| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 9, Number 1-2, April 2020, pages 13-17
Novel Translocation in Acute Myeloid Leukemia: Case Report and Review of Risk-Stratification and Induction Chemotherapy in Patients With Acute Myeloid Leukemia
George M. Jehaa, Tiffany Wesleya, b, Vince D. Cataldob, c, d
aLouisiana State University Health Sciences Center, New Orleans, LA, USA
bLouisiana State University Health Sciences Center, Baton Rouge, LA, USA
cHematology-Oncology Clinic, Baton Rouge, LA, USA
dCorresponding Author: Vince D. Cataldo, Louisiana State University Health Sciences Center, Baton Rouge, LA, USA
Manuscript submitted January 9, 2020, accepted February 27, 2020
Short title: Novel AML Translocation: Case Report and Review
doi: https://doi.org/10.14740/jh605
| Abstract | ▴Top |
Identification of chromosomal abnormalities in patients with acute myeloid leukemia (AML) has contributed substantially to our current understanding of the molecular pathogenesis underlying leukemogenesis, and risk-stratification based on molecular abnormalities both influences treatment strategies and aids in determining prognosis. While over 300 established mutations have been documented in AML, the enhanced availability of genetic analysis and the increase in awareness of uncommon chromosomal translocations have made it possible for rare, apparently unique translocations to become recognized and to ultimately gain prognostic significance. Hence, we present a case of AML with a novel, balanced 2;12 translocation involving breakpoints previously undescribed. Although the patient required second induction, first remission was ultimately achieved. While the prognostic significance of this translocation is not fully elucidated, it is our hope that documentation of this patient’s presentation will help to characterize the significance of a yet undefined cytogenetic abnormality in AML.
Keywords: Acute myeloid leukemia; Translocation; t(2; 12)
| Introduction | ▴Top |
Acute myeloid leukemia (AML), the most common of the acute leukemias, is a complex, heterogeneous malignancy with marked genetic, epigenetic and phenotypic variation [1, 2]. In most cases of AML, chromosomes of malignant cells harbor specific, non-random and often recurrent abnormalities which have important implications in terms of clinical and pathological presentation, prognosis and therapeutic response. Pretreatment cytogenetic abnormalities, morphology, immunophenotype and clinical features define the prognostic categories of AML and predict induction success, cumulative incidence of relapse and overall survival in adults with de novo leukemia [2, 3]. The presence of certain genetic abnormalities, usually detected by fluorescence in situ hybridization (FISH) analysis, polymerase chain reaction (PCR) and next generation sequencing makes it possible to risk stratify patients at the time of initial presentation with AML.
| Case Report | ▴Top |
Patient characteristics
A 64-year-old female patient with a medical history of essential hypertension, obesity and osteoarthritis presented to the emergency department (ED) after an episode of lightheadedness and near-syncope while using her workplace commode. On admission, the patient had a white blood cell (WBC) count of 1.2 × 103/µL, a hemoglobin level of 8.7 g/dL and a platelet count of 20 × 103/µL. A review of her medical records revealed an ED admission 5 months prior with similar complaints and a platelet count of 121 × 103/µL, but at that time, the etiology for her thrombocytopenia was not pursued. Once common causes of pancytopenia, including nutritional, infectious, immune-mediated and medication-related etiologies were excluded, a bone marrow aspirate and biopsy was performed which revealed bone marrow hypercellularity (60%) with 70% blasts (Fig. 1). Immunophenotypic analysis using flow cytometry highlighted a prominent blast population (45.3% of cells) highly expressing CD34, CD13, CD33, CD117, CD71, CD22 (partial), and partial human leukocyte antigen D related (HLA-DR), consistent with myeloblasts. No Auer rods or promyelocytic features were identified.
