| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 1, February 2021, pages 30-34
Spontaneous Tumor Lysis Syndrome: A Rare Presentation in Plasmablastic Lymphoma
Joana Infantea, b, d, Andre Casadob, Antonio Almeidac, Antonio Messiasb
aIntensive Care Unit, Hospital da Luz Lisboa, Av. Lusiada 100, 1500-650 Lisbon, Portugal
bDepartment of Hematology and Marrow Transplantation, Hospital de Santa Maria, Av. Prof. Egas Moniz MB, 1649-028 Lisbon, Portugal
cDepartment of Hematology, Hospital da Luz Lisboa, Av. Lusiada 100, 1500-650 Lisboa, Portugal
dCorresponding Author: Joana Infante, Intensive Care Unit, Hospital da Luz Lisboa, Av. Lusiada 100, 1500-650 Lisbon, Portugal
Manuscript submitted March 13, 2020, accepted April 4, 2020, published online February 6, 2021
Short title: PBL Presenting as STLS
doi: https://doi.org/10.14740/jh620
| Abstract | ▴Top |
Tumor lysis syndrome is an oncological emergency, which can ultimately lead to death if not recognized early and treated accordingly. The institution of adequate prophylactic measures can decrease its incidence and severity; but very rarely, a highly aggressive neoplasm such as acute lymphoblastic leukemia or Burkitt’s lymphoma can present with spontaneous tumor lysis syndrome (sTLS). We present the case of a 58-year-old male with newly diagnosed plasmablastic lymphoma with a retroperitoneal bulky mass invading the bladder, who presented with severe sTLS and was admitted to an intensive care unit due to acute renal failure and hyperkalemia requiring emergent renal replacement therapy. With urgent chemotherapy, several hemodialysis sessions and rasburicase, all the metabolic derangements were corrected and the patient fully recovered a normal renal function. This report highlights the importance of early recognition of sTLS in any patient presenting with severe and de novo multiple metabolic derangements involving uric acid, phosphorus, calcium and creatinine, even in patients with tumors not usually presenting with this complication.
Keywords: Spontaneous tumor lysis syndrome; Plasmablastic lymphoma; Dialysis
| Introduction | ▴Top |
Tumor lysis syndrome (TLS) is an oncological emergency resulting in renal damage, seizures and arrhythmias, which can lead to death if not recognized and treated rapidly [1]. It occurs most frequently after initiation of chemotherapy for hematological neoplasms, but very rarely it can occur spontaneously in highly proliferative hematological malignancies such as Burkitt’s lymphoma or acute lymphoblastic leukemia, further complicating the acute management of these patients.
Spontaneous tumor lysis syndrome (sTLS) is even rarer in other neoplastic diseases, and reports of sTLS occurring in plasmablastic lymphoma (PBL) are extremely scarce.
| Case Report | ▴Top |
A 58-year-old male presented with a 1-month history of anorexia, hematuria and abdominal distension. He had been diagnosed with human immunodeficiency virus (HIV)-1 infection 8 months previously and treated with abacavir, lamivudine and dolutegravir and with undetectable viral load at the time of presentation.
Blood tests revealed only a slight normochromic normocytic anemia (hemoglobin 11.7 g/dL) and slight elevation of creatinine (1.65 mg/dL). He underwent abdominal and pelvic computed tomography (CT) scan, which showed a large mass (7 × 8 cm) emerging from the bladder wall and projecting into the bladder cavity and outwards towards the pelvic region. The patient underwent a cystoscopy of the bladder and the lesion was biopsied.
