| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 9, Number 1-2, April 2020, pages 18-22
Spontaneous Remission in a Patient With Acute Myeloid Leukemia Leading to Undetectable Minimal Residual Disease
Daniel Helbiga, c, d, Andres E. Quesadab, c, Wenbin Xiaob, Mikhail Roshalb, Martin S. Tallmana, David A. Knorra
aDepartment of Medicine, Leukemia Service, Memorial Sloan Kettering Cancer Center, NYC, NY, USA
bDepartment of Pathology, Hematopathology Service, Memorial Sloan Kettering Cancer Center, NYC, NY, USA
cThese authors contributed equally to this work.
dCorresponding Author: Daniel Helbig, Department of Medicine, Leukemia Service, Memorial Sloan Kettering Cancer Center, 530 E 70thStreet, M-507, NYC, NY 10021, USA
Manuscript submitted January 15, 2020, accepted February 17, 2020
Short title: Spontaneous Remission of AML
doi: https://doi.org/10.14740/jh606
| Abstract | ▴Top |
Although rare, spontaneous remission (SR) of acute myeloid leukemia (AML) has been reported in the literature, the underlying mechanisms driving remission remain unknown. However, it is most commonly associated with a preceding severe infection. We present a case of a 40-year-old man with no past medical history who presented to our hospital with severe left hip pain and fevers and was found to have AML. Chemotherapy was delayed because the patient required extensive debridement and fasciotomy of his left hip and a prolonged course of antibiotics. After his acute illness had stabilized, a repeat bone marrow biopsy was performed which showed no abnormal myeloid blasts and resolution of his original cytogenetic and molecular abnormalities. At the time of this writing, our patient remains in remission with undetectable minimal residual disease (MRD), now 14 months from his initial diagnosis of AML.
Keywords: AML; Spontaneous remission; Minimal residual disease
| Introduction | ▴Top |
Acute myeloid leukemia (AML) is defined by the presence of > 20% blasts in the peripheral blood or bone marrow and treatment with chemotherapy is typically promptly initiated once a patient is diagnosed. While rare, spontaneous remission (SR) of AML prior to chemotherapy has been reported, the underlying mechanisms driving remission remain unknown. It was first described in the literature by Eisenlohr in 1878 after a patient had regression of “marked leukocytosis” after a severe typhoid infection [1]. More recently, Rashidi and Fisher reviewed 46 cases of SR of AML from 1951 to 2014 and there have been several other case reports since 2014 describing this phenomenon [2-7].
Herein, we report an exceptional case of a patient who had SR of AML after a severe infection that led to complete remission (CR) with undetectable minimal residual disease (MRD) and resolution of his previous cytogenetic and molecular abnormalities. To our knowledge, this is the first report of a patient with SR in AML who had multiple sensitive MRD testing over several time points to confirm CR with undetectable MRD. Additionally, we evaluate the potential mechanisms of SR in AML and review the previous literature on the topic.
| Case Report | ▴Top |
A 40-year-old man with no past medical history presented to an outside hospital with severe left hip pain and fevers that started abruptly the day prior. On arrival to the hospital, he was febrile (105 °F) and tachycardiac in the 120s. On physical exam, he had significant erythema and tenderness to the left hip area. He underwent a computed tomography (CT) scan that demonstrated an acute tear of the left gluteal medius muscle. Initial blood cultures grew methicillin-susceptible Staphylococcus aureus (MSSA) so the patient was initiated on broad-spectrum antibiotics. Initial labs also revealed pancytopenia with a white blood cell (WBC) count of 0.2 × 109/L, hemoglobin level of 7.1 g/dL, and platelet count of 28 × 109/L. Lactate dehydrogenase (LDH) was 539 units/L and creatine kinase (CK) was 2,063 units/L. The remainder of the labs were within normal limits.