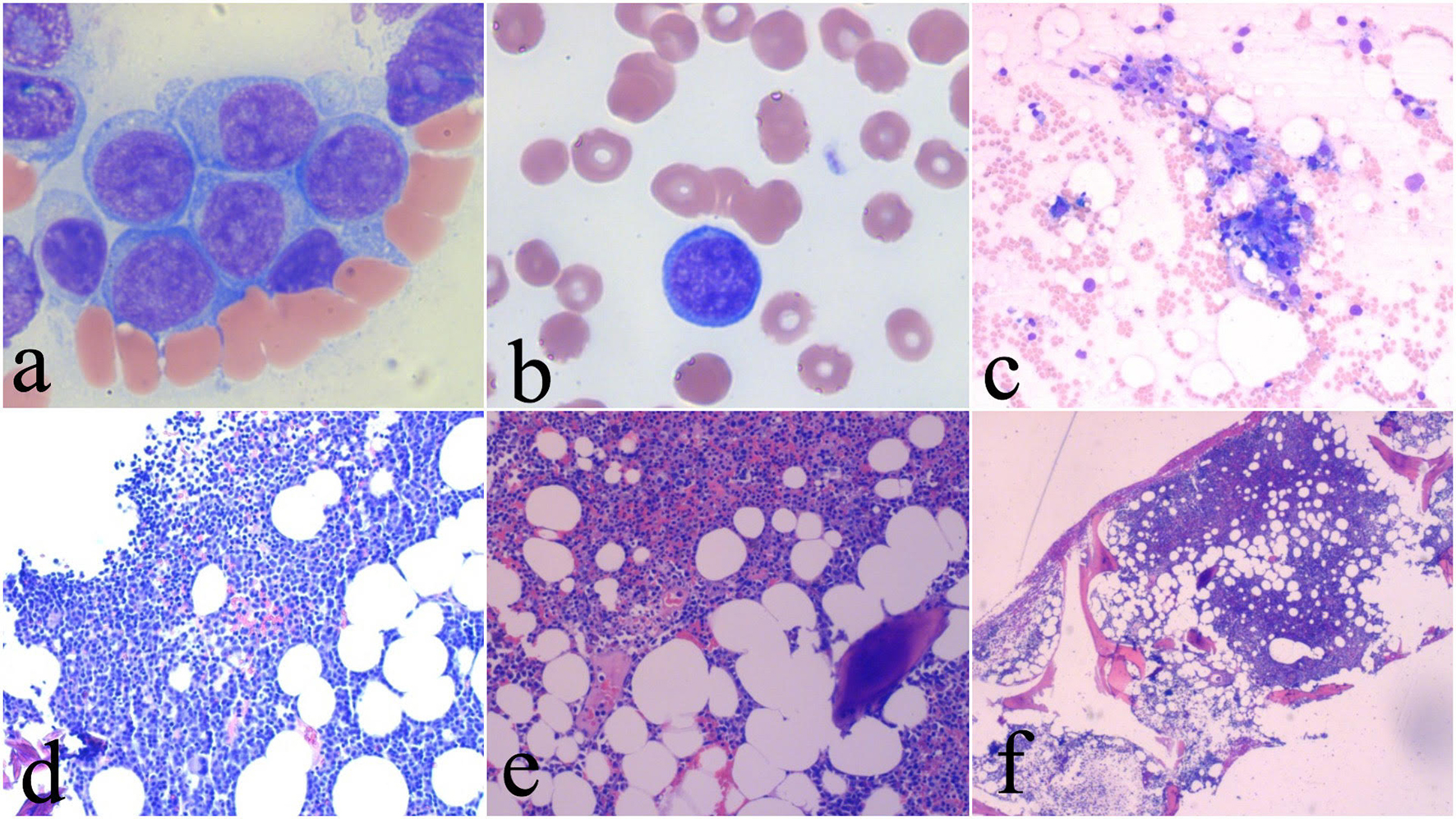 Click for large image | Figure 1. Pretreatment images showing blasts in the bone marrow and peripheral blood (a, b) and bone marrow hypercellularity (c). Bone marrow aspirate after two cycles of chemotherapy showing marked hypocellularity consistent with chemoablation (d). Post-recovery bone marrow aspirate and core biopsy showing recovered bone marrow (e, f). |
Chromosomal analysis
Chromosomal analysis demonstrated a balanced 2;12 translocation with the following karyotype: 46, XX, t(2;12) (q35;q24.1). This translocation was noted in all metaphases examined on karyotype analysis. FISH for AML (Mayo Clinic Laboratories) was negative for all cytogenic abnormalities included in the assay. As this is an unknown translocation in AML, a probe for this specific translocation was not utilized.