In the following week, the patient complained of weight gain, worsening of anorexia, progressive abdominal distension, and oliguria. Ten days after the cystoscopy, the routine blood tests requested for the Urology appointment revealed critical decline in renal function, with a creatinine of 14.03 mg/dL and blood urea nitrogen of 90 mg/dL. There were multiple metabolic disturbances (Table 1), namely hyperkalemia (K+ 6.6 mmol/L), hyperphosphatemia (serum phosphate 7.7 mg/dL), hyperuricemia (uric acid 24.9 mg/dL), hypocalcemia (8.5 mg/dL), metabolic acidemia (pH 7.32 and HCO3 18 with pCO2 27), and high Lactate dehydrogenase (LDH) (2,012 UI/L). Electrocardiogram showed no acute abnormalities and the chest X-ray revealed a bilateral pleural effusion. A follow-up CT scan (Fig. 1) revealed a marked growth of the bladder wall mass (10 × 10 cm); now most of it occupying the retroperitoneum and invading the bladder. New lesions were detected, such as supra- and infradiaphragmatic lymph nodes and a diffuse peritoneal infiltration. Ureterohydronephrosis was absent.
 Click to view | Table 1. Blood Workup at Presentation, Showing Kidney failure and Multiple Metabolic Derangements |
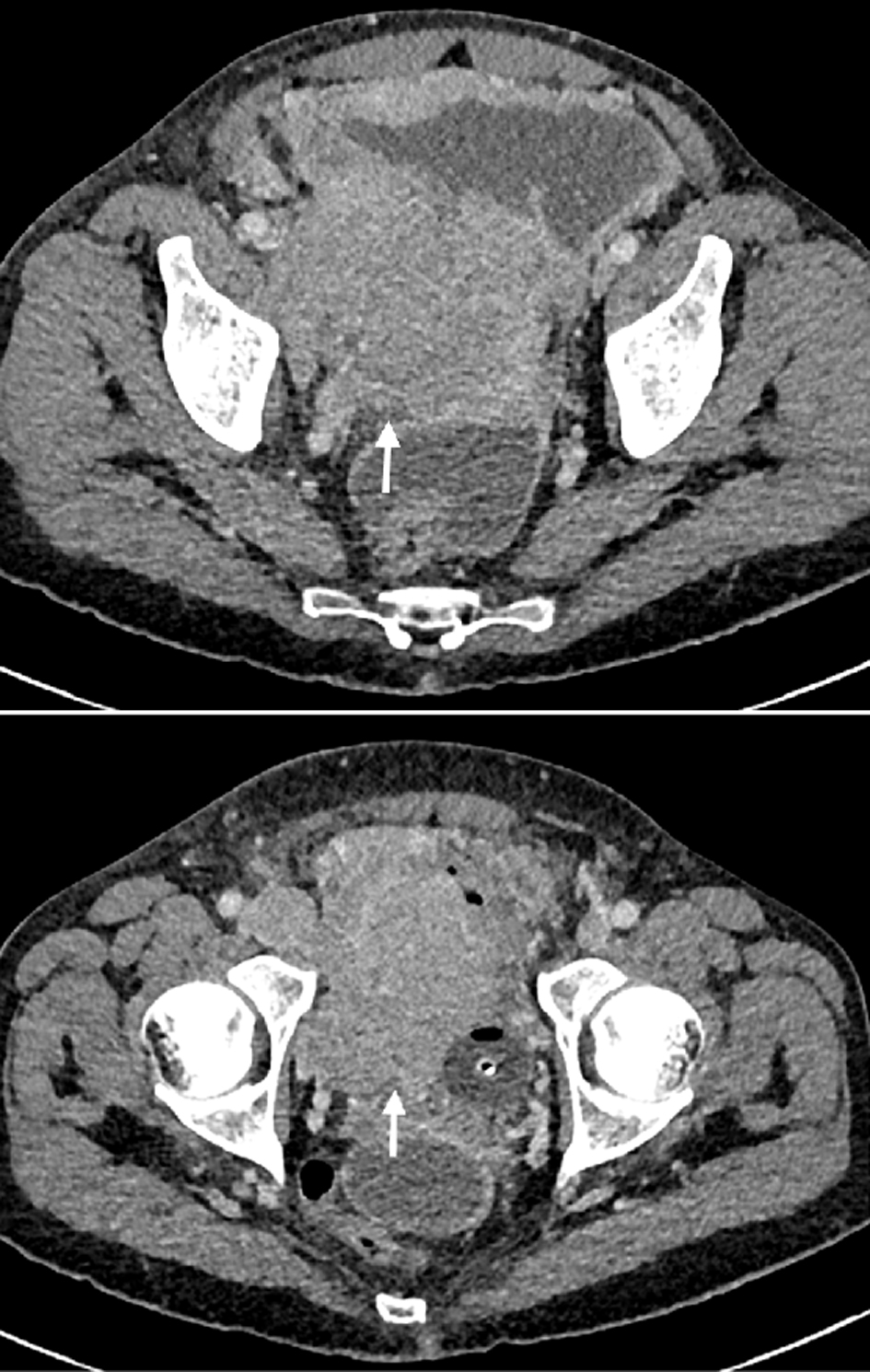 Click for large image | Figure 1. Abdominopelvic CT scan displaying a solid polylobulated retroperitoneal mass which invades the bladder wall, measuring approximately 10 × 10 centimeters. CT: computed tomography. |
The patient was urgently admitted to the intensive care unit (ICU) and was treated with 0.15 mg/kg rasburicase, and underwent urgent hemodialysis for oliguria, refractory hyperkalemia and a phosphorus-calcium product above 70.
