| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 1, February 2021, pages 8-13
Use of a Mobile Intermittent Pneumatic Compression Device (Vekroosan) in Mobile Patients With Chronic Venous Disease
Raj Ramakrishnaa, b, c, d, g, William Alexandera, e, g, Lina Baytiehd, f
aIllawarra Private Cancer Care Centre, Wollongong, NSW, Australia
bSouthern IML Pathology, Wollongong, NSW, Australia
cThe Graduate School of Medicine, University of Wollongong, Wollongong, NSW, Australia
dWollongong Hospital, Wollongong, NSW, Australia
eOceania University of Medicine, Wollongong, NSW, Australia
fIllawarra Shoalhaven Health District, Wollongong, NSW, Australia
gCorresponding Author: Raj Ramakrishna and William Alexander, Illawarra Private Cancer Care Centre, 35 Denison St, Wollongong, NSW 2500, Australia
Manuscript submitted May 27, 2020, accepted November 27, 2020, published online February 6, 2021
Short title: Vekroosan in Mobile Patients With CVD
doi: https://doi.org/10.14740/jh684
| Abstract | ▴Top |
Background: Compression therapy is an essential part of chronic venous disorder (CVD) treatment in reducing associated complications. This observational study aimed to note the use, effects and tolerance of a mobile intermittent pneumatic calf compression (IPC) device, Vekroosan® (DVT Solution P/L).
Methods: In 56 patients, Doppler ultrasonography was used to measure venous blood peak flow velocity (PFV) at external iliac, common femoral, distal superficial femoral and popliteal vein levels both before and after application of Vekroosan calf compressor for comparison.
Results: Vekroosan was able to show significant clinical benefit in 45 patients (80%). There was a significant increase in femoral PFV pressure in post-compression measurement when compared to the pre-compression measurement (43.1 vs. 32.4 cm/s, P < 0.001), even when patients mobilize. On average, the PFV pressure increased by 10.7 cm/s when compared to baseline. A significant decrease was seen in calf swelling after calf compression (31.3 vs. 21.9 cm, P < 0.01), also with mobilization. Eighty-seven percent of patients tolerated the device well.
Conclusions: Our study shows that use of mobile IPC devices, such as Vekroosan, is safe and effective in the treatment of CVD, can be used while mobilizing and can achieve results comparable to non-mobile devices.
Keywords: Vekroosan; Calf compression; Chronic venous disease
| Introduction | ▴Top |
Chronic venous disease (CVD) of the lower limbs is caused by abnormalities of the venous wall and valves that lead to obstruction or reflux of blood flow in the veins [1]. Clinical consequences of CVD include chronic edema, lower limb pain and discomfort, reduced mobility, hyperpigmentation, stasis dermatitis and venous ulcers [2]. Severe and persistent edema leads to secondary lymphedema and marked lower leg swelling [2]. The calf can become permanently enlarged and hard, and venous ulcers occur more frequently and can be often difficult to heal [3]. Consequently, it adds to the individual’s suffering, increases morbidity and places a significant burden on the health care system. The direct and indirect costs of CVD have been estimated at three billion US dollars per annum [4].
Sustained calf compression is an important part of chronic venous leg ulcer therapy to promote ulcer healing and prevent recurrence [2, 5, 6]. This has been used as the basis for preventative measures in patients with CVD using calf compression stockings, which can be very difficult to wear, and has had recent doubt cast over their efficacy in certain subpopulations [7, 8]. This has prompted the current study in the observed use, effects and tolerance of mobile sequential pneumatic calf compression.
Various intermittent pneumatic calf compression (IPC) devices are widely used, and have shown improvement in leg swelling and associated pain in patients with CVD or patients undergoing orthopedic or spinal surgery [6]. However, there are no large studies to substantiate its use, nor the utility of mobile IPC for the management of advanced CVD, deep venous thrombosis (DVT) and patients having undergone high-risk surgical procedures for thrombosis such as joint replacement surgeries and abdominal and pelvic surgeries.
This study therefore aimed to observe the use of a mobile (battery operated) sequential calf compression device, Vekroosan® (DVT Solution P/L), in the management of patients with CVD, to explore the hemodynamic effects of IPC, and to note the immediate clinical outcomes observed when IPC is used in these patients.
| Materials and Methods | ▴Top |
Participant recruitment
Patients with CVD symptoms were invited to participate in this study in the following way: during regular consultation in an outpatient clinic, patients who met the criteria for the study were offered a chance to participate and given a participant information sheet/consent form and an appointment for enrolment. Patients were recruited in this multi-center study on a voluntary basis, at our community-based outpatient clinics.