Chemotherapy and outcome
The patient underwent standard induction chemotherapy (7 + 3 therapy) with a 7-day cycle of cytarabine (100 mg/m2 per day, days 1 - 7) plus idarubicin (12 mg/m2 per day, days 1 - 3). Bone marrow aspirate and biopsy performed 14 days after the initiation of induction chemotherapy revealed persistent AML with 60-70% blasts present, and the patient was administered a 5-day cycle of salvage reinduction chemotherapy with cytarabine (100 mg/m2/day, days 1 - 5) plus idarubicin (12 mg/m2/day, days 1 - 2). On day 14 following the initiation of the second induction, bone marrow aspirate and biopsy assessment revealed a markedly hypocellular bone marrow (10%) with panhypoplasia consistent with chemoablation. Flow cytometric analysis at this time identified less than 0.1% of cells as CD34 positive myeloblasts.
| Discussion | ▴Top |
Impact of patient age on therapeutic decision-making
While AML may present at any age, it is most common in the elderly with a median age at diagnosis of 65 - 70 years. In older patients, comorbid medical conditions may limit treatment options, and outcomes are often worse than those seen in younger patients. Compared to younger AML patients, older patients tend to have a lower percentage of favorable cytogenetics (e.g. t(8;21), t(15;17) or inv(16)), higher percentage of unfavorable cytogenetics (e.g. complex cytogenetics or abnormalities of chromosomes 5 or 7), higher incidence of multidrug resistance, lower clinical response rates, shorter remission durations and shorter medial overall survival. Prior myelodysplastic syndrome, myeloproliferative disorders, or other hematologic disorders, which are unfavorable risk factors for outcomes, are also more frequent in older patients. These factors, in combination with fears of significant toxicity from chemotherapy, have led to a tendency for physicians to offer less intensive therapy or palliative care only to older patients with AML [3-5]. Table 1 summarizes prognostic risk factors for adults with AML [6].
 Click to view | Table 1. Favorable and Unfavorable Risk Factors for Outcomes in Adults With Acute Myeloid Leukemia [6] |
A review of Medicare records from 2000 to 2009 found that chemotherapy was administered within 3 months of diagnosis in less than half of patients over the age of 65 years, even though elderly patients receiving chemotherapy lived longer than those receiving supportive care only [5]. In an international study involving 488 patients aged > 65 years with newly diagnosed AML, physicians were asked whether they would choose best supportive care only, low-dose cytarabine, or intense chemotherapy (conventional 7 + 3), and only 18% of patients were chosen to receive intensive chemotherapy, compared to 18% for best supportive care only and 64% for low-dose cytarabine [4]. The risk of treatment-related mortality (TRM) may be a deterrent for physicians to offer intensive therapy to elderly patients, but it has been shown that age is not the most important factor determining TRM and should be considered only in the context of other covariates when choosing intensity of induction therapy [3, 7, 8]. In fact, excluding age from certain multivariate models designed to predict the risk of TRM did not significantly alter the predictive ability of these models, suggesting that older age is a surrogate for other prognostic factors and by itself should not be a contraindication to treatment [3, 9]. These data have significant real-world implications because, despite the tendency for physicians to pursue less aggressive therapy in elderly patients, intensive therapy improves both early death rates and long-term survival compared to palliative therapy, and some investigators argue that AML patients up to 80 years of age should be considered for standard intensive therapy [3, 5, 8]. Ultimately, the decision to undergo treatment must be weighed against the potential for significant diminishment in patient quality of life.
