| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 2, Number 1, June 2013, pages 27-30
Coronary Vasospasm Mimicking ST-Elevation Myocardial Infarction in a Patient With ATRA-Induced Differentiation Syndrome: A case Report and Review of Literature
Khawar Maqsooda, d, Ghazi Mirranib, Nosheen Sarwarb, James F. Burkec
aDepartment of Medicine, Division of Hospital Medicine, Baystate Medical Center, Tufts University School of Medicine, 759 Chestnut Street, Springfield MA 01199, USA
bDepartment of Medicine, Division of Internal Medicine, Lankenau Medical Center, 100 E Lancaster Avenue, Wynnewood PA 19096, USA
cDepartment of Medicine, Division of Cardiology, Lankenau Medical Center, 100 E Lancaster Avenue, Wynnewood PA 19096, USA
dCorresponding author: Khawar Maqsood, Department of Medicine, Division of Hospital Medicine, Baystate Medical Center, Tufts University School of Medicine, 759 Chestnut Street, Springfield, MA 01199, USA
Manuscript accepted for publication March 15, 2013
Short title: Coronary Vasospasm Mimicking ST-Elevation Myocardial Infarction
doi: https://doi.org/10.4021/jh58w
| Abstract | ▴Top |
All-trans retinoic acid (ATRA) has revolutionized the treatment of acute promyelocytic leukemia (APL). Differentiation syndrome is a frequent side effect of ATRA seen in patients with APL. We describe a case of chest pain with inferior ST elevations in a patient with ATRA induced differentiation syndrome. Patient was rushed for cardiac catheterization but his pain and EKG changes resolved after nitroglycerine administration in the catheterization lab. His coronary angiogram did not show any significant lesions raising concern for coronary vasospasm as the underlying cause for pain and EKG changes. Only one case of ATRA related MI has been described in literature before.
Keywords: All-trans retinoic acid; Coronary vasospasm; Differentiation syndrome
| Introduction | ▴Top |
Coronary vasospasm has been described in association with chemotherapy such as 5-FU and capecitabine [1-4]. All-trans retinoic acid (ATRA) is used in treatment of acute promyelocytic leukemia (APL) [5]. Differentiation syndrome is an unpredictable but frequent side effect of ATRA seen in patients with APL [6]. We describe a case of coronary vasospasm mimicking inferior ST-elevation myocardial infarction (STEMI) in a patient with ATRA-induced differentiation syndrome.
| Case Report | ▴Top |
A 65-year-old Caucasian gentleman with past medical history of hypertension, ulcerative colitis and group-B streptococcal sepsis was admitted for induction chemotherapy for a new diagnosis of acute promyelocytic leukemia (APL). He was initially started on cytarabine, doxorubicin and all-trans retinoic acid (ATRA) but he developed dyspnea which was thought to be secondary to differentiation syndrome. He was started on dexamethasone 10 mg BID. This was tapered to 4 mg daily over several days. He had a prolonged hospitalization due to neutropenic fever. Two weeks after initiation of chemotherapy the patient complained of chest pain and worsening dyspnea. EKG performed showed ST elevations in leads II, III and aVF with premature atrial complexes (Fig. 1).
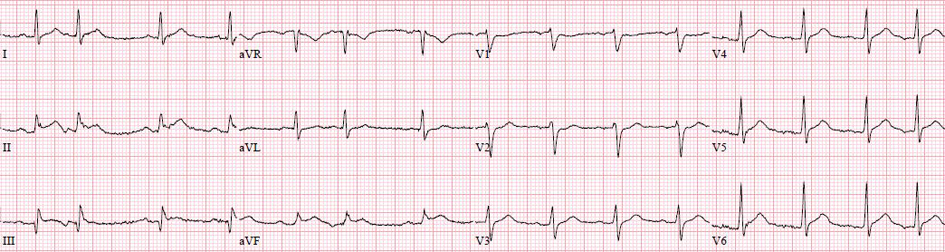 Click for large image | Figure 1. EKG performed showed ST elevations in leads II, III and aVF with premature atrial complexes. |
The patient was given nitroglycerine and intravenous metoprolol tartrate. He was taken to cardiac catheterization laboratory for a suspected myocardial infarction. In the laboratory, his EKG changes reversed and his symptom of chest pain resolved. Coronary arteriogram showed patent coronary arteries. Echocardiogram showed a normal ejection fraction without any regional wall motion abnormalities or pericardial effusion. He became hypotensive post procedure (possibly secondary to nitroglycerine and beta-blocker) and was started on dopamine infusion and transferred to the ICU.