During the enrolment appointment, participants were given information on the study and the chance to ask questions and were also informed that if they chose not to be involved, there would be no repercussions.
Patients with acute DVT, acute pulmonary emboli, leg ulcerations, or any open wound, history of malignancies requiring active chemotherapy, and those unable to consent or who were under the age of 18, were excluded.
Methods
Patients underwent Doppler velocity measurements using Doppler ultrasonography (General Electric Logic S8, using ML 6-15 linear array probe, 9L linear array for larger patients; Phillips IU 22x Matrix L 12-5 50 mm linear probe, L 9-3 linear array probe for larger patients). B mode imaging was performed utilizing CrossX beam and spatial compounding tool that combines multiple images from different angles into a single real-time image was used to help achieve a clearer view to assess for acute and chronic thrombosis. Color Doppler imaging with spectral Doppler was used to demonstrate flow velocity rate and augmentation at external iliac, common femoral, distal superficial femoral and popliteal vein levels. Vekroosan® was applied around patients’ calves and the three chambers were inflated sequentially (Figs. 1 and 2). Doppler recordings of femoral peak flow velocity (PFV) were performed using the Doppler ultrasonography as above. Baseline pressure was recorded prior to applying the mobile calf compressor device, Vekroosan® and repeated pressure measurement was done at an average pressure of 40 mm Hg and varied between 30 and 80 mm Hg [1]. Patients were allowed to mobilize to assess the utility of the device (Fig. 3). All measurements were performed by a qualified and experienced ultrasonographer. Measurements at each time point were the average of five repeated determinations of the PFV. Measurements were performed after 20-min rest with the device inactive on the leg as a baseline measurement and immediately after the device was turned on the measurements were repeated sequentially after activation of each chamber. The PFV measurements were collected for chamber 1 (lower), chamber 2 (mid) and chamber 3 (upper) which were activated.
 Click for large image | Figure 1. Chamber 1 (lower) inflated. |
 Click for large image | Figure 2. All chambers inflated. |
 Click for large image | Figure 3. Patient mobilizing. |
All patients were asked as to their perception of tolerance for the device, including pain and discomfort, and this information was recorded.
Further analysis of calf swelling changes was made in 46 patients who were able to complete 1 h of calf compressions. Ten patients who consented could not complete the second part of the study due to logistic reasons. Calf measurements were made at the same calf level (e.g., 15 - 18 cm below knee cap) before and after an hour of mobile calf compression using Vekroosan®.
Data analysis
Statistical analyses were performed using the IBM SPSS Statistics Package. An independent samples t-test was conducted to compare the means of the pooled post-calf compression groups and the pre-compression group. Logistic regression was conducted to analyze the incremental difference between lower, mid and upper chambers.
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Institutional review board approval was granted.
| Results | ▴Top |
For the study, 60 patients consented and 56 were able to complete the study. Four patients were not able to participate in the study due to an open lower limb wound at the time of the study. There were 31 female and 25 male evaluable patients with a mean age of 68 years (range 46 - 80 years). There were 13 patients on anticoagulation. There was a significant increase in PFV pressure when using the mobile IPC Vekroosan post-compression (M = 43.1, SD = 27.6) when compared to the pre-compression measurement (M = 32.4, SD = 20.6, t(1,222) = 2.7, P = 0.008). On average, the PFV pressure increased by 10.7 cm/s when compared to the pre-compression (baseline) measurement (Fig. 4). Patients on anticoagulation had a greater difference in pre- and post-measurements than those who were not (11.8 vs. 9.6 cm/s). A total of 168 measurements were taken.