A bone marrow biopsy was performed, and the patient was transferred to our institution for further workup given the concern for an underlying leukemia. The initial bone marrow biopsy revealed a hypercellular marrow (70-80%) with sheets of blasts and scattered small islands of erythroid elements and reduced megakaryocytes (Fig. 1a, b). Flow cytometry of the bone marrow aspirate revealed blasts positive for CD34 and CD117 (partially), comprising 80% of the total cells analyzed. Flow cytometry of the peripheral blood identified a CD34 positive blast population (3.7% of total WBCs) with abnormal expression of CD11b, CD56 (subset), CD13 (absent), CD33 (mostly absent), and CD117 (absent to dim) (Fig. 2a-d). Cytogenetics demonstrated trisomy 8 and molecular testing revealed BCOR (p.Val1112fs), RUNX1 (p.Ile364fs), and IDH2 (p.Arg172Lys) mutations. Based on the above, a diagnosis of AML was made.
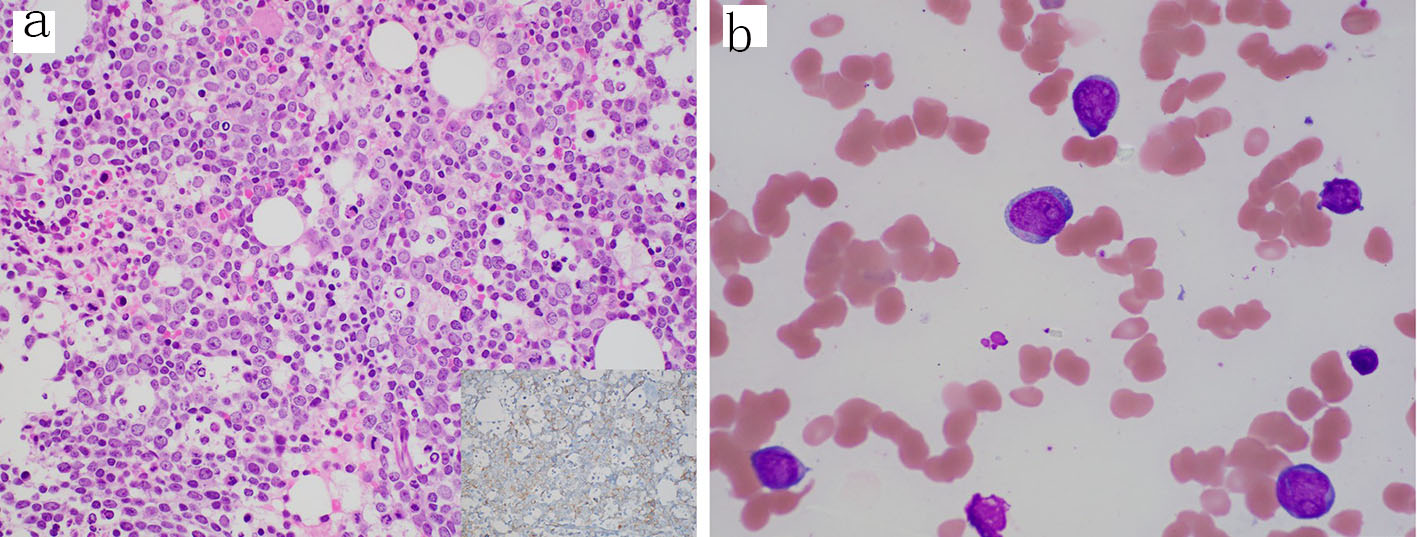 Click for large image | Figure 1. Diagnostic bone marrow biopsy and aspirate with acute myeloid leukemia. (a) Core biopsy showing sheets of blasts and decreased trilineage hematopoiesis (H&E, 400 ×); inset, CD34 stain (400 ×) highlighting the increased blasts. (b) Aspirate smear showing blasts with irregular nuclei, fine chromatin, prominent nucleoli and scant cytoplasm (Wright-Giemsa, 1000 × oil immersion). H&E: hematoxylin-eosin staining. |