He complained of ongoing dyspnea but had no other complaints. His blood pressure was 100/56, pulse 71/min and regular and pulse oximetry of 99% on 3 liters oxygen via nasal cannula. Pulmonary examination showed a few rhonchi and crackles at the right base. Rest of his physical examination was normal. Laboratory data showed a hemoglobin of 6.6 g/dL, WBC count 400/UL with absolute neutrophil count of 300/µL, platelets 37,000/µL, BUN 30 mg/dL, creatinine 1.5 mg/dL, troponin I 0.28 ng/mL and CPK 91 U/L. Chest x-ray showed pulmonary vascular prominence compatible with fluid overload versus differentiation syndrome. A ventilation perfusion lung scan showed low probability for a pulmonary embolism.
He was receiving ceftazidime for neutropenic fever. Dexamethasone was increased to 10 mg BID for the differentiation syndrome. He received transfusion of one unit of packed red blood cells. He was weaned off the dopamine the next day and was transferred to telemetry. His hospital course was complicated by pancytopenia requiring multiple transfusions of packed red blood cells and platelets. Ceftazidime was discontinued once his fevers subsided, and his cultures came back negative. He was restarted on amoxicillin for prophylaxis against his previous group-B streptococcal sepsis. Initially, he did not receive diuretics due to hypotension. Once his blood pressure stabilized, he was diuresed. His dyspnea improved with combination of diuresis, steroids and packed red blood cell transfusion. He was discharged home with a slowly tapering dose of dexamethasone.
| Discussion | ▴Top |
Cardiotoxicity is a not uncommon side effect of chemotherapy. Coronary vasospasm mimicking myocardial infarction has been described in patients receiving 5-fluorouracil and capecitabine, a pro-drug of 5-fluorouracil [7]. Hemorrhagic myocarditis caused by high-dose cyclophosphamide, potentiation of the cardiotoxic effect of anthracyclines by dacarbazine and plicamycin and serious ventricular and supraventricular arrhythmias induced by amsacrine have been reported [8-10]. Ischemic VT and polymorphic PVCs associated with a prolongation of QT interval secondary to 5-fluorouracil-induced vasospasm have been reported [11, 12].
ATRA has revolutionized the treatment of APL. Addition of ATRA to anthracyclines has been shown to improve disease-free survival and overall survival [13]. Although, ATRA is considered a safe medication with mild adverse reactions, retinoic acid syndrome (RAS) or differentiation syndrome is a serious and potentially fatal side effect seen in up to 2-25% of patients undergoing induction chemotherapy [14]. The onset of the syndrome ranges from 2 to 21 days (median period of 10 days) after initiating ATRA therapy.
The characteristic features of RAS include unexplained fever, pulmonary infiltrates and effusions associated respiratory distress, weight gain, elevated white blood cells, pericardial effusion, episodic hypotension and acute renal failure. The reported mortality from RAS is up to 2% [15-17]. At least three of the following signs and/or symptoms should be present to diagnose RAS: fever, weight gain, pulmonary infiltrates, pleural or pericardial effusions, respiratory distress, hypotension and renal failure. The exact pathogenesis of RAS is unclear. It is theorized that ATRA-treated APL cells release inflammatory cytokines including interleukin IL-1b, IL-6, IL-8 and tumor necrosis factor alpha (TNF-α). This release of cytokines can lead to endothelial damage. Tissue infiltration of APL cells may contribute to the syndrome [18].
