| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 2, Number 1, June 2013, pages 14-21
Efficacy of Ribavirin to Prevent Hepatitis Reactivation in Hepatitis C Virus-Infected Patients Treated for Non-Hodgkin Lymphoma, Single Egyptian Center Study
Ashraf M. El-Hefnia, b, Tarek A Elgoharya, Ahmed Kotba, Fouad M. Abu-Taleba
aHematology and Medical Oncology Unit, Internal Medicine Department Faculty of Medicine, Zagazig University, Egypt
bCorresponding author: Ashraf M. El-Hefni, Hematology/Medical Oncology Unit, Internal Medicine Department, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Manuscript accepted for publication May 14, 2013
Short title: Efficacy of Ribavirin
doi: https://doi.org/10.4021/jh72w
| Abstract | ▴Top |
Background: Reports had found an association between B cell non-Hodgkin lymphomas (NHL) and Hepatitis C virus (HCV) infection. However, data on acute exacerbation and reactivation of chronic HCV infection following chemotherapy are very limited. We studied the efficacy of ribavirin to prevent hepatitis reactivation in HCV-infected patients treated for NHL.
Methods: This study was carried out at Hematology and Medical Oncology unit, Internal Medicine Department, Zagazig University Hospitals and it included 57 patients with B-cell NHL who were naive to chemotherapy, among them 24 patients were positive for HCV antibody and 33 patients were negative for HCV (group C). The HCV infected group was subdivided into 11 patients who received ribavirin (group A) and 13 patients did not receive ribavirin (group B)and routine investigations for NHL were done, HCV RNA was measured for HCV infected patients before and after the end of chemotherapy.
Results: HCV infection occurred in 42% of patients with B cell NHL. Acute hepatic enzyme exacerbation occurred in 8 (14%) of all patients with the highest percentage was 29.2 % among HCV infected patients (7/24), while only one patient (3%) in the HCV negative group (P = 0.007). Among the 24 NHL patients with HCV positivity, we compared group A and group B during chemotherapy as regards to hepatic enzyme flare, it was (27% and 30%, respectively, P = 0.6). Five (20.8%) of 24 NHL patients with HCV positivity were developed HCV PCR reactivation.
Conclusions: The frequency of HCV infection in patients with B cell NHL was higher than in the general population and acute exacerbation and reactivation of chronic HCV infection occurred in a sizeable subset of patients with NHL during chemotherapy, However the use of ribavirin did not decrease hepatic enzyme flare or HCV PCR reactivation during chemotherapy.
Keywords: Non-Hodgkin lymphomas; Hepatitis C virus (HCV) infection; Ribavirin
| Introduction | ▴Top |
Hepatitis C virus (HCV) infection is endemic in Egypt and many reports had found an association between B cell non-Hodgkin lymphomas (NHL) and HCV infection. The role of HCV infection in lymphomagenesis may be related to chronic antigenic stimulation of HCV [1]. However, little is known about acute exacerbation and reactivation of chronic HCV infection in patients with cancer [2]. Most of the reported cases of liver dysfunction in HCV-infected cancer patients occur in non-Hodgkin lymphomas [3]. Authors have reported a reactivation of HCV replication in patients with CD20-positive B-cell NHL under Rituximab-based chemotherapy [4].
Also, limited studies indicate that episodes of acute exacerbation of chronic HCV seem to be less severe than similar episodes of chronic hepatitis B virus (HBV) exacerbation [5]. Following reactivation of the HBV after cytotoxic chemotherapy is common in clinical practice. Thus, prophylactic antiviral (lamivudine) treatment should be started at the initiation of chemotherapy and maintained for at least 6 months following the completion of chemotherapy [6].
We sought to determine the frequency of HCV infection among B cell NHL patients, also to determine the efficacy of ribavirin to prevent hepatitis reactivation in HCV-infected patients treated for NHL.
In our study, in analogy with the use of lamivudine as prophylaxis against HBV reactivation, we tried to use Ribavirin (a synthetic nucleoside analogue) as a prophylactic antiviral treatment to reduce the risk of HCV reactivation and severe hepatitis flares.
| Patients and Methods | ▴Top |
The study was a randomized controlled intervention trial, which carried out at Hematology and Medical Oncology unit, Internal Medicine Department, Zagazig University Hospitals between July 2010 and August 2012. It included 57 patients with NHL who were naive to chemotherapy, among them 24 patients were infected by HCV infection and 33 patients were not infected by HCV (group C). The HCV infected group was subdivided into 11 patients who received ribavirin (group A) and 13 patients did not received ribavirin (group B) (Fig. 1).
 Click for large image | Figure 1. The distribution of the study groups. |
Inclusion criteria: 1), age: > 18 years; 2), sex: both sexes were eligible; 3), pathological proof of non- Hodgkin’s lymphoma; 4), adequate bone marrow reserve; 5), adequate liver and kidney functions; 6), Eastern Cooperative Oncology Group performance status (PS) of ≤ 2; 7), all patients were naive for anti-HCV treatment; 8), all patients were chemotherapy naive.
