| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Editorial
Volume 9, Number 1-2, April 2020, pages 1-4
Abnormal Hemostatic Parameters and Risk of Thromboembolism Among Patients With COVID-19 Infection
Roberto Castellia, b, Antonio Gidaroa
aDepartment of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Luigi Sacco Hospital, Milan, Italy
bCorresponding Author: Roberto Castelli, Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Luigi Sacco Hospital, Milan, Italy
Manuscript submitted April 15, 2020, accepted April 17, 2020
Short title: Hemostatic Parameters and Risks in COVID-19
doi: https://doi.org/10.14740/jh636
Since December 2019, a newly identified coronavirus disease (COVID-19) has spread in China and the rest of World. The pathogen has been identified as a novel enveloped RNA beta-coronavirus [1], with a phylogenetic similarity to SARS-CoV named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) has recently declared COVID-19 a public health emergency of international concern [2].
At the time of writing, the COVID-19 epidemic is still ongoing. Latest numbers estimate > 1,614,405 people infected worldwide, with 1,155,207 active cases (49,115 critical cases, 4%) and 96,789 deaths [3]. In Italy, 143,626 infected people (8.9% of all infected people worldwide), 18,279 death people, 96,877 active cases with 3.7% of critical cases have been reported to date [4]. The clinical feature of disease is very wide and ranges from minor unspecified symptoms (such as fever, dry cough and diarrhea) to severe pneumonia, lung insufficiency and death. Fall in respiratory insufficiency needing mechanical ventilation, or in multiple organ failure occurs in 5% of the infected patients, mainly according to age and comorbidities [5]. Disease severity also correlates with pro-inflammatory cytokines (i.e. interleukin (IL-2), IL-6, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), interferon γ-induced protein (IP)-10, monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1A and tumor necrosis factor (TNF)-α), although it is not yet clear what is the cause of such a cytokine storm [6]. Among the several clinical and biochemical parameters associated with poor prognosis, increased D-dimer levels have gained particular attention and represent a predictor of the development of acute respiratory distress syndrome (ARDS). Tang et al [7] described the outcome of 183 patients admitted to the Tongji Hospital in Wuhan with RNA detection confirmed 2019-nCoV pneumonia. The authors observed that disseminated intravascular coagulation (DIC) is a frequent occurrence in worsening of COVID-19 pneumonia. Coagulation abnormalities and marked increase of D-dimer were often associated with increased mortality or concomitant severe thromboembolic complications. Surprisingly, the authors noted that in the Chinese population, the prevalence of thromboembolic events was particularly elevated among patients with severe lung failure. Similar prevalence was observed by Cui et al in 81 patients admitted to intensive care units (ICUs), where the prevalence of major thromboembolism was around 25% [8]. Low molecular weight heparin (LMWH) or unfractionated heparin (UFH) at prophylactic doses are associated with a reduced 28-day mortality in more severe COVID-19 patients [9], displaying a sepsis-induced coagulopathy (SIC) score ≥ 4 (40.0% vs. 64.2%, P = 0.029) or D-dimer levels more than six-fold the upper limit of normal (32.8% vs. 52.4%, P = 0.017). Comparably, but slightly higher records of thromboembolic complications (up to about 30%) were observed in Italian patients admitted in ICU (unpublished data). These numbers will be smaller but not less than 2% in all Italian COVID-19 patients hospitalized. Normal incidence of deep vein thrombosis in hospitalized Italian patients without COVID-19 is reported 0.025% [10]. One of the possible reasons for the increased frequency of thromboembolic complications in Italian patients could be attributed to higher median age of 45.5 [4] compared to China at 37.4 [5]. The thromboembolic complications observed in Italian patients affected by severe COVID-19 include venous thromboembolism and pulmonary embolism (VTE). All thromboembolic complications are documented by compressive ultrasonography (CUS) or computerized axial tomography (CT) scan.