Cardiac manifestations reported with ATRA-induced differentiation syndrome include pericardial effusion (19%), cardiac tamponade with cardiogenic shock [19], and myocarditis [20-24]. Reversible myocardial stunning has been described in a patient with non-obstructive coronary artery disease [25]. One patient receiving ATRA developed an acute myocardial infarction and lacunar cerebral infarction [26]. The exact pathogenesis of these events is unknown. One possibility is ATRA-induced coronary vasospasm resulting in myocardial infarction and myocardial stunning. Thromboembolism is a well-recognized complication associated with APL [27-29]. ATRA can increase the pro-coagulant state of APL, and the risk of thromboembolism rises to 5% [30, 31]. The release of inflammatory cytokines seen with RAS leads to endothelial injury and dysfunction, which may increase this risk.
ATRA has revolutionized the treatment of APL. In most patients, only mild adverse effects are noted. However, RAS is a potentially fatal complication. ATRA has been reported to cause pericardial effusion with cardiac tamponade, myocarditis, myocardial stunning and myocardial infarction. We report a case of ATRA-induced differentiation syndrome resulting in clinical presentation of inferior STEMI based on EKG and troponin I results. Coronary arteriography showed non-obstructive coronary artery disease. EKG changes were transient and reversed by nitrates. Coronary vasospasm, possibly induced by ATRA is the likely mechanism.
Disclosure
Authors have not received any funding or grants to support this project.
| References | ▴Top |
- Brestescher C, Pautier P, Farge D. [Chemotherapy and cardiotoxicity]. Ann Cardiol Angeiol (Paris). 1995;44(8):443-447.
- Mariani G, Giaccon G, Mastore M. Acute severe coronary spasm associated with initial first dose of 5-fluorouracil chemotherapy. Ital Heart J. 2003;4(8):568-570.
pubmed - Spencker S, Schmittel A, Westermann D, Marek A, Schultheiss HP, Witzenbichler B. [Angina pectoris and ST-elevation after chemotherapy with 5-fluorouracil]. Internist (Berl). 2007;48(1):69-72, 74.
doi pubmed - Scott PA, Ferchow L, Hobson A, Curzen NP. Coronary spasm induced by capecitabine mimicks ST elevation myocardial infarction. Emerg Med J. 2008;25(10):699-700.
doi pubmed - Park JH, Tallman MS. Treatment of acute promyelocytic leukemia without cytotoxic chemotherapy. Oncology (Williston Park). 2011;25(8):733-741.
pubmed - Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33(4):331-338.
doi pubmed - Ilveskoski E, Nikus K, Tanner M, Eskola M. [Coronary artery disease attack caused by cytotoxic chemotherapy]. Duodecim. 2009;125(9):997-1001.
pubmed - Birchall IW, Lalani Z, Venner P, Hugh J. Fatal haemorrhagic myocarditis secondary to cyclophosphamide therapy. Br J Radiol. 2000;73(874):1112-1114.
pubmed - Fenaux P, Tertian G, Castaigne S, Tilly H, Leverger G, Guy H, Bordessoule D, et al. A randomized trial of amsacrine and rubidazone in 39 patients with acute promyelocytic leukemia. J Clin Oncol. 1991;9(9):1556-1561.
pubmed - Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. 2009;11(12):1579-1586.
doi pubmed - Hrovatin E, Viel E, Lestuzzi C, Tartuferi L, Zardo F, Brieda M, Dametto E, et al. Severe ventricular dysrhythmias and silent ischemia during infusion of the antimetabolite 5-fluorouracil and cis-platin. J Cardiovasc Med (Hagerstown). 2006;7(8):637-640.
doi pubmed - Keefe DL, Roistacher N, Pierri MK. Clinical cardiotoxicity of 5-fluorouracil. J Clin Pharmacol. 1993;33(11):1060-1070.
doi pubmed - Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021-1028.
doi pubmed - Warrell RP, Jr., de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329(3):177-189.