The clinical and laboratory features were highly suggestive of sTLS. Preliminary histopathology reports from the bladder biopsies showed large immunoblastic cells which expressed CD138, CD38, MUM1 and EBER, and with a very high proliferative rate (Ki67 > 96%), consistent with a PBL. The patient was begun on a 5-day course of 1,000 mg methylprednisolone and emergent debulking chemotherapy with 500 mg cyclophosphamide intravenous (IV) after the first hemodialysis session.
The patient remained in the ICU for 7 days, during which he required three further hemodialysis sessions due to severe hyperphosphatemia which aggravated after chemotherapy (Fig. 2), yielding a phosphorus-calcium product still above 70. The uric acid rapidly declined and the patient started allopurinol. Diuresis eventually recovered, and the patient’s creatinine stabilized in the range of 2 mg/dL.
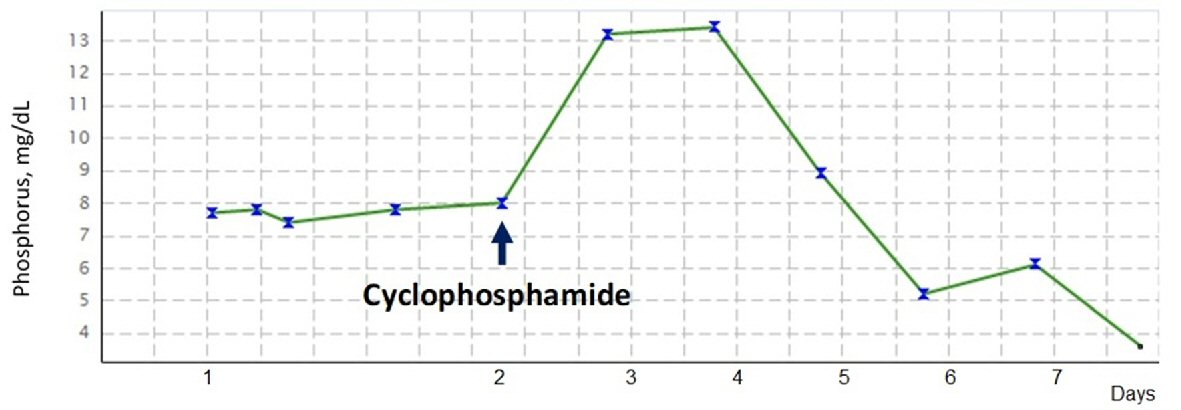 Click for large image | Figure 2. Evolution of serum phosphate concentration over time, since intensive care admission (day 1). The hyperphosphatemia, although significant, was not as proportionately elevated as the remaining tumor lysis parameters. This, however, changed once chemotherapy was initiated, with a spike from 8 mg/dL to approximately 13 mg/dL, and hence requiring two hemodialysis sessions to correct this serious hyperphosphatemia. |
However, despite the improvement in most characteristics, the phosphorous rose to a maximum value of 13 mg/dL after chemotherapy, requiring hemodialysis until its resolution.