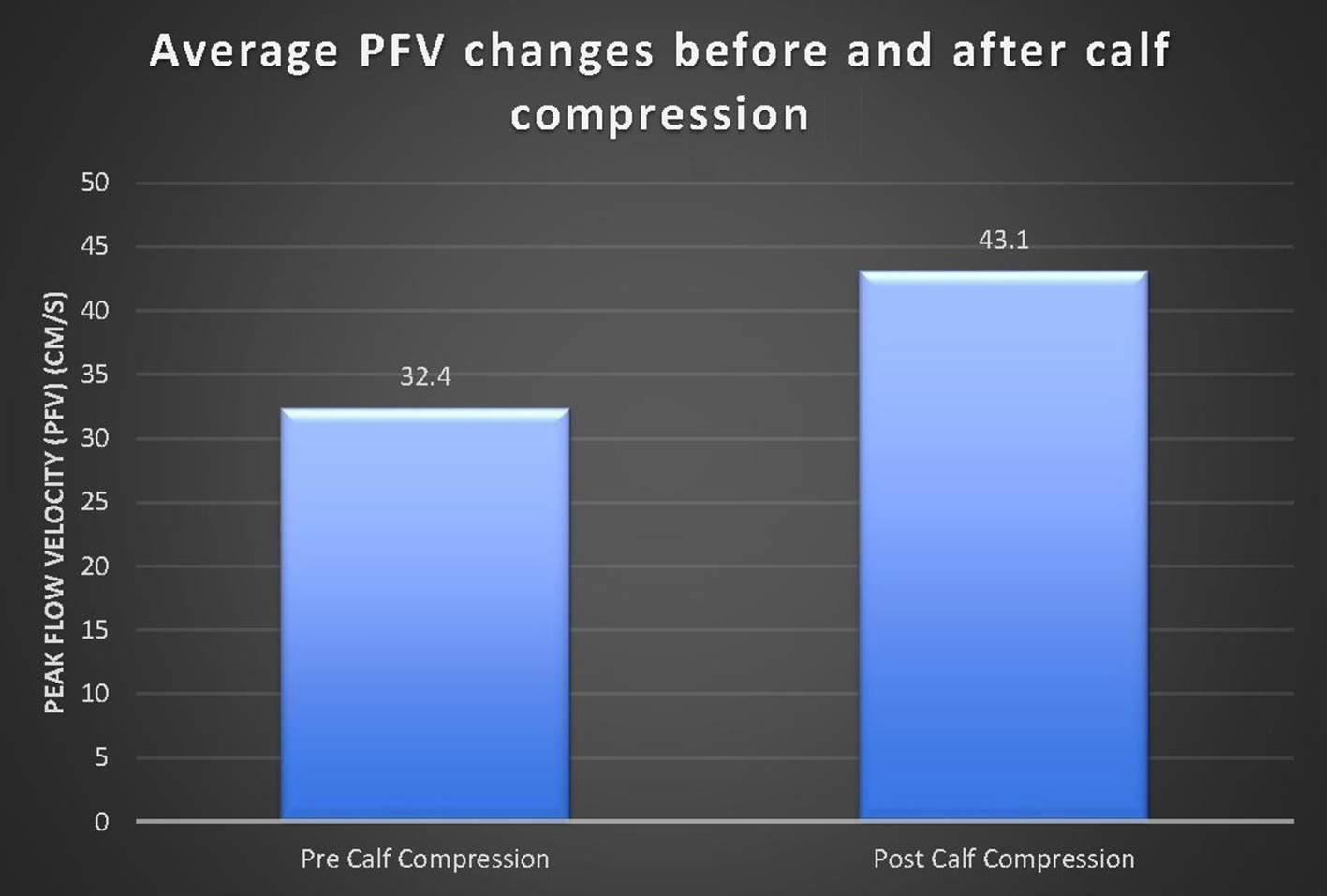 Click for large image | Figure 4. Comparison of PFV before and after calf compression. There was a significant increase in average PVF after calf compression (P < 0.001). |
The PFV results were compared between baseline and post-calf compression measurements. As logistic regression showed no significant difference between the three chambers, the data were combined for statistical analysis.
A paired sample t-test was then conducted to compare the calf circumference before and after a minimum of 1 h of calf compression using Vekroosan. There was a significant decrease in the calf circumference from before compression (M = 31.3, SD = 4.1) to after compression (M = 29.9, SD = 4.0). There was evidence of reduced calf measurements before and after calf compression (at least 1 h) by an average of 1.4 cm (t(1,45) = 13.1, P < 0.001) (Fig. 5). There was greater reduction in calf size in the group not on anticoagulation (1.6 vs. 1.2 cm).
