| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 9, Number 1-2, April 2020, pages 33-36
Lenalidomide Plus Decitabine Treatment in a Myelodysplastic Syndrome Patient With Deletion 5q and Excess Blasts
Istemi Serina, c, Rafet Erenb, Mehmet Hilmi Dogua
aDepartment of Hematology, University of Health Science, Istanbul Training and Research Hospital, Istanbul, Turkey
bDepartment of Hematology, University of Health Science, Okmeydani Training and Research Hospital, Istanbul, Turkey
cCorresponding Author: Istemi Serin, Department of Hematology, University of Health Science, Istanbul Training and Research Hospital, Istanbul 34098, Turkey
Manuscript submitted March 1, 2020, accepted March 23, 2020
Short title: Lenalidomide and Decitabine in MDS-RAEB-1
doi: https://doi.org/10.14740/jh618
| Abstract | ▴Top |
This is the first case of decitabine plus lenalidomide treatment for a myelodysplastic syndrome (MDS) patient with 5q deletion (del(5q)) and elevated number of blasts. Upon bone marrow aspiration and biopsy with conventional cytogenetical studies she was diagnosed with MDS with del(5q) and refractory anemia with excess blasts (RAEB-1). Decitabine was started at a daily dose of 20 mg/m2 1 - 5 days and lenalidomide was started at daily doses of 10 mg 6 - 20 days a month. After two cycles, her hemoglobin level increased and transfusion dependency ceased. After four cycles, bone marrow aspiration showed blast ratio of < 5%. Decitabine and lenalidomide were applied for three more cycles. Decitabine was terminated after seven cycles and lenalidomide has been continued for 12 months. Latest blood values (February 2020) were as follows: white blood cells (WBCs) of 8,670/mm3, neutrophil count of 3,470/mm3, hemoglobin (Hb) level of 11.7 g/dL and platelet count of 203,000/mm3, and the patient continues to follow-up without treatment. In conclusion, combination of lenalidomide and decitabine seems to be an effective treatment modality without notable side effects in MDS patients with del(5q) and excess blasts. The efficacy of this combination should be validated with studies including large patient groups and with longer follow-up periods.
Keywords: Myelodysplastic syndrome; Del(5q); Decitabine; Lenalidomide
| Introduction | ▴Top |
Myelodysplastic syndrome (MDS), which is characterized by variable degrees of leukopenia and anemia, is one of the most common bone marrow failure syndromes and displays a heterogeneous clinical course including transformation to acute leukemia [1]. Presence of cytogenetic anomalies has a substantial role in determining the prognosis of MDS [2, 3]. Among them, isolated 5q deletion (del(5q)) leads to the occurrence of relatively benign form of the disease called 5q-syndrome, which is not usually accompanied with a blast increase in the bone marrow and manifests itself with typical dismegakaryopoiesis, thrombocytosis and severe anemia [4]. In this paper, we present an MDS patient with del(5q) and refractory anemia with excess blasts (10%) (RAEB-1) who was treated successfully with the combination of decitabine and lenalidomide treatment. This is the first case of decitabine plus lenalidomide treatment for an MDS patient with del(5q) and elevated number of blasts.
| Case Report | ▴Top |
A 55-year-old woman with weakness, fatigue and anemia requiring transfusion monthly was referred to our hematology department for further investigation. Her hematologic parameters were as follows: white blood cell (WBC) count of 4,940/mm3, neutrophil count of 890/mm3, hemoglobin (Hb) level of 8.1 g/dL, mean corpuscular volume (MCV) of 116 fL, and platelet count of 343,000/mm3. Because her nutritional and hemolysis parameters were normal, bone marrow biopsy was performed with the provisional diagnosis of MDS. Her bone marrow aspiration showed elevated blast ratio of 10% with dismegakaryopoietic signs. Bone marrow biopsy revealed increase in the number of megakaryocytes, signs of dismegakaryopoiesis. Upon conventional cytogenetical studies she was diagnosed with 5q-syndrome. Decitabine was started at a daily dose of 20 mg/m2 1 - 5 days and lenalidomide was started at daily doses of 10 mg 6 - 20 days in a month. After two cycles, her hemoglobin value increased and transfusion dependency ceased. After four cycles, her hematologic parameters were as follows: WBC of 5,860/mm3, neutrophil count of 1,430/mm3, Hb level of 10.5 g/dL and platelet count of 201,000/mm3. Bone marrow aspiration showed blast ratio of < 5%. Decitabine and lenalidomide were applied for three more cycles. Hemogram values after seven cycles were as follows: WBC count of 7,150/mm3, neutrophil count of 2,780/mm3, Hb level of 12.1 g/dL and platelet count of 168,000/mm3. Decitabine was terminated after seven cycles and lenalidomide has been continued for 12 months. Most recent blood values (February 2020) were as follows: WBC of 8,670/mm3, neutrophil count of 3,470/mm3, Hb level of 11.7 g/dL and platelet count of 203,000/mm3, and the patient continues to follow-up without treatment (Figs. 1-4).