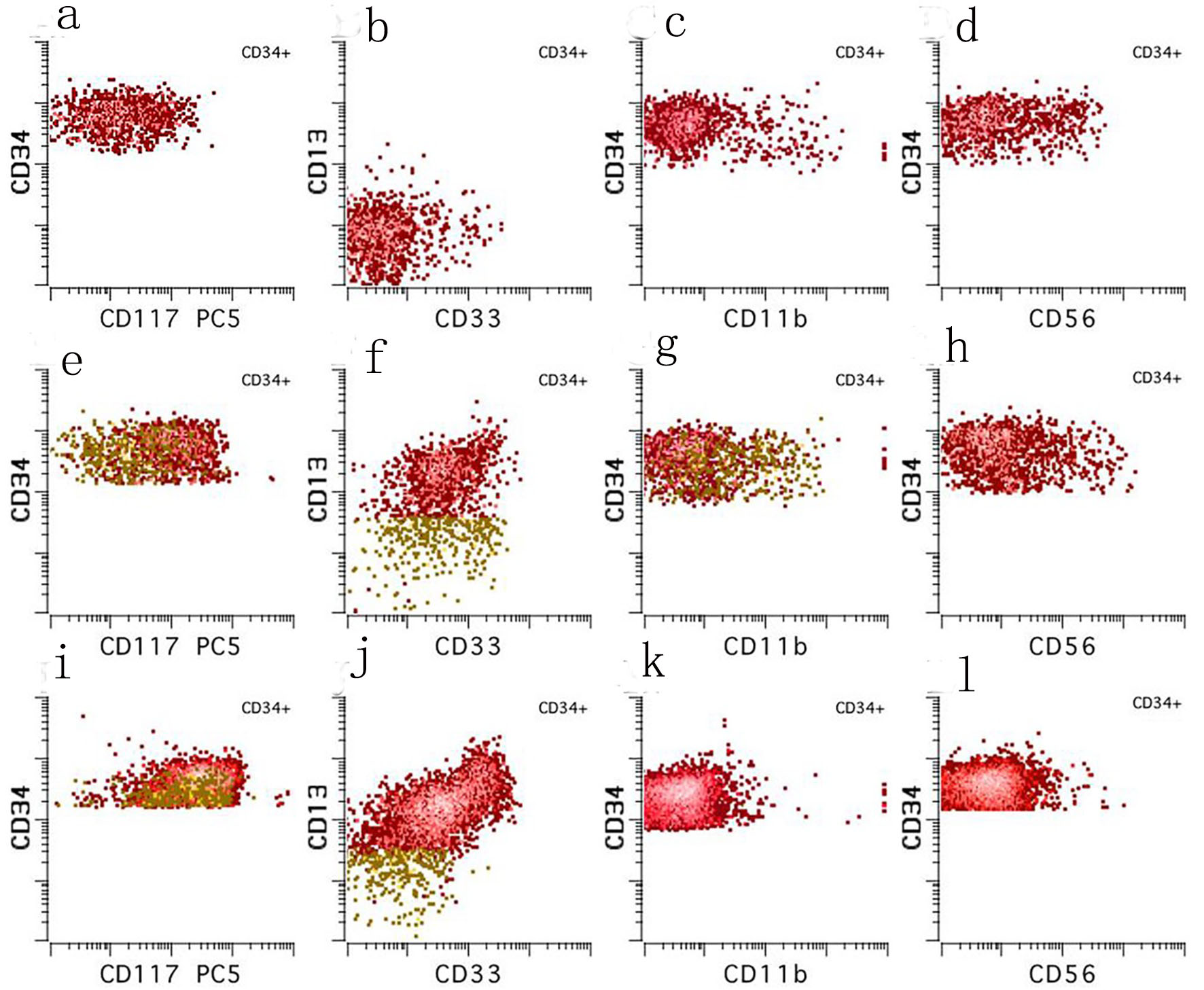 Click for large image | Figure 2. Ten color multiparameter flow cytometry. (a-d) Flow cytometry performed on the peripheral blood at our institution shortly after (day 3) the diagnostic bone marrow was taken showing a CD34 positive blast population (3.7% of total white blood cells) with abnormal expression of CD117 (absent to dim), CD13 (absent), CD33 (mostly absent), and CD56 (subset). (e-h) Flow cytometry performed on the day 21 bone marrow aspirate showing a small abnormal blast population (orange, 0.08% of total white blood cells) with a similar immunophenotype to those seen in the peripheral blood on day 3. (i-l) Flow cytometry performed on the day 49 bone marrow aspirate showing no abnormal myeloid blast population. |
On arrival to our institution, the patient continued to experience daily fevers and required frequent transfusions of red blood cells. He also continued to complain of severe left hip pain despite broad-spectrum antibiotics and anti-fungal coverage. Magnetic resonance imaging (MRI) of the left hip revealed an underlying fluid collection and possible abscess within the gluteus medius muscle. Drainage by interventional radiology was attempted but unsuccessful. A repeat MRI several days later showed worsening myositis, myonecrosis, and fasciitis so the patient underwent extensive debridement and fasciotomy and was transferred to the intensive care unit (ICU) afterwards for closer monitoring. He eventually stabilized and was transferred out of the ICU to the leukemia floor in preparation for starting induction chemotherapy, but it was postponed to allow time for his left leg infection to heal after his extensive surgery.