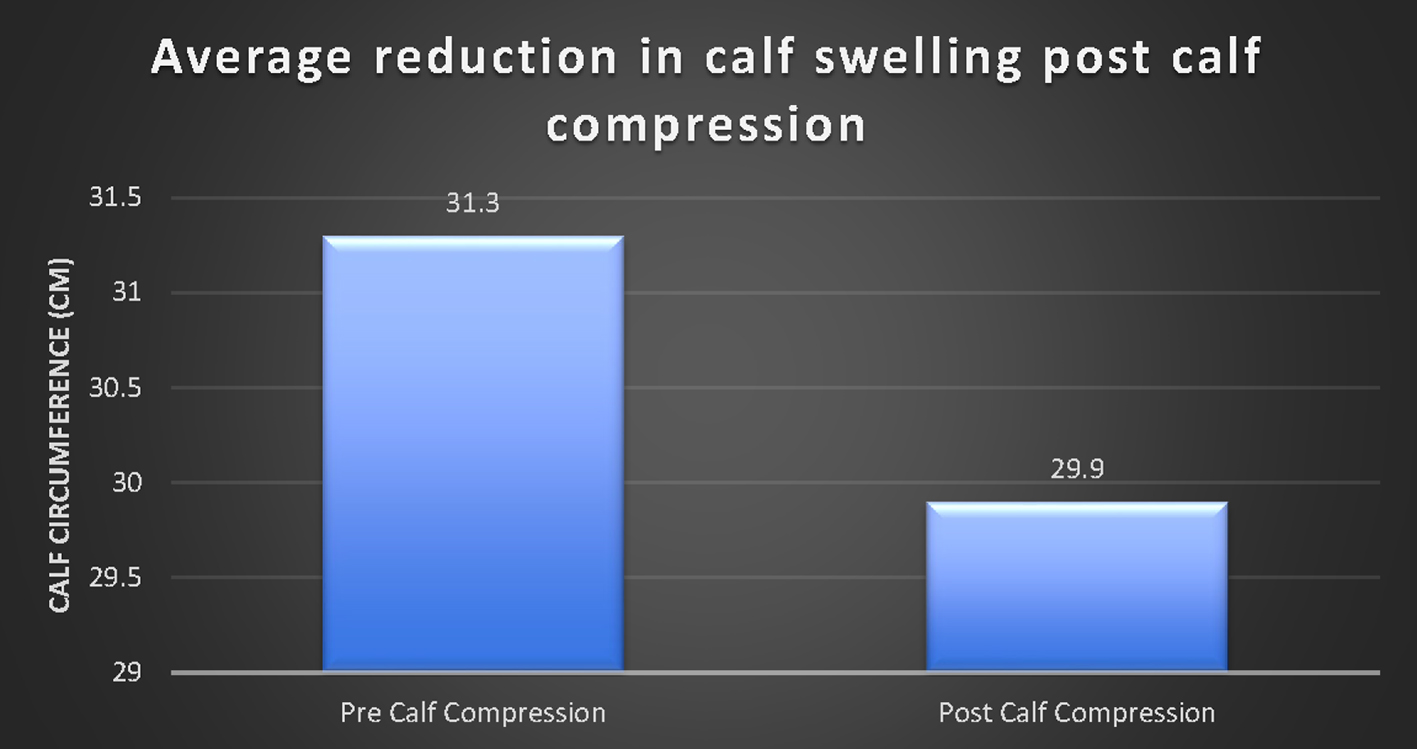 Click for large image | Figure 5. A significant decrease in calf swelling (as measured by calf circumference) after calf compression (P < 0.01). |
There were no significant differences found between the three chamber pressure changes with the calf compressor using logistic regression.
Out of 46 patients who were questioned as to their tolerance, six responded that they had experienced mild pain or discomfort. The remaining 40 tolerated it well (87%).
| Discussion | ▴Top |
Compression therapy is an important part of treatment for CVD in reducing related complications. Often patients do not comply with compression therapies, such as stockings, bandages and non-mobile IPC units because of difficulty with use of these therapies [3]. In addition, such therapies as compression stockings have come under scrutiny in recent studies, and, although there is no conclusive evidence, have raised the question as to their efficacy [7, 8].
The use of IPC in patients with DVT or CVD has been discussed in a review of its application [9]. It was concluded by the author and further supported by relevant literature that the full potential of IPC has probably not yet been realised, and its use and application requires better quality research [9]. Intermittent compression used in immobile patients is well established, and effective in the prevention of DVT and reduction of lymphedema. Our study has refined the use of an IPC that can be modified to an individual’s needs that allows mobilization, using a programmable sequential calf compression (Vekroosan®). Doppler ultrasonography has been used to study the effect of calf compression and the use of sequential and individualized calf pressure changes to achieve significant clinical outcomes.