All patients share alterations of hemostatic parameters showing: 1) elevated levels of D-dimer (> 1,000 at admission rapidly rising); 2) elevated fibrinogen which may be consumed in the late stage of disease; 3) thrombocytopenia, but less commonly than in classical DIC; and 4) normal or somewhat prolonged partial prothrombin time (PTT) and international normalized ratio (INR). Additional coagulation tests have shown elevated levels of von Willebrand factor and factor VIII, and normal levels of natural anticoagulants such protein C, protein S and antithrombin III (unpublished data).
Table 1 shows the most frequent hemostatic alterations observed among Italian patients admitted to ICU for respiratory failure.
 Click to view | Table 1. Coagulation Parameters in COVID-19 DIC, Acute DIC and Chronic DIC |
COVID-19 produces a sort of DIC which is usually characterized by hypercoagulability. The exact pathogenetic mechanism of COVID-19-related DIC is unclear, although inflammatory drive and cytokine release may be responsible for the observed coagulation impairment leading to thromboembolic complications. IL-6 may stimulate up-regulation of fibrinogen synthesis by the liver and the virus may bind directly to endothelial cells causing alveolar damage.
DIC appears to be a driver of disease severity and correlates with poor outcome [7]. Furthermore, from 40 autopsies, a quarter presented lung specimens macro-thrombosis with typical pattern of pulmonary embolism and more than two-thirds showed thrombi in microvessels, associated with diffuse alveolar damage and interstitial lung infiltrates of granulocytes, macrophages and giant platelets (unpublished data). These reports are in agreement with those of Luo et al [11]. All these findings suggest a mechanism of inflammatory-mediated macro- and micro-thrombosis as a pathogenetic mechanism of damage in the setting of severe COVID-19 infection. The hypothesis of improving the clinical outcome of COVID-19 patients by simple and inexpensive antithrombotic drugs is very appealing, but several issues need to be addressed and clarified before adopting an aggressive approach, in particular defining the appropriate timing for the start of the treatment and the dosage, which may impact concomitant medications.
While the scientific community is waiting for more robust evidence on the role of heparin or antiplatelet therapy in coagulopathies associated with COVID-19 infection, the Italian Society on Thrombosis and Hemostasis suggests some recommendations [12], based on expert consensus, for the management of the hemostasis derangement in COVID-19 patients (Figs. 1-3). 1) Individual risk evaluation together with laboratory tests should always include hemostasis function, D-dimer and platelet count; deep vein thrombosis (DVT) ultrasound screening. In case of elevated levels of D-dimer, this latter should be strictly monitored. 2) The use of LMWH, UFH, or fondaparinux at doses indicated for prophylaxis of VTE is strongly advised in all COVID-19 hospitalized patients; patients with anticoagulant contraindications should be treated with limb compression. Thromboprophylaxis should be administered throughout the hospital stay. This should also be maintained at home for 7 - 14 days after hospital discharge. 3) The use of intermediate-dose LMWH (i.e. enoxaparin 4,000 IU subcutaneously every 12 h) can be considered on an individual basis in patients with multiple risk factors for VTE (i.e. body mass index > 30, previous VTE, active cancer, etc.). Activated Xa should be strictly monitored especially in patients with renal failure. 4) Therapeutic doses of UFH or LMWH are currently not supported by evidence outside of established diagnoses of VTE or as a bridging strategy in patients on vitamin K antagonists (VKAs), and cannot be recommended as a standard treatment for all COVID-19 patients. 5) Attention should be paid for VKAs and direct oral anticoagulants (DOACs) due to interference with antiviral agents. 6) Strict cooperation between hematologist and all physicians involved in the management of COVID-19 patients is recommended.