While waiting to begin induction chemotherapy, his fevers resolved and his peripheral blood counts improved. A repeat bone marrow biopsy was performed 3 weeks from the original bone marrow biopsy (day 21), revealing a normocellular marrow (60-70%) with trilineage maturing hematopoiesis with no overt increase in blasts (Fig. 3). Flow cytometry identified a small abnormal myeloid blast population comprising only 0.08% of the total cells analyzed, having abnormal expression of CD11b, CD56 (partial), CD13 (absent), CD33 (dim to absent), and CD117 (dim to absent) (Fig. 2e-h).
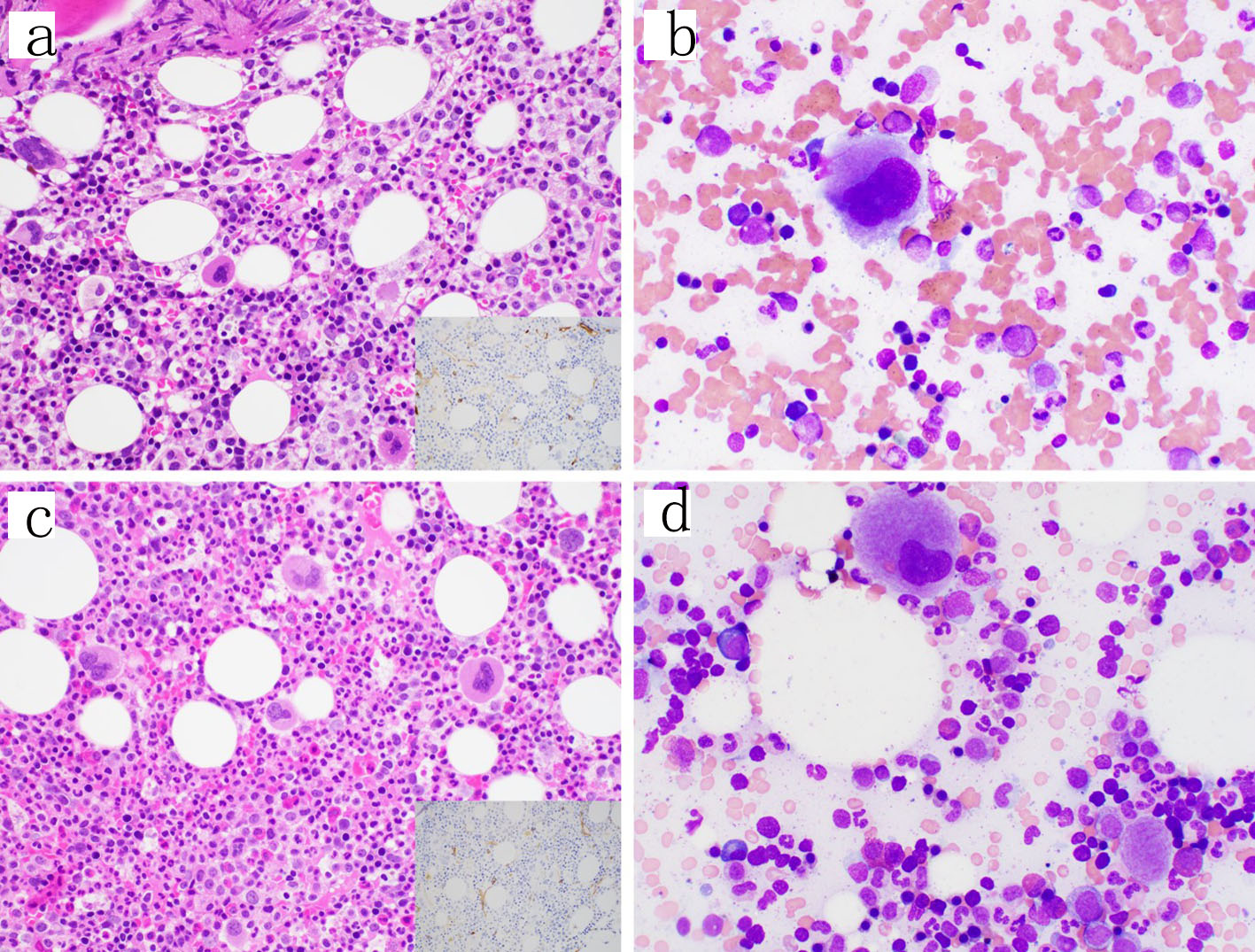 Click for large image | Figure 3. Follow-up bone marrow biopsies and aspirates. (a, b) Day 21 from the original bone marrow biopsy. The core biopsy showed trilineage maturing hematopoiesis and no increase in blasts by H&E (400 ×) or CD34 immunohistochemical stain (inset, 400 ×) or the aspirate smear (1000 × oil immersion); however, a small abnormal blast population was detected by flow cytometry. (c, d) Day 49 from the original bone marrow biopsy similarly showing trilineage maturing hematopoiesis and no increase in blasts on the core biopsy by H&E (400 ×) or CD34 immunohistochemical stain (inset, 400 ×) or the aspirate smear (1000 × oil immersion) and no abnormal blast population detected by flow cytometry. H&E: hematoxylin-eosin staining. |
Given this remarkable improvement, no treatment was given and he was discharged home. A repeat bone marrow was performed a month later (day 49) as an outpatient, which showed a normocellular marrow (50-60%) with trilineage maturing hematopoiesis with no abnormal myeloid blasts identified by flow cytometry (Fig. 2i-l). Intriguingly, cytogenetics showed no abnormalities and molecular testing using a next-generation sequencing (NGS) assay revealed no abnormalities. He underwent a fourth bone marrow biopsy a month later in order to continue to monitor for evidence of disease, which once again showed no evidence of leukemia using flow MRD testing. He has had multiple repeat bone marrows as an outpatient since diagnosis with no evidence of leukemia.
