et al, requires: confinement to the bone marrow, the absence of bone trabeculae destruction in the marrow, exclusion of any other leukemia/lymphoma which is known to arise primarily in the marrow, and no other evidence of extra bone marrow involvement such as lymph nodes, spleen, or liver [2]. Our patient did fit into these criteria and was thus considered to have PBML, of the DLBCL subtype. PBML is very rare, accounting for less than 1% of all non-Hodgkin lymphomas [1]. The majority of the reported cases of PBML are of the DLBCL subtype (71% in one series), although follicular lymphoma and peripheral T-cell lymphomas have also been reported [1]. The prognosis of this rare subtype of lymphoma is very poor, with a reported 2-year overall survival of 45% in one series [1] and a 20% overall survival rate with a maximum follow-up of 107 months in another meta-analysis [3]. The most common presentations for PBMLs are B-symptoms (67%), bone pain (33%), and frequent anemia and/or thrombocytopenia (89% for either), likely due to the involvement of the bone marrow [1, 2].
The vast majority of the reported PBML cases were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (prior to rituximab approval) or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) type regimens. The addition of rituximab to the CHOP regimen appears to improve outcomes but patient numbers remain too small to say with certainty at this time. Given the reportedly poor prognosis of this disease despite the use of CHOP regimens and our patient’s young age, we opted for a more intensive regimen for our patient with R-Hyper-CVAD. We believed this regimen might provide better outcomes and better central nervous system (CNS) protection given the patient’s relatively higher risk of CNS relapse with stage IV disease and elevated LDH. Hyper-CVAD was first described in 1997 by Koller et al at MD Anderson [4]. This regimen alternates hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone with methotrexate and cytarabine for a total of eight courses, each 21 days or less in length. In 2006, rituximab was tested with hyper-CVAD by Thomas et al, again at MD Anderson, and appeared efficacious [5]. When rituximab is added to the regimen, it is given only during the first four courses. This R-Hyper-CVAD regimen was completed with our patient. In one of the published case series, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (VACOP-B) (another intensive regimen) was used and CR was achieved for 8.8 years at the time of publication [1]. The VACOP-B regimen differs from R-Hyper-CVAD in that it includes etoposide and bleomycin and does not incorporate methotrexate or cytarabine for CNS coverage.
A review of published PBML cases and case series did not reveal any other patients with this lymphoma that had been treated with R-Hyper-CVAD. We plan to continue to monitor this patient very closely and hope that with further use of intensive chemotherapy regimens in this dismal subtype of lymphoma, we may be able to determine if these provide better outcomes for this group of patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
AR and DS were responsible for manuscript preparation. All authors have approved the final transcript.
| References | ▴Top |
- Martinez A, Ponzoni M, Agostinelli C, Hebeda KM, Matutes E, Peccatori J, Campidelli C, et al. Primary bone marrow lymphoma: an uncommon extranodal presentation of aggressive non-hodgkin lymphomas. Am J Surg Pathol. 2012;36(2):296-304.
doi pubmed - Nishida H, Suzuki H, Hori M, Obara K. Primary isolated bone marrow diffuse large B cell lymphoma with long-term complete remission. Leuk Res Rep. 2018;10:11-15.
doi pubmed - Chang H, Hung YS, Lin TL, Wang PN, Kuo MC, Tang TC, Wu JH, et al. Primary bone marrow diffuse large B cell lymphoma: a case series and review. Ann Hematol. 2011;90(7):791-796.
doi pubmed - Koller CA, Kantarjian HM, Thomas D, O'Brien S, Rios MB, Kornblau S, Murphy S, et al. The hyper-CVAD regimen improves outcome in relapsed acute lymphoblastic leukemia. Leukemia. 1997;11(12):2039-2044.
doi pubmed - Thomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569-1580.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