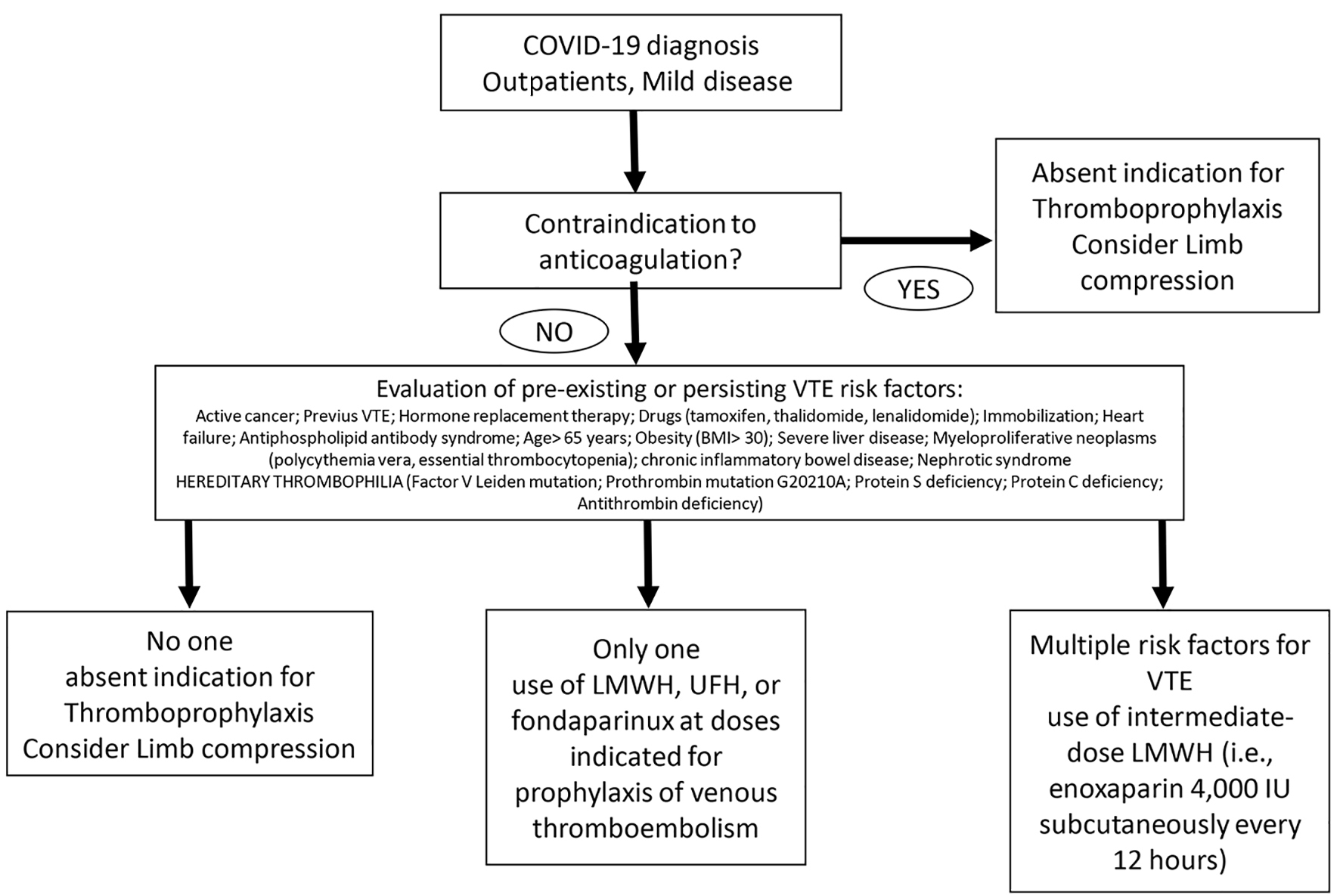 Click for large image | Figure 1. Prophylaxis of venous thromboembolism therapy scheme for COVID-19 diagnosed outpatients with mild disease. |
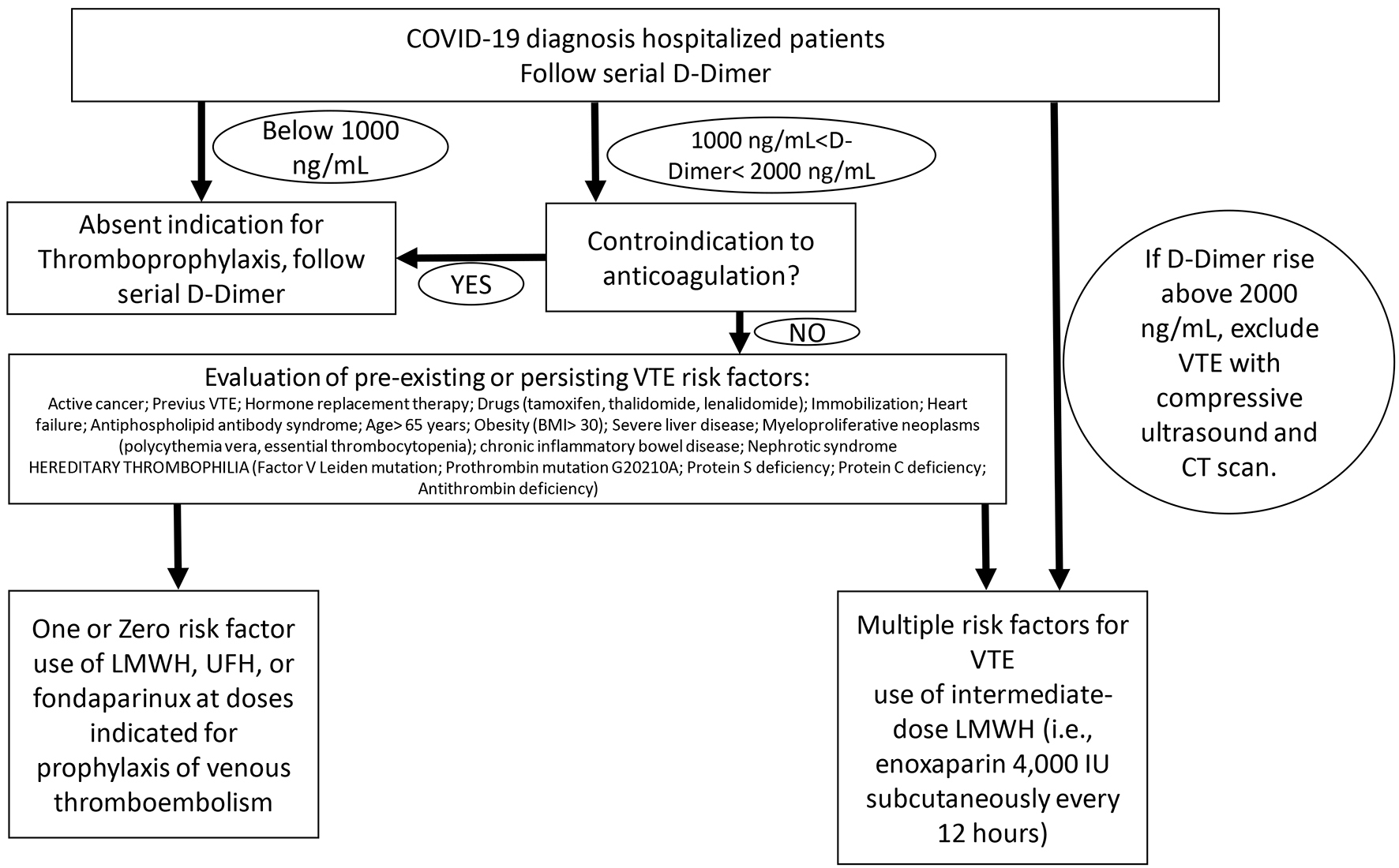 Click for large image | Figure 2. Prophylaxis of venous thromboembolism therapy scheme for COVID-19 hospitalized patients. |
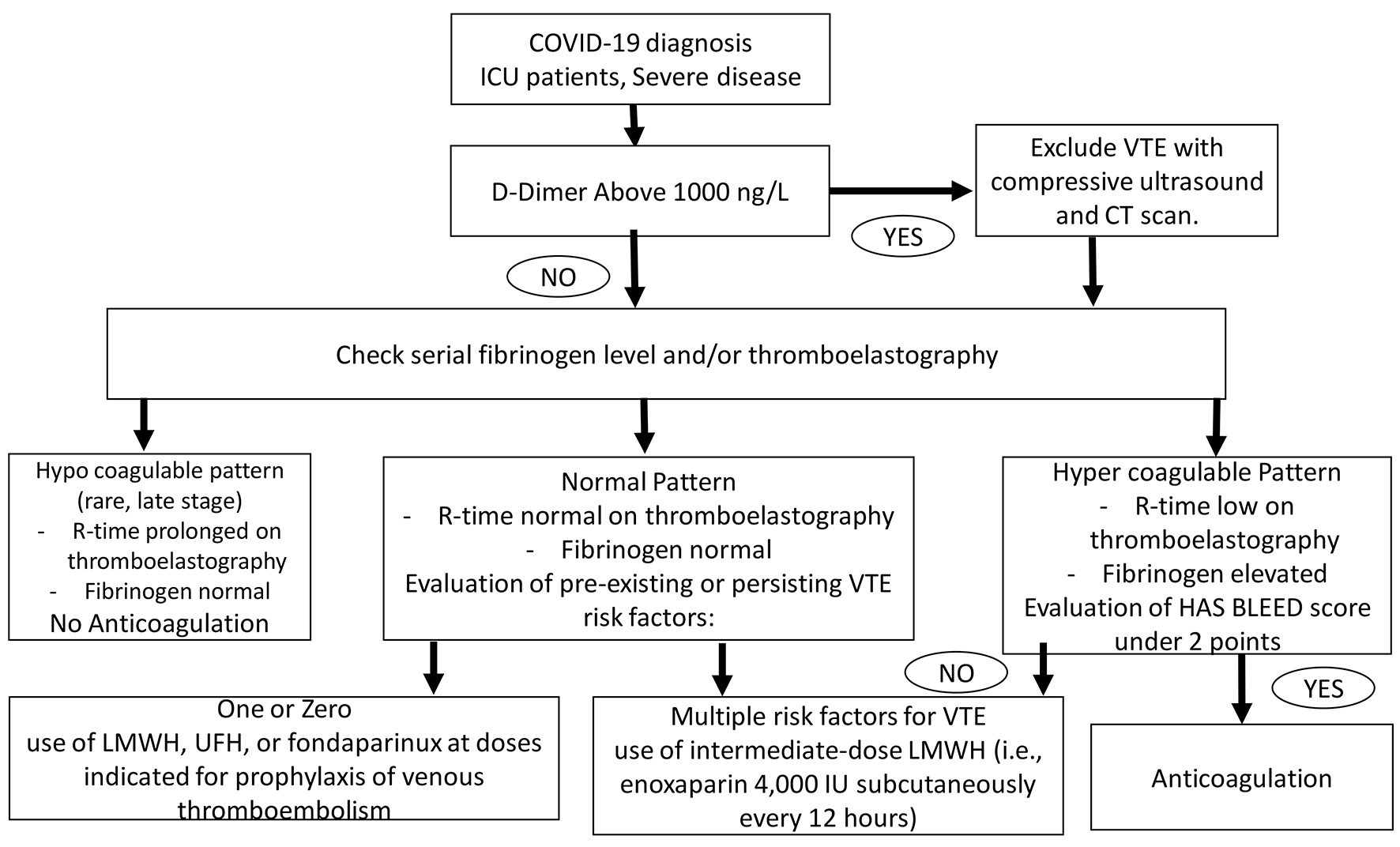 Click for large image | Figure 3. Prophylaxis of venous thromboembolism therapy scheme for COVID-19 ICU patients. ICU: intensive care unit. |
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Both the authors equally contributed to analyzing data, writing text and figures.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574.
doi - World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int.
- https://www.worldometers.info/coronavirus/.
- Italian ministry of health website, http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5351&area=nuovoCoronavirus&menu=vuoto.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242.
doi pubmed - Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, Antinori S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337-342.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847.
doi pubmed - Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020.
doi - Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020.
doi - Loffredo L, Arienti V, Vidili G, Cogliati C, Battaglia S, Perri L, Di Giulio R, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43.
doi pubmed - Luo W, Yu H, Gou J, Li X, Sun Y, Li J, Liu L. Clinical pathology of critical patient with novel coronavirus Pneumonia (Covid-19), in press.
- Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, Marcucci R, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