| Discussion | ▴Top |
At the time of this writing, our patient remains in remission with undetectable MRD and has remained off treatment to this date, now 6 months from his initial diagnosis of AML. SR in AML remains a rare phenomenon, and is typically associated more with solid rather than liquid malignancies [8, 9]. However, the vast majority of patients with AML receive intensive chemotherapy immediately after establishing the diagnosis, which eliminates the possibility of an SR. Therefore, only a small subset of patients, such as our patient with an overwhelming infection, have the potential to demonstrate an SR as their treatment is often delayed.
The mechanism of SR remains unclear but one of the most frequently proposed is an immune-mediated cause secondary to a preceding severe infection. In the paper by Rashidi and Fisher that reviewed 46 cases of SR of AML, 42 patients (91.3%) had a fever prior to SR and 32 patients (71.1%) had a documented infection with the most common infections being pneumonia, bacteremia, and skin/soft tissue infections [2]. A potential mechanism by which a severe infection may contribute to remission of leukemia is through the excessive activation and productive of pro-inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin 2 (IL-2) leading to increased activity of T lymphocytes, macrophages, and natural killer (NK) cells all leading to an anti-leukemia effect. In fact, previous case reports have shown elevated levels of TNF and IL-2, as well as increased activity of NK cells, during active infections in patients who had an SR of AML [10, 11].