The patient has currently completed four cycles of cyclophosphamide, doxorubicin, etoposide, vincristine and prednisolone, and four cycles of weekly rituximab given the associated Epstein-Barr virus (EBV) infection, and his renal function has fully recovered.
| Discussion | ▴Top |
TLS is an oncological emergency which is potentially lethal due to several metabolic derangements caused by the release of intracellular components of chemotherapy-destroyed tumor cells. According to the Cairo-Bishop definition [2], TLS is present if two or more of the following criteria are met, 3 days before or 7 days after cytotoxic therapy: serum uric acid ≥ 8 mg/dL, serum potassium ≥ 6.0 mEq/L, serum phosphorus ≥ 4.5 mg/dL, and/or calcium ≤ 7 mg/dL. Clinical TLS, the pathophysiology of which is described in Figure 3, is defined by laboratorial TLS plus at least one of the following clinical complications: increase in serum creatinine 1.5 times the upper limit of normal, cardiac arrhythmia or seizures.
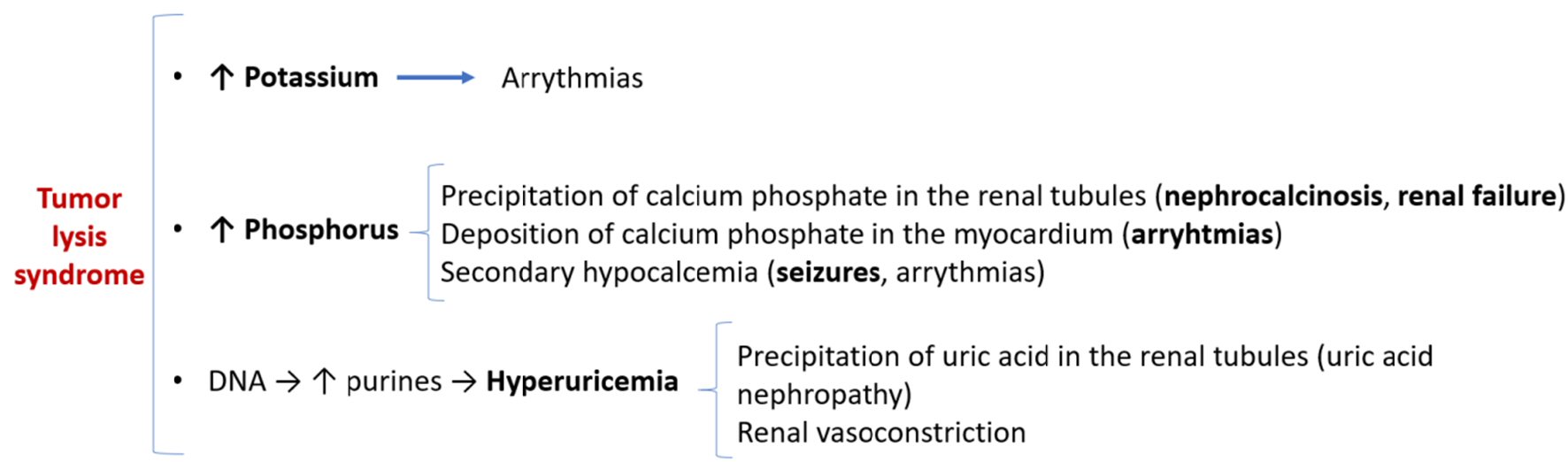 Click for large image | Figure 3. Pathophysiology of tumor lysis syndrome and its clinical implications. |
Since this is a preventable complication, both patient-related and disease-related risk factors for TLS have been identified (Table 2, [3]) in order to help the clinician implement adequate prophylactic measures before the beginning of chemotherapy [1]. TLS usually occurs after standard cytotoxic therapy, but some cases have been described after treatment with corticosteroids (particularly in acute lymphoblastic leukemia, [4]), monoclonal antibodies, tyrosine kinase inhibitors, BCL-2 inhibitors or other targeted therapies [5].