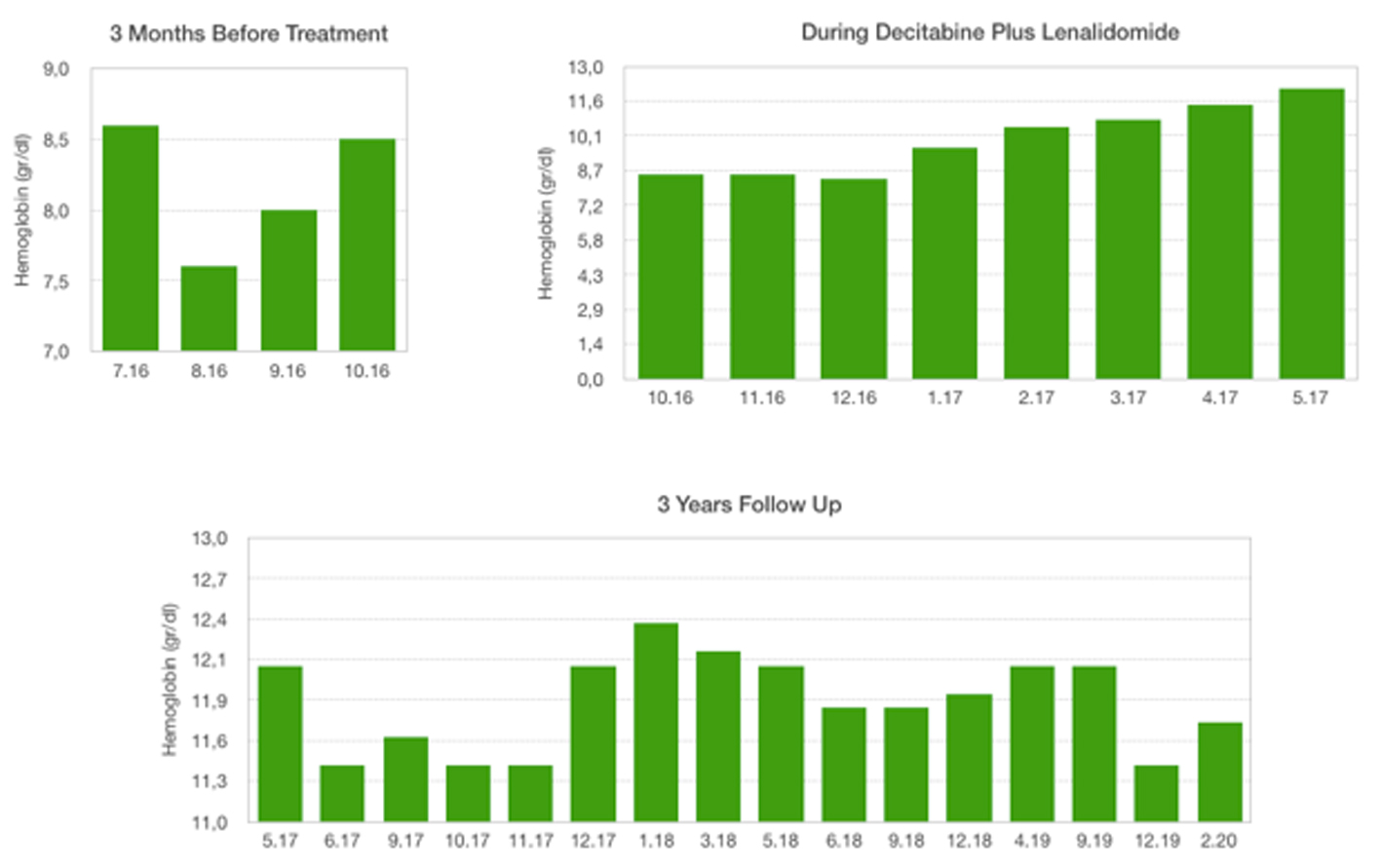 Click for large image | Figure 1. Hemoglobin levels: 3 months before treatment, during decitabine plus lenalidomide, 3 years follow-up. |
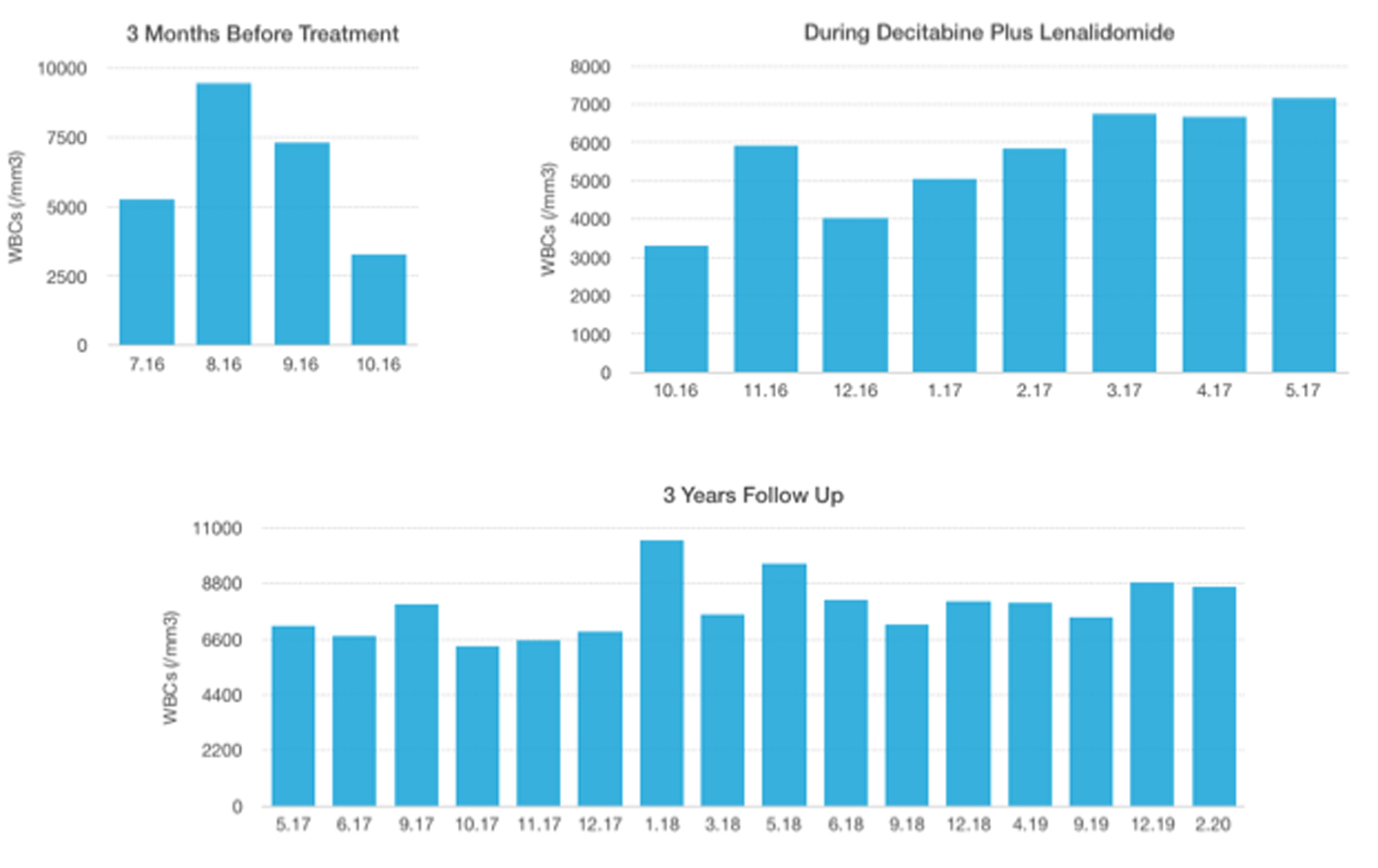 Click for large image | Figure 2. WBC levels: 3 months before treatment, during decitabine plus