The role of the immune system in eradicating malignancy was pioneered centuries ago by Dr. William Coley in the 1890s. Considered the “father of cancer immunotherapy”, Dr. Coley read about a patient who developed regression of their sarcoma after they developed a severe erysipelas infection caused by Streptococcus pyogenes. He then created his own mixture of Streptococcus pyogenes and Serratia marcescens, called Coley’s toxin, using it to treat sarcoma patients for decades [12, 13]. In fact, it was trialed as a potential treatment for leukemia patients in the early 1900s as well [14]. More recently, the field of immunotherapy in hematologic malignancies has been rapidly evolving with the emergence of chimeric antigen receptor (CAR) T-cells, Bi-specific T-cell engagers (BiTEs), and checkpoint inhibitors, which are all designed to stimulate the patient’s own immune system to eliminate the malignant cells.
However, it is unclear whether infection alone could explain SR of AML, especially because a large percentage of patients with AML present with fever and documented infection yet their leukemia does not spontaneously remit. In fact, in one study, up to 72% of patients who presented to the hospital at one institution with de novo AML had a concomitant fever at presentation [15]. In addition, there have been several case reports with patients who had an SR of their AML without a preceding infection, making it unlikely that infection alone could explain all cases of SR of AML [2-7].
At this point in time, it remains unknown what the optimal treatment should be for this rare group of patients, but the comprehensive review by Rashidi and Fisher does provide some evidence [2]. Of the 46 patients identified in the paper, 39 patients (88.6%) achieved a CR while the rest only had a partial response (PR). However, the median duration of remission was only 5 months and the vast majority of these patients eventually relapsed with one relapse occurring as late as 36 months after initial diagnosis. At the time of their publication, eight patients (17.4%) had not relapsed, although three of those patients still had less than a year of follow-up. Only three patients (6.5%) remained in remission up to 4 years after their initial diagnosis of AML and were likely cured of their AML without needing chemotherapy.
Of note, the patients in the review by Rashidi and Fisher were only in a morphologic CR, defined as < 5% blasts in the bone marrow. However, management of leukemia has evolved in recent years as MRD testing is now frequently obtained to assist in decision making. Uniquely, our patient had resolution of his previous BCOR, RUNX1, and IDH2 mutations on the NGS panel. BCOR and RUNX1 mutations are both adverse prognostic markers and are associated with higher risk disease in AML, whereas IDH2 mutations are generally thought to have a neutral impact but have become more clinically relevant recently with the discovery of novel IDH1/2 inhibitors [16-18]. To our knowledge, resolution of BCOR, RUNX1, or IDH2 mutations has not been described in previous case reports of SR in AML; however, monitoring of RUNX1 and IDH2 mutation status by NGS during CR has been shown to identify patients at risk of relapse [19-21].
Conclusions
In conclusion, we present a case of SR of AML leading to CR with undetectable MRD and resolution of previous cytogenetic and molecular abnormalities. While rare, SR provides evidence for the role that the patient’s own immune system can have in AML and offers opportunity to explore novel immunotherapy approaches in the treatment of AML. Future research is still needed to determine what the optimal treatment strategy should be for these patients. For now, we propose that patients achieving CR with undetectable MRD can be safely monitored with frequent serial bone marrow biopsies to assess for disease relapse.
Acknowledgments
We would like to acknowledge the multidisciplinary team at Memorial Sloan Kettering Cancer Center who were involved in the care of this patient.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The manuscript has been sufficiently de-identified to protect the patient.