Studies have shown improved PFV measurements in volunteers that are statistically significant with a fivefold increase, relative to baseline [10, 11]. This increase in PFV is consistent with increased PFV reported by others, for IPC devices, with a flow velocity of 35 - 60 cm/s [12, 13]. Hence there is evidence to show that increased PFV may be one of the possible mechanisms for thrombosis prophylaxis and may explain findings that suggest IPC is effective in peri-operative prophylaxis of venous thrombosis [14]. Increased PFV produced by this device (Vekroosan) through mechanical, pneumatic compression of the calf muscles may have an equivalent prophylactic effect. Therefore, this device may have the potential to reduce the risk for DVT in the treated leg. Other compression devices were proven clinically effective in the prevention of DVT with their major effect being on the PFV rather than the total volume flow (TVF), similar to the Vekroosan device. Studies have shown that pressures as low as 40 mm Hg provide clinically significant improvement in venous flow [15, 16]. The unique profile of the device including pressure sequencing and sharp pressure increase within 5 s with its sharp pressure rise and fall affects the flow not only at the calf level, but actually significantly increases the PFV proximal to device location, at the superficial femoral vein level as demonstrated in the superficial flow measurements. Our study has seen significant changes in PFV changes (P < 0.001) (Fig. 4) in this mobile device, and this is consistent with the literature [10-13].
Using the Vekroosan, there was an increase of nearly 11 cm/s PFV (mean increase from 32.7 to 43.1 cm/s, pre- and post-calf compression using the device) with more than 30% increase from baseline PFV. There appears to be a significant flow improvement even with mobilization. There was no statistically significant difference with the compression of lower chamber compared to middle and upper chambers (Figs. 1 and 2). However, it was noted that if the compression pressures in the lower and middle chambers were not maintained before compressing the upper chamber, in patients with venous reflux, it would increase swelling and pain around the ankles. Hence, it is important to maintain pressure in the lower and middle chambers whilst inflating the upper chamber in order to achieve proper sequencing and thereby improve the flow from calf to inferior vena cava. The deflation sequencing is also important to ensure that there is no risk of venous reflux, by deflating upper, mid and lower chambers, respectively. As stated, unlike the hospital bedbound IPC, this mobile unit has the capacity to be used whilst mobilizing (Fig. 3).
The advantage of this device was that it was easy to apply and patients could mobilize with it. Furthermore, the pressures could be altered in each chamber individually, if required, to maximize the femoral flow velocity. External calf pressures could be altered depending on the individual tolerance and needs of the patient.
Another clinical benefit of Vekroosan® was the statistically significant reductions in the calf swelling measurements, after its use (Fig. 5). With application of Vekroosan® for at least 1 h, the majority of patients found benefit. It appeared more noticeable in patients with larger circumferences and marked CVD. With reduced swelling, consequently, patients also experienced less lower limb aches and pains. Although some patients found discomfort and mild pain using IPC, the vast majority of the patients (87%) tolerated the therapy quite well. This device, in particular, has shown this clinical benefit of reduced lower limb swelling and, hence, pain associated with CVD in the mobile state. Longitudinal and a larger cohort studies are warranted to assess the extended benefits of DVT prevention and reduced risk of chronic venous ulceration.
Venous leg ulcer is a significant health problem that afflicts 1% of the population at some point during their lifetime. IPC is already widely used to prevent DVT, although our study did not include patients with this affliction. Nevertheless, it has been purported that the intermittent nature of pulsatile external compression produces beneficial physiologic changes, which include hematologic, hemodynamic and endothelial effects, which should theoretically promote healing of venous ulcers. Clinical studies of the management of venous ulcers show that IPC increases overall healing and accelerates the rate of healing [17], leading to current guideline recommendations for care of these patients.
It appears that application of mobile IPC in combination with sustained graduated compression improves outcome in patients with the most advanced venous disease [2].
Although one of the limitations to our study was a relatively small sample size, with this cohort we were able to show clinical and statistical significance with application of Vekroosan in patients who could choose to mobilize with it.
A strategic limitation was that of not having had a control group in one sense or another. A control group could have been a cohort whose sole “control” measure was the use of either compression stockings or bandages.
Another limitation could be the clinical approach itself to our study; it would be interesting to note how patients fair with Vekroosan in the community.