lenalidomide, 3 years follow-up. WBC: white blood cell. |
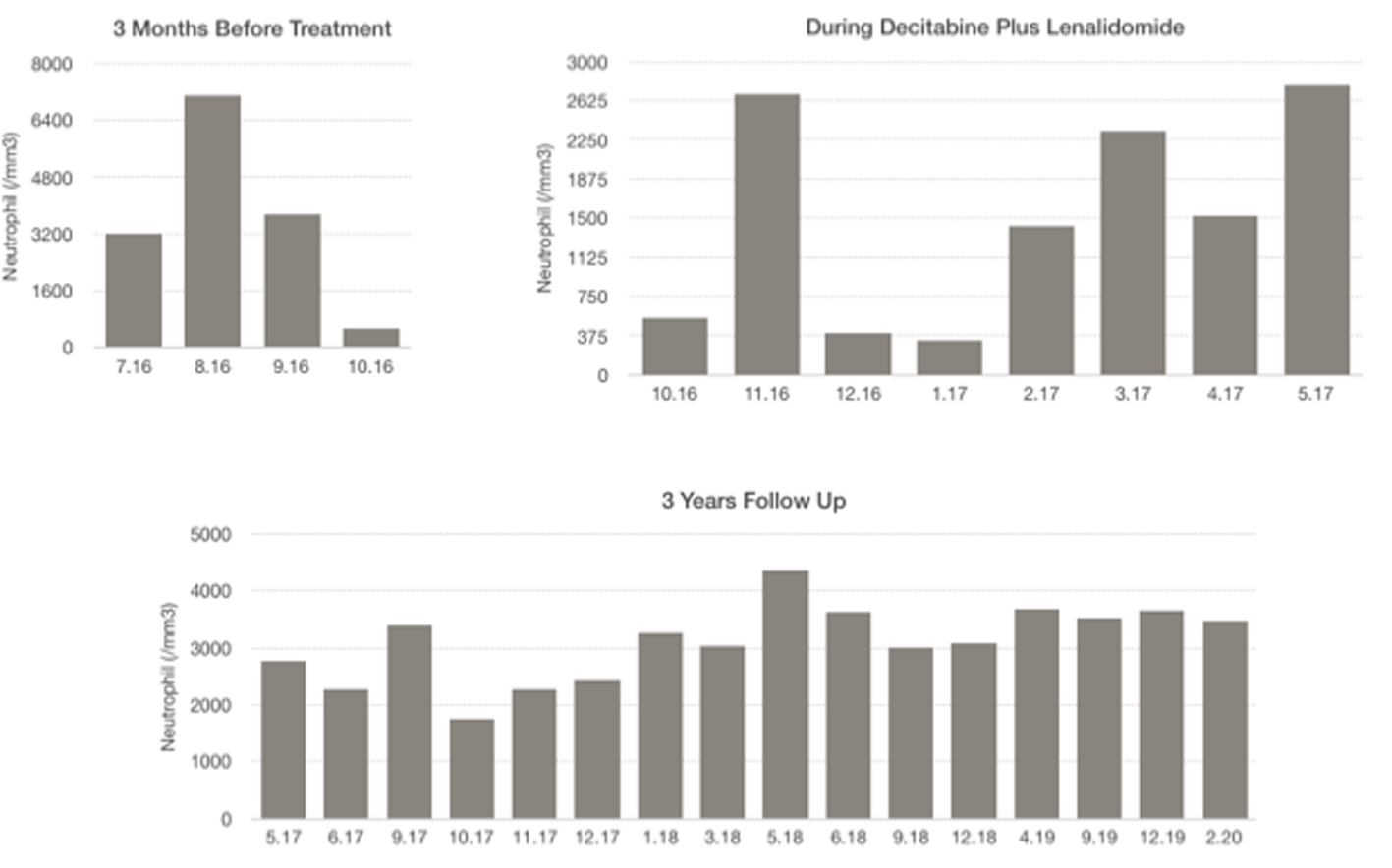 Click for large image | Figure 3. Neutrophil levels: 3 months before treatment, during decitabine plus lenalidomide, 3 years follow-up. |
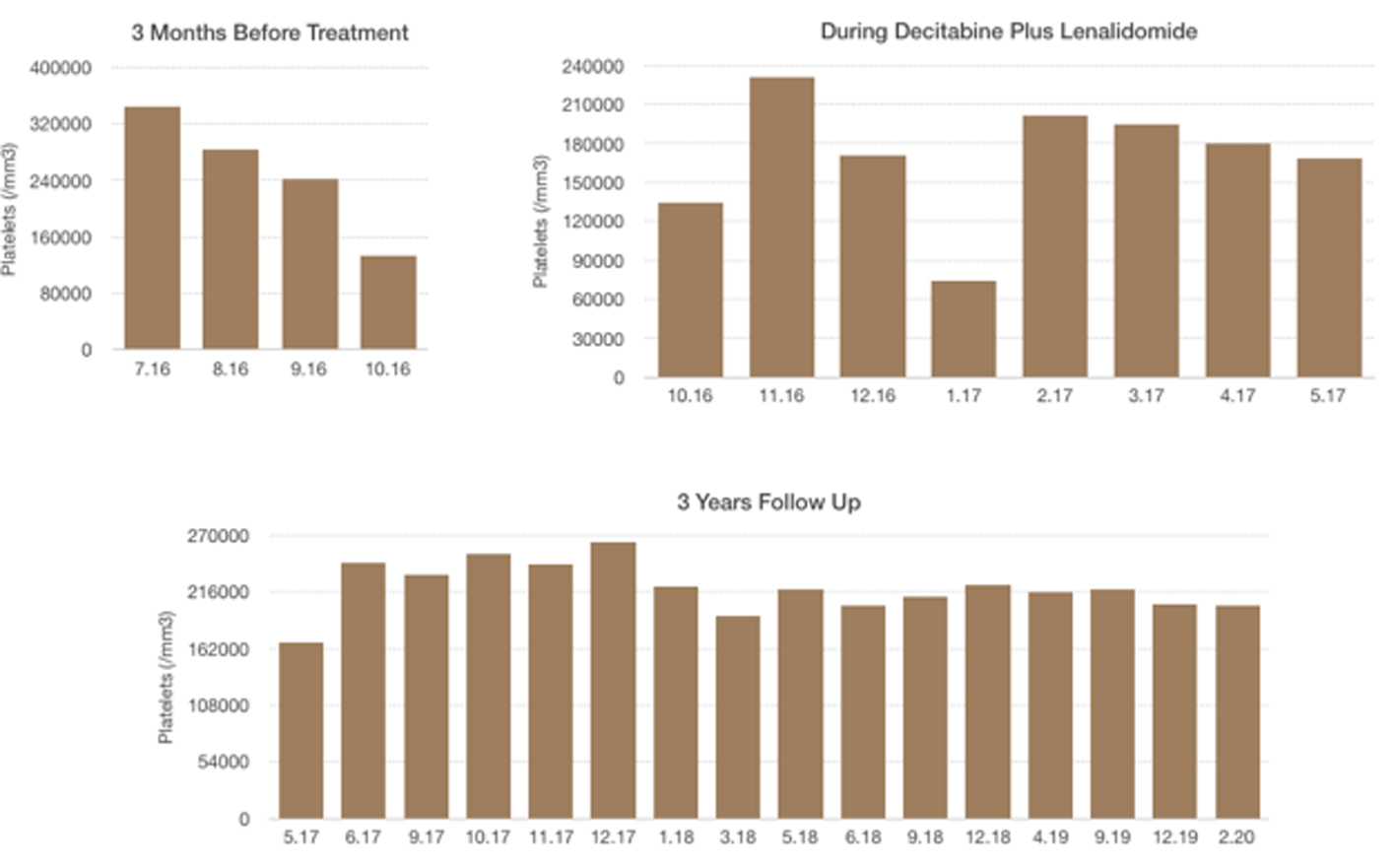 Click for large image | Figure 4. Platelet levels: 3 months before treatment, during decitabine plus lenalidomide, 3 years follow-up. |
| Discussion | ▴Top |
Decitabine is a hypomethylating agent that reactivates tumor suppressor genes by demethylating them. In recent years, decitabine treatment has emerged as a promising therapy in patients with MDS-RAEB and response is usually obtained after 2 - 4 cycles in most of the cases [5, 6]. Although decitabine has been mostly used as a monotherapy, several studies showed that combination of decitabine with various agents such as vorinostat, thalidomide and gemtuzumab improved the outcomes in MDS patients [7-9].