Author Contributions
DH, DAK, and AEQ wrote the manuscript. All authors contributed to the editing of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Eisenlohr C. Leucaemia lienalis, lymphatica et medullaris mit multiplen Gehirnnervenlahmungen. Archiv fur Pathologie, Anatomie und Physiologie und fur Klinische Medizin. 1878;73:56-73.
doi - Rashidi A, Fisher SI. Spontaneous remission of acute myeloid leukemia. Leuk Lymphoma. 2015;56(6):1727-1734.
doi pubmed - Kazmierczak M, Szczepaniak A, Czyz A, Rupa-Matysek J, Komarnicki M. Spontaneous hematological remission of acute myeloid leukemia. Contemp Oncol (Pozn). 2014;18(1):67-69.
doi pubmed - Camus V, Etancelin P, Jardin F, Lenain P, Contentin N, Daliphard S, Buchonnet G, et al. Spontaneous remission in three cases of AML M5 with NPM1 mutation. Clin Case Rep. 2015;3(11):955-959.
doi pubmed - Vachhani P, Mendler JH, Evans A, Deeb G, Starostik P, Wallace PK, Wang ES. Spontaneous remission in an older patient with relapsed FLT3 ITD mutant AML. Case Rep Hematol. 2016;2016:1259759.
doi pubmed - Mozafari R, Moeinian M, Asadollahi-Amin A. Spontaneous Complete Remission in a Patient with Acute Myeloid Leukemia and Severe Sepsis. Case Rep Hematol. 2017;2017:9593750.
doi pubmed - Rautenberg C, Kaivers J, Germing U, Haas R, Schroeder T, Kobbe G. Spontaneous remission in a patient with very late relapse of acute myeloid leukemia 17 years after allogeneic blood stem cell transplantation. Eur J Haematol. 2019;103(2):131-133.
doi pubmed - Jessy T. Immunity over inability: The spontaneous regression of cancer. J Nat Sci Biol Med. 2011;2(1):43-49.
doi pubmed - Salman T. Spontaneous tumor regression. Journal of Oncological Science. 2016:2(1):1-4.
doi - Musto P, D'Arena G, Melillo L, Cascavilla N, La Sala A, Ladogana S, Carotenuto M. Spontaneous remission in acute myeloid leukaemia: a role for endogenous production of tumour necrosis factor and interleukin-2? Br J Haematol. 1994;87(4):879-880.
doi pubmed - Jimemez C, Ribera JM, Abad E, Pintos G, Milla F, Junca J, Feliu E. Increased serum tumour necrosis factor during transient remission in acute leukaemia. Lancet. 1993;341(8860):1600.
doi - Hoption Cann SA, van Netten JP, van Netten C, Glover DW. Spontaneous regression: a hidden treasure buried in time. Med Hypotheses. 2002;58(2):115-119.
doi pubmed - Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672-680.
- Larrabee R. The treatment of leukemia with the mixed toxins of Coley. Boston Medical and Surgical Journal. 1908;158:183-187.
doi - Kulsoom B, Shamsi TS, Ahmed N, Hasnain SN. Clinical presentation of acute myeloid leukaemia - A decade-long institutional follow-up. J Pak Med Assoc. 2017;67(12):1837-1842.
- Terada K, Yamaguchi H, Ueki T, Usuki K, Kobayashi Y, Tajika K, Gomi S, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408.
doi pubmed - Schnittger S, Dicker F, Kern W, Wendland N, Sundermann J, Alpermann T, Haferlach C, et al. RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood. 2011;117(8):2348-2357.
doi pubmed - DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, Routbort M, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736.
doi pubmed - Kohlmann A, Nadarajah N, Alpermann T, Grossmann V, Schindela S, Dicker F, Roller A, et al. Monitoring of residual disease by next-generation deep-sequencing of RUNX1 mutations can identify acute myeloid leukemia patients with resistant disease. Leukemia. 2014;28(1):129-137.
doi pubmed - Ok CY, Loghavi S, Sui D, et al. Persistent IDH1/2 mutation in remission can predict relapse in patients with acute myeloid leukemia. Haematologica. 2018;104(2):305-311.
doi pubmed - Petrova L, Vrbacky F, Lanska M, Zavrelova A, Zak P, Hrochova K. IDH1 and IDH2 mutations in patients with acute myeloid leukemia: Suitable targets for minimal residual disease monitoring? Clin Biochem. 2018;61:34-39.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