Goals of treatment
Goals of care for patients with AML are individualized and should be based on discussions between the patient and physicians. Influencers of goals of care include age, medical comorbidities, performance status, prognostic genetic features of the leukemic cells and individual goals of care. Most medically fit patients are treated with the goal of achieving long-term survival with the intention of cure. For medically frail patients, goals of care involve prolongation of life, alleviation of symptoms and improvement of quality of life. In patients with significant debility, severe comorbid medical conditions, or advanced age, treatment is often supportive with avoidance of disease-modifying therapy [6].
Pretreatment risk stratification
While a multitude of cytogenetic abnormalities exist in AML, many of these abnormalities are nonoverlapping and are associated with distinct clinical presentations, response to therapy, rates of relapse and overall survival [8, 10-13]. Thus, it is not surprising that cytogenetic abnormalities form the foundation for the classification and risk stratification systems created by the World Health Organization (WHO) and the European Leukemia Net (ELN) [10]. The 2017 ELN guidelines utilize cytogenetics to classify patients into “favorable”, “intermediate” and “adverse” risk groups (Table 2) [8]. Using this classification scheme, AML patients harboring cytogenetic abnormalities not included in the “favorable” or “adverse” categories should be classified as “intermediate”. The patient we present in this report was classified as “intermediate” risk due to her lacking cytogenetic classification criteria for the “favorable” or “adverse” risk groups. With the increased utility of next generation sequencing, the ongoing replacement of single gene assays by gene panel analysis, and the development of exome sequencing and genome wide assays, it is likely that the future will see the development of more sophisticated prognostic systems than ELN 2017 [3].
 Click to view | Table 2. The 2017 ELN Risk Stratification by Geneticsa |
Initial induction chemotherapy
With the exception of patients with acute promyelocytic leukemia, most patients with AML receive two courses of chemotherapy: induction and consolidation (often called post-remission therapy). The goal of induction therapy is the eradication of as many leukemic cells as possible from the bone marrow. In previous decades, induction therapy for most cases of AML was essentially invariant and consisted of a 7-day infusion of cytarabine and 3 days of daunorubicin or idarubicin (7 + 3 therapy). Today, this regimen remains the standard of care for most patients with favorable or intermediate prognostic features and for select patients with intermediate fitness. Treatment with a liposomal formulation of cytarabine and daunorubicin may be advisable as an alternate to 7 + 3 in patients with adverse prognosis AML, therapy-related AML, or AML with myelodysplasia-related changes [6]. Addition of a third agent to 7 + 3 may be indicated in some cases of AML. For example, gemtuzumab ozogamicin (GO), an antibody-drug conjugate consisting of a monoclonal antibody against CD33 linked to a cytotoxic agent, is approved for newly diagnosed and relapsed/refractory cases of AML in which cells express CD33. Midostaurin often is added to 7 + 3 for older patients harboring FLT3 mutation. In patients who are not candidates for intensive therapy, the hypomethylating agents decitabine and azacitidine may represent more appropriate treatment options. Current guidelines also recommend the consideration of investigational therapies and clinical trials in all patients with AML, and this is especially true in poor risk patients as the likelihood of achieving clinical response with conventional 7 + 3 is particularly low in these individuals. Additional studies in progress aim to define the most appropriate and successful induction strategies for newly diagnosed AML patients of various risk categories.
Allogeneic stem cell transplantation (allo-SCT)
The goal of initial therapy in AML is achievement of complete remission [14]. While over half of patients enter complete remission, without additional post-remission therapy, relapse of disease is nearly inevitable in the majority of these cases [14-16]. Post-remission therapy, which is based on cytogenetic analysis and risk of relapse, includes consolidation chemotherapy, myeloablative allo-SCT and autologous transplant [15]. In selected patients who have achieved first complete remission, especially poor-risk patients, allo-SCT is the preferred consolidation therapy [17-20]. Hematopoietic stem cell transplantation represents the only curative option for patients who fail to achieve clinical response or who relapse after achieving clinical response, and should be offered to patients with poor-risk cytogenetics or patients requiring second induction [17, 20].