 Click to view | Table 2. Risk Factors for the Development in TLS in Hematological Malignancies, Which Can Be Divided in Tumor-Related or Patient-Related Factors [12] |
More rarely, tumors can present with spontaneous tumor lysis. STLS is defined as TLS occurring in the context of a neoplastic disease before any type of therapy. This occurs when the proliferative rate is such that some tumor cells do not withstand the selective pressure and undergo apoptosis while others massively replicate; and it is not preventable. The actual incidence of sTLS is unknown, although it is suggested to be a rare occurrence. To date it has been described in hematologic malignancies such as Burkitt’s lymphoma [6], acute lymphoblastic leukemia [7], or plasma cell leukemia [8]; and there are sporadic case reports in solid cancers such as metastatic melanoma [9], metastatic prostate cancer [10] and hepatocellular carcinoma [11].
Reports of sTLS before any type of therapy in PBL are scarce. We highlight a case of a HIV-positive male with PBL lung involvement [12], and a case series of HIV-positive PBL patients [3], two of which presented with sTLS and died before chemotherapy could be started. All three cases are remarkable for a concomitant HIV infection, and for not having oral cavity involvement; both characteristics were also a feature of our reported case.
PBL is a rare variant of diffuse large B cell lymphoma; and it is commonly associated with HIV infection (comprising 5% of all HIV-associated lymphomas) and EBV infection [13], as is the case of our patient. Its median proliferative rate is estimated to be a Ki67 > 80% [13].The oral cavity is the primarily involved site in HIV-positive patients, and an extranodal presentation is common. In this case, the primary lesion was a bulky retroperitoneal mass invading the bladder, but there was also diffuse infiltration of the peritoneum and supradiaphragmatic disease. Its aggressiveness is displayed by its multiple organ invasions and its high proliferative rate, thus increasing the risk of a sTLS.
Adding to the diagnosis of a PBL, this patient presented with at least four disease-related risk factors for TLS [14]: high proliferative index (Ki67 > 95%), bulky disease, LDH twice above upper limit of normal and organ (bladder) infiltration.
The pattern of evolution of the hyperphosphatemia of this patient was particularly peculiar. The hyperphosphatemia, although significant, was out of proportion to the marked elevation of creatinine and uric acid in this patient. This, however, changed once chemotherapy was initiated, with a rise from 8 mg/dL to approximately 13 mg/dL, requiring two hemodialysis sessions to be corrected. This phenomenon of a less severe hyperphosphatemia in sTLS than that which is seen in chemotherapy-induced TLS is thought to be due to the hyperproliferative malignant cells that are uptaking the released intracellular phosphorus for resynthesis of new tumor cells. On the other hand, after chemotherapy, tumor cell growth is blocked and phosphorus is no longer reutilized, so the serum phosphorus concentration climbs unhindered [15] and eventually precipitates with calcium in the renal tubules (nephrocalcinosis), worsening renal failure if not treated urgently.
In cases of severe renal failure, arrhythmias and/or seizure, admittance to ICU may be warranted. Extensive guidelines for prophylaxis and treatment of TLS have been published [2], and include vigorous hydration with IV fluids, allopurinol or the recombinant urate oxidase, and rasburicase. Renal replacement therapy (RRT) may be needed if the standard indications for emergent RRT are present, but also if the phosphorus-calcium product is above 70 in the TLS setting [16], as illustrated by our case report.
The occurrence of sTLS can be detrimental to the patient in two dimensions: first, its potential lethality; secondly, even if the metabolic derangement is managed, the renal failure and clinical instability may limit dosages of chemotherapy in the first cycle and preclude the use of renally excreted cytotoxic drugs.
Intensive care medicine plays a critical role in the care of patients with this aggressive form of presentation of tumors. Management of patients in tertiary units in close collaboration with hematologists enables close monitoring and organ support and thus improves patient outcomes, with the goal of stabilizing the patient and improving his renal function in order to enable the best possible treatment for the neoplastic disease. If acted upon promptly, the metabolic derangements of TLS can be corrected and renal function fully recovered, although some cases may still be fatal.