Exclusion criteria: 1), prior or concurrent second malignancy; 2), pregnant, lactating females; 3), medical contraindication for receiving the study treatment as patients with active or uncontrolled infection; 4), positivity for HBs Ag or HIV; 5), non-viral causes of liver affection.
| Methods | ▴Top |
Informed consent was obtained from participants, (females must accept to use contraception during treatment) and protocols of chemotherapy were approved by our institutional board and all were subjected to: 1), thorough history taking, clinical examination; 2), complete blood counts; 3), serum lactate dehydrogenase (LDH); 4), erythrocyte sedimentation rate (ESR); 5), liver and kidney function tests (ALT, AST, serum billirubin, serum albumin, INR and serum creatinine); 6), viral markers (HBs Ag, HBcAb, HCV Abs) and HCV RNA in serum by PCR if HCV antibodies were positive; 7), autoimmune hepatitis antibodies (antinuclear antibody (ANA), anti-smooth muscle antibody (SMA), liver/kidney microsomal antibody (LKM), anti soluble liver antigen (SLA/LP) and anti-mitochondrial antibody (AMA)); 8), serum electrolytes (Na, K and Ca), serum uric acid and fasting blood sugar; 9), bone marrow biopsy, echocardiography and radiological studies were performed including: chest radiograph, CT scan of abdomen and pelvis and CT scan of the neck and thorax if any abnormality was noted or suspected on the routine chest radiograph.
HCV DNA examination
HCV RNA in serum was done at the beginning and at the end of chemotherapy. It was quantified using a commercially available polymerase chain reaction method (COBAS Taqman HCV Test; Roche Molecular Systems, Branchburg, NJ) with a quantification range from 43 to 69,000,000 IU/mL [7].
Treatment plan
Chemotherapy were given based on the pathological sub-types either indolent or aggressive NHL. Detailed history, full clinical examination and Laboratory assessment were performed before each treatment cycle. All patients who ended the first 4 cycles of treatment were eligible for reevaluation within 2 to 3 weeks, and those who responded (complete response (CR) or partial response (PR)) were completed the therapy to a total 6 cycles.
We randomly divided the patients with positive HCV infection into two groups, one group received Ribavirin 1,000 - 1,200 mg daily orally during the course of chemotherapy and the other did not.
Definitions
Acute exacerbation of chronic HCV infection was defined as a 3-fold or greater increase in serum ALT level in the absence of the use of hepatotoxic drugs (other than chemotherapeutics), or other systemic infections (including hepatitis A, HBV, cytomegalovirus, adenovirus, and human immunodeficiency virus infections) [5].
HCV reactivation was defined as an increase in HCV viral load of at least 1 log10 IU/mL over baseline following chemotherapy or immunosuppressive therapy, as chronically infected patients have stable HCV RNA levels that may vary by 0.5 log10 IU/mL [8].
Response criteria
According to Revised response evaluation criteria is in solid tumors (version 1.1) [9].
Statistical analysis
All the data were managed using SPSS-version version 20.0. A two-sided P value of less than 0.05 was considered to indicate statistical significance. The association between categorical data was tested by Chi-square and Fisher exact tests. The t-test was used to assess whether the means of two groups were statistically different from each other. To compare between more than two groups, one way analysis of variance (ANOVA) was used. Survival analysis was done according to Kaplan-Meier method, and compared by log-rank test.
Overall survival (OS) was calculated as interval (by months) between date of randomization (pathology date) till date of death or date of last follow up.
Disease free survival (DES) was calculated as the period of time patient lived without evidence of disease relapse (for responding patients). It is the interval (by months) between date of the complete response till date of disease progression or date of last follow up [1].
| Results | ▴Top |
The base line characters of all patients are described in (Table 1). In our study, HCV infection occurred in 42% of patients with B cell NHL. Acute hepatic enzyme exacerbation occurred in 8 (14%) of all patients, and with the highest percentage (29.2%) among HCV infected patients (7/24), while only one patient (3%) in the HCV -ve group (P = 0.007). Among the 24 NHL patients with HCV positivity, we compare patients who received ribavirin (group A) versus those did not receive ribavirin (group B) during chemotherapy as regards to hepatic enzyme flare which was (27% and 30%, respectively, P = 0.6), five (20.8%) of 24 NHL patients with HCV positivity developed HCV PCR reactivation; 2 patients of group A and 3 of group B patients (Table 2, 3). Only 3 patients (37.5%) form those who developed hepatic enzyme flare stopped their chemotherapy, while none from the other group stopped chemotherapy, there was a statistical significant difference (P = 0.002). There was a significant relation between HCV reactivation and hepatic enzyme flare (P < 0.001). Addition of ribavirin to chemotherapy did not have any significance effect in NHL infected patients (Table 4, 5). The overall survival and disease-free survival in the three patients groups were comparable, although many patients among the HCV infected NHL group showed some delay in the treatment schedule, but this follow up was short (Fig. 2).