Another treatment of choice in MDS patients is lenalidomide, an immunomodulatory drug that has additional benefits in the regulation of erythropoiesis and reorganization of erythroid production in patients with MDS [10]. Within the subtypes of MDS, lenalidomide has been initially used in isolated 5q-syndrome, a rare form of MDS presenting itself particularly with anemia that requires frequent transfusions. In clinical studies, it has been shown that lenalidomide therapy decreased transfusion dependency prominently in this group of patients [11, 12].
The novel data related to the simultaneous administration of lenalidomide and azacytidinin in MDS patients with del(5q) and excess blasts has begun to rise recently [13-15]. On the other hand, there is inadequate data about the combination of lenalidomide with decitabine, another hypomethylating agent, in those patients. This is the first case of decitabine plus lenalidomide treatment for MDS patient with del(5q) and elevated number of blasts. Although thrombocytopenia and leukopenia have been often observed with the use of either lenalidomide or decitabine [16, 17], we did not encounter grade 3/4 neutropenia and thrombocytopenia or any other considerable non-hematological side effects.
In conclusion, combination of lenalidomide and decitabine seems to be an effective treatment modality without a notable side effect in MDS patients with del(5q) and excess blasts. The efficacy of this combination should be validated with studies including large patient groups and with longer follow-up periods.
Acknowledgments
The case report was accepted for a poster during the 14th International Symposium on Myelodysplastic Syndromes (MDS 2017).
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
An informed consent was obtained from the patient.
Author Contributions
All authors contributed to the editing of the manuscript. IS wrote the manuscript and made the accompanying figures.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872-1885.
doi pubmed - Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088.
doi pubmed - Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465.
doi pubmed - Germing U, Lauseker M, Hildebrandt B, Symeonidis A, Cermak J, Fenaux P, Kelaidi C, et al. Survival, prognostic factors and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia. 2012;26(6):1286-1292.
doi pubmed - Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794-1803.
doi pubmed - Luo XP, Xu ZF, Qin TJ, Zhang Y, Zhang HL, Fang LW, Pan LJ, et al. [Analysis of the impact of decitabine treatment cycles on efficacy and safety in patients of myelodysplastic syndrome-refractory anemia with excess blasts]. Zhonghua Xue Ye Xue Za Zhi. 2016;37(10):870-875.
- Kirschbaum M, Gojo I, Goldberg SL, Bredeson C, Kujawski LA, Yang A, Marks P, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167(2):185-193.
doi pubmed - Zhao WH, Zeng QC, Huang BT, Li BS, Chen RL. Decitabine plus thalidomide yields more sustained survival rates than decitabine monotherapy for risk-tailored elderly patients with myelodysplastic syndrome. Leuk Res. 2015;39(4):424-428.
doi pubmed - Daver N, Kantarjian H, Ravandi F, Estey E, Wang X, Garcia-Manero G, Jabbour E, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30(2):268-273.
doi pubmed - Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, Verma A. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36.
doi pubmed - Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5-10.
doi pubmed - Dogu MH, Sari I, Hacioglu S, Keskin A. Dramatic response in the dependency to transfusion after low doses of lenalidomide treatment in a 5q-syndrome patient: a case report. North Clin Istanb. 2014;1(3):191-193.
doi - DiNardo CD, Daver N, Jabbour E, Kadia T, Borthakur G, Konopleva M, Pemmaraju N, et al. Sequential azacitidine and lenalidomide in patients with high-risk myelodysplastic syndromes and acute myeloid leukaemia: a single-arm, phase 1/2 study. Lancet Haematol. 2015;2(1):e12-20.
doi - Zeidan AM, Gore SD, Komrokji RS. Higher-risk myelodysplastic syndromes with del(5q): is sequential azacitidine-lenalidomide combination the way to go? Expert Rev Hematol. 2013;6(3):251-254.
doi pubmed - Platzbecker U, Braulke F, Kundgen A, Gotze K, Bug G, Schonefeldt C, Shirneshan K, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27(6):1403-1407.
doi pubmed - List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549-557.
doi pubmed - Sekeres MA, Tiu RV, Komrokji R, Lancet J, Advani AS, Afable M, Englehaupt R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120(25):4945-4951.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