Conclusion
IPC device Vekroosan has added a mobile and pulsatile function to managing CVD patients. Its use has shown statistically significant results both in terms of achieving PFV increment and reduction in leg swelling with continuous use while mobilizing. Mechanical devices offer a major advantage over pharmaceutical items that are not only more expensive to the health system but also a burden to the individual with potential adverse events and inconveniences. IPC can only move forward in the treatment of CVD patients while striving to maintain normalcy of lifestyle in patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Raj Ramakrishna is affiliated with DVT Solutions Pty. Ltd.
Informed Consent
Informed consent was obtained from all participants.
Author Contributions
Raj Ramakrishna: study design, recruited participants, collected data, analyzed data, co-wrote manuscript, final article approval prior to submission; William Alexander: collected data, analyzed data, statistical analysis, co-wrote manuscript, final article approval prior to submission; Lina Baytieh: study design, analyzed data, statistical analysis, co-wrote manuscript, final article approval prior to submission.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Nicolaides AN, Cardiovascular Disease, Educational, Research, Trust, European Society of Vascular, Surgery, The International Angiology Scientific Activity Congress Organization, International Union of, Angiology, Union Internationale de Phlebologie at the Abbaye des Vaux de, Cernay. Investigation of chronic venous insufficiency: A consensus statement (France, March 5-9, 1997). Circulation. 2000;102(20):E126-163.
doi pubmed - Comerota AJ. Intermittent pneumatic compression: physiologic and clinical basis to improve management of venous leg ulcers. J Vasc Surg. 2011;53(4):1121-1129.
doi pubmed - Berliner E, Ozbilgin B, Zarin DA. A systematic review of pneumatic compression for treatment of chronic venous insufficiency and venous ulcers. J Vasc Surg. 2003;37(3):539-544.
doi pubmed - Ramakrishna R, Gupta S, Sarathy K, Batterham M, Kirsh G. Combined short course low molecular weight heparin and calf compression therapy in prevention of deep vein thrombosis after total knee replacement: a randomized prospective study. J Hematol Thromb Dis. 2013;1:118.
doi - Vicaretti M. Compression therapy for venous disease. Australian Prescriber. 2010;33:186-190.
doi - Milic D. J. Chronic Venous Disease. Medicographia. 2011;33:253-258.
- Caprini JA. Stockings or no stockings: that is the question. Ann Surg. 2010;251(3):397-398.
doi pubmed - Morris RJ, Woodcock JP. Intermittent pneumatic compression or graduated compression stockings for deep vein thrombosis prophylaxis? A systematic review of direct clinical comparisons. Ann Surg. 2010;251(3):393-396.
doi pubmed - Morris RJ. Intermittent pneumatic compression - systems and applications. J Med Eng Technol. 2008;32(3):179-188.
doi pubmed - Malone MD, Cisek PL, Comerota AJ, Jr., Holland B, Eid IG, Comerota AJ. High-pressure, rapid-inflation pneumatic compression improves venous hemodynamics in healthy volunteers and patients who are post-thrombotic. J Vasc Surg. 1999;29(4):593-599.
doi - Galili O, Mannheim D, Rapaport S, Karmeli R. A novel intermittent mechanical compression device for stasis prevention in the lower limbs during limited mobility situations. Thromb Res. 2007;121(1):37-41.
doi pubmed - Ricci MA, Fisk P, Knight S, Case T. Hemodynamic evaluation of foot venous compression devices. J Vasc Surg. 1997;26(5):803-808.
doi - Whitelaw GP, Oladipo OJ, Shah BP, DeMuth KA, Coffman J, Segal D. Evaluation of intermittent pneumatic compression devices. Orthopedics. 2001;24(3):257-261.
- Pavon JM, Adam SS, Razouki ZA, McDuffie JR, Lachiewicz PF, Kosinski AS, Beadles CA, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical patients: a systematic review. J Arthroplasty. 2016;31(2):524-532.
doi pubmed - Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, Tormene D, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141(4):249-256.
doi pubmed - Maton B, Thiney G, Ouchene A, Flaud P, Barthelemy P. Intramuscular pressure and surface EMG in voluntary ankle dorsal flexion: Influence of elastic compressive stockings. J Electromyogr Kinesiol. 2006;16(3):291-302.
doi pubmed - Nelson EA, Hillman A, Thomas K. Intermittent pneumatic compression for treating venous leg ulcers. Cochrane Database Syst Rev. 2014;5:CD001899.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