 Click to view | Table 1. Patient Characteristics of the Studied Groups |
 Click to view | Table 2. Comparison Between HCV + ve Patients Who Received Ribavirin (Group A) Versus Those Did Not Receive Ribavirin (Group B) Regarding Hepatic Enzyme Flare and HCV PCR Reactivation |
 Click to view | Table 3. Frequency Treatment Disruption Among the Studied Groups of Patients According to Hepatic Enzyme Flare |
 Click to view | Table 4. Relation Between HCV PCR Reactivation in NHL Patients With HCV + ve and Hepatic Enzyme Flare |
 Click to view | Table 5. Distribution of Treatment Response Among the Studied Groups of Patients According to HCV Status |
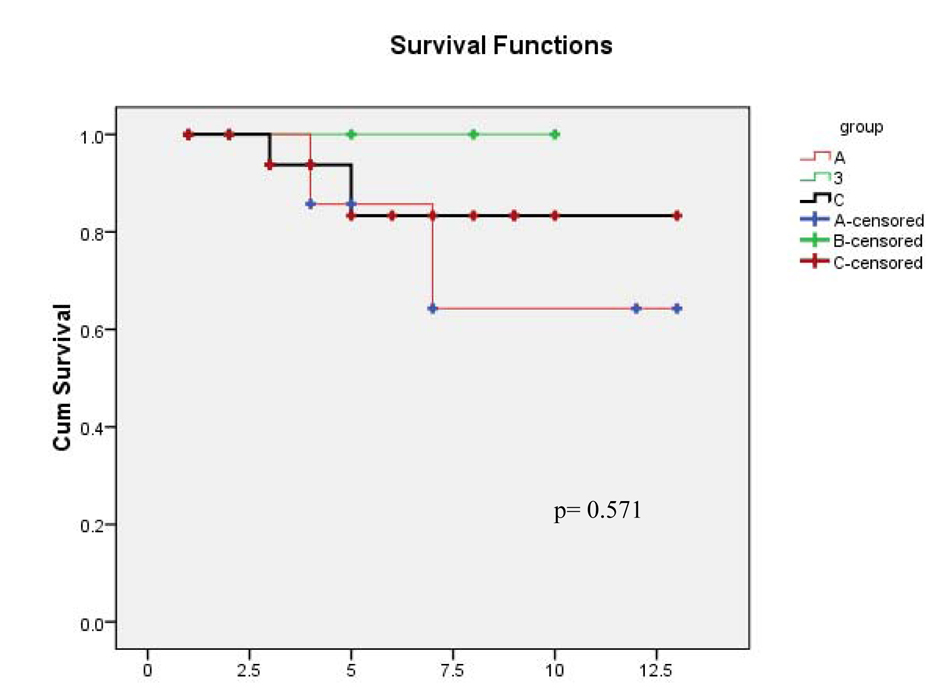 Click for large image | Figure 2. One year disease-free survival. |
As regards overall response, 63% of our patients achieved complete response (CR) and 28% partial response (PR); 76% of DLBCL (30/39) patients obtained a CR, while between indolent NHL patients 21% (3/14 patients) achieved CR. While, among HCV infected NHL patints, 73.3% (11/15 patients) of DLBCL achieved CR, whereas, 33.3% (3/9 patients) of an indolent NHL achieved CR (P-value = 0.07).
| Discussion | ▴Top |
The percentage of HCV infection among our B cell NHL patients is 42% (24 out of 57 cases) which is nearly equal to results obtained by Cowgill et al [10] (a study conducted at Cairo University hospitals, Egypt on 220 patients and the prevalence of HCV infection was 42.7%), while in Nicola et al study which conducted in Italy on 36 patients and the prevalence of HCV infection was 22 % [11].
The interesting finding in our study is that percentage (42%) of HCV infection which is higher than that observed in the general population (15-20%) [12]. This comes in agreement with Nosotti et al [3], who stated that the prevalence of HCV infection among NHL patients was 9.2%, this prevalence is also higher than that observed in the general population in Italy (3%) and this association between B cell non-Hodgkin lymphomas (NHL) and HCV infection may be related to chronic antigenic stimulation of HCV.
The comparison between HCV infected patients and HCV uninfected patients with NHL showed no statistically significant difference as regard age, sex, clinical presentation, stage, IPI score, PS status, LDH level, pathological type, chemotherapy regimen and this was in concordance with results of Marignani et al [13]. Most of our HCV infected patients (75%) presented with advanced stage (III/IV) compared to 42.5% among HCV negative patients (P-value = 0.09) and this result was also similar to the study of Luppi et al [14] who reported that the percentage of advanced stage (III/IV) was 74% of HCV positive patients, while 64% of HCV negative patients.
In our work, fourteen patients (24.5%) had an indolent NHL and forty three patients (75.4%) had an aggressive NHL. The most frequent pathological type was DLBCL and the highest percentage (37.5%) of Indolent NHL type was found among HCV infected NHL cases (9/24 patients), especially marginal zone lymphoma (3 patients). This result was in concordance with Arcaini et al [1] who reported that 37% of HCV infected NHL cases (59/160 patients) had an indolent NHL type, while 62% of the cases had a DLBCL type.
In the terms of overall response, 63% of our patients