Conclusion
Characterization of chromosomal abnormalities in AML has greatly contributed to our understanding of leukemogenesis. Risk-stratification based on molecular abnormalities both influences treatment strategies and aids in determining prognosis. With the enhanced availability of genetic analysis and the increase in awareness of rare chromosomal translocations, it is possible that previously uncharacterized genetic abnormalities may become recognized and ultimately gain prognostic significance. To our knowledge, the t(2;12) (q25;q24.1) translocation has not been previously described. Although this patient required second induction, first remission was ultimately achieved. While the prognostic significance of this translocation is not fully elucidated, it is our hope that documentation of this patient’s presentation will help to characterize the significance of a yet undefined cytogenetic abnormality in AML.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
GJ, VC, and TW analyzed and interpreted the patient data regarding the hematological disease and hospital course. GJ wrote the manuscript with support from VC and TW. VC and TW supervised the project. All authors discussed the results and contributed to the writing of the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Lowenberg B, Rowe JM. Introduction to the review series on advances in acute myeloid leukemia (AML). Blood. 2016;127(1):1.
doi pubmed - Sandhya DG, Ahmed F, Khadke K, Murthy SS, Rajappa SJ. A novel 11;18 translocation in a case of acute myeloid leukemia with maturation. Indian J Hum Genet. 2012;18(3):369-372.
doi pubmed - Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267-1291.
doi pubmed - Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299.
doi pubmed - Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127-1138.
doi pubmed - Schiffer CA, Larson RA, Rosmarin AG. Prognosis of acute myeloid leukemia. In: UpToDate (Post TW). 2019.
- O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, Bhatt V, et al. Acute myeloid leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(7):926-957.
doi pubmed - Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Ebert BL, et al. Global acute myeloid leukemia epidemiology and patient flow analysis 2016. Blood. 2017;129(4):424-448.
doi pubmed - Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, Appelbaum FR, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417-4423.
doi pubmed - Moarii M, Papaemmanuil E. Classification and risk assessment in AML: integrating cytogenetics and molecular profiling. Hematology Am Soc Hematol Educ Program. 2017;2017(1):37-44.
doi pubmed - Morra E, Barosi G, Bosi A, Ferrara F, Locatelli F, Marchetti M, Martinelli G, et al. Clinical management of primary non-acute promyelocytic leukemia acute myeloid leukemia: Practice Guidelines by the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation. Haematologica. 2009;94(1):102-112.
doi pubmed - Amriah Buang. WHO Klassifikation 2016. Blood [Internet]. 2006;2(20):58-71. Available from: http://www.ukm.my/geografia.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951.
doi pubmed - Kanate AS, Pasquini MC, Hari PN, Hamadani M. Allogeneic hematopoietic cell transplant for acute myeloid leukemia: Current state in 2013 and future directions. World J Stem Cells. 2014;6(2):69-81.
doi pubmed - Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349-2361.
doi pubmed - Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, Bennett JM, et al. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol. 1988;6(4):583-587.
doi pubmed - Ganapule A, Nemani S, Korula A, Lakshmi KM, Abraham A, Srivastava A, Balasubramanian P, et al. Allogeneic Stem Cell Transplant for Acute Myeloid Leukemia: Evolution of an Effective Strategy in India. J Glob Oncol. 2017;3(6):773-781.
doi pubmed - Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, Willman C, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649-1656.
doi pubmed - Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, van Marwijk Kooy M, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9):3658-3666.
doi pubmed - Hamilton BK, Copelan EA. Concise review: the role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells. 2012;30(8):1581-1586.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