doi pubmed - Warrell RP, Jr. Retinoid resistance in acute promyelocytic leukemia: new mechanisms, strategies, and implications. Blood. 1993;82(7):1949-1953.
pubmed - Larson RS, Tallman MS. Retinoic acid syndrome: manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol. 2003;16(3):453-461.
doi - Fenaux P, Chomienne C, Degos L. Acute promyelocytic leukemia: biology and treatment. Semin Oncol. 1997;24(1):92-102.
pubmed - Fenaux P, De Botton S. Retinoic acid syndrome. Recognition, prevention and management. Drug Saf. 1998;18(4):273-279.
doi pubmed - Larrea L, de la Rubia J, Jimenez C, Martin G, Sanz MA. Cardiac tamponade and cardiogenic shock as a manifestation of all-trans retinoic acid syndrome: an association not previously reported. Haematologica. 1997;82(4):463-464.
pubmed - Fabbiano F, Magrin S, Cangialosi C, Felice R, Mirto S, Pitrolo F. All-trans retinoic acid induced cardiac and skeletal myositis in induction therapy of acute promyelocytic leukaemia. Br J Haematol. 2005;129(3):444-445.
doi pubmed - Klein SK, Biemond BJ, van Oers MH. Two cases of isolated symptomatic myocarditis induced by all-trans retinoic acid (ATRA). Ann Hematol. 2007;86(12):917-918.
doi pubmed - Manna A, Cadenotti L, Motto A, Ballo P. Reversible cardiac dysfunction without myocytolysis related to all-trans retinoic acid administration during induction therapy of acute promyelocytic leukemia. Ann Hematol. 2009;88(1):91-92.
doi pubmed - van Rijssel RH, Wegman J, Oud ME, Pals ST, van Oers MH. A case of ATRA-induced isolated myocarditis in the absence of circulating malignant cells: demonstration of the t(15;17) translocation in the inflammatory infiltrate by in situ hybridisation. Leuk Res. 2010;34(7):e142-144.
doi pubmed - Choi S, Kim HS, Jung CS, Jung SW, Lee YJ, Rheu JK, Jo JR, et al. Reversible Symptomatic Myocarditis Induced by All-Trans Retinoic Acid Administration during Induction Treatment of Acute Promyelocytic Leukemia: Rare Cardiac Manifestation as a Retinoic Acid Syndrome. J Cardiovasc Ultrasound. 2011;19(2):95-98.
doi pubmed - De Santis GC, Madeira MI, de Oliveira LC, Falcao RP, Rego EM. Cardiac stunning as a manifestation of ATRA differentiation syndrome in acute promyelocytic leukemia. Med Oncol. 2012;29(1):248-250.
doi pubmed - Miyoshi T, Otsuki T, Omine K, Kirito K, Nagai T, Izumi T, Komatsu N, et al. [Acute promyelocytic leukemia accompanied by retinoic acid syndrome with complications of acute myocardial infarction and cerebral infarction during treatment with all-trans retinoic acid]. Rinsho Ketsueki. 2002;43(10):954-959.
pubmed - Pogliani EM, Rossini F, Casaroli I, Maffe P, Corneo G. Thrombotic complications in acute promyelocytic leukemia during all-trans-retinoic acid therapy. Acta Haematol. 1997;97(4):228-230.
doi pubmed - Ziegler S, Sperr WR, Knobl P, Lehr S, Weltermann A, Jager U, Valent P, et al. Symptomatic venous thromboembolism in acute leukemia. Incidence, risk factors, and impact on prognosis. Thromb Res. 2005;115(1-2):59-64.
doi pubmed - Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009;113(17):3911-3917.
doi pubmed - Montesinos P, Bergua JM, Vellenga E, Rayon C, Parody R, de la Serna J, Leon A, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775-783.
doi pubmed - Breccia M, Avvisati G, Latagliata R, Carmosino I, Guarini A, De Propris MS, Gentilini F, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21(1):79-83.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