Conclusions
This report highlights the importance of early recognition and treatment of sTLS, even in patients with tumors not usually presenting with this complication. STLS should be suspected in any patient presenting with severe and de novo multiple metabolic derangements involving uric acid, phosphorus, calcium and creatinine, and medical history and physical examination should be directed at searching for a subjacent neoplastic disease.
Acknowledgments
The authors would like to acknowledge all the physicians and nurses involved in the patient’s care in the Intensive Care Unit of Hospital da Luz Lisboa.
Financial Disclosure
No funding was received. None of the authors have disclosures relevant to this manuscript.
Conflict of Interest
None to declare.
Informed Consent
Not applicable. The manuscript has been sufficiently de-identified to protect the patient.
Author Contributions
All authors contributed to the editing of the manuscript. JI wrote the manuscript and made the accompanying figures. AC, AA and AM thoroughly revised the content of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Cairo MS, Coiffier B, Reiter A, Younes A, Panel TLSE. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578-586.
doi pubmed - Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778.
doi pubmed - Jorge VM, Tiu A, Gupta S, Dourado C, Varadi G. Plasmablastic lymphoma in a community-based cancer center: a 10-year analysis of this rare disease in a underserved urban area. Blood. 2019;134(Supplement_1):5361-5361.
doi - Duzova A, Cetin M, Gumruk F, Yetgin S. Acute tumour lysis syndrome following a single-dose corticosteroid in children with acute lymphoblastic leukaemia. Eur J Haematol. 2001;66(6):404-407.
doi pubmed - Howard SC, Trifilio S, Gregory TK, Baxter N, McBride A. Tumor lysis syndrome in the era of novel and targeted agents in patients with hematologic malignancies: a systematic review. Ann Hematol. 2016;95(4):563-573.
doi pubmed - Tsokos GC, Balow JE, Spiegel RJ, Magrath IT. Renal and metabolic complications of undifferentiated and lymphoblastic lymphomas. Medicine (Baltimore). 1981;60(3):218-229.
doi pubmed - Kobayashi D, Wofford MM, McLean TW, Lin JJ. Spontaneous tumor lysis syndrome in a child with T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(5):773-775.
doi pubmed - Kollathodi SB, Parameswaran KK, Madhavan L, Kuruvilla S. Hematological malignancies presenting as spontaneous tumor lysis syndrome: A case series. J Family Med Prim Care. 2018;7(5):1116-1119.
- Durham CG, Herrington J, Seago S, Williams C, Holguin MH. From skin to spontaneous lysis: A case of spontaneous tumor lysis syndrome in metastatic melanoma. J Oncol Pharm Pract. 2018;24(3):221-225.
doi pubmed - McGhee-Jez A, Batra V, Sunder T, Rizk S. Spontaneous tumor lysis syndrome as presenting sign of metastatic prostate cancer. Cureus. 2018;10(12):e3706.
doi pubmed - Kekre N, Djordjevic B, Touchie C. Spontaneous tumour lysis syndrome. CMAJ. 2012;184(8):913-916.
doi pubmed - Khalid L, Parajuli R, Holt GE, Vosiughi A. Spontaneous tumor lysis: an unusual complication of a rare case of plasmablastic lymphoma with lung involvement. In: C80 thoracic oncology: case reports [Internet]. American Thoracic Society; 2016. p. A6045-A6045. American Thoracic Society International Conference Abstracts. Available from: https://doi.org/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6045.
- Tchernonog E, Faurie P, Coppo P, Monjanel H, Bonnet A, Algarte Genin M, Mercier M, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group. Ann Oncol. 2017;28(4):843-848.
doi pubmed - Larson RA. Tumor lysis syndrome: Definition, pathogenesis, clinical manifestations, etiology and risk factors. UpToDate. 2019; Post TW E.
- Kjellstrand CM, Cambell DC, 2nd, von Hartitzsch B, Buselmeier TJ. Hyperuricemic acute renal failure. Arch Intern Med. 1974;133(3):349-359.
doi pubmed - Mirrakhimov AE, Voore P, Khan M, Ali AM. Tumor lysis syndrome: A clinical review. World J Crit Care Med. 2015;4(2):130-138.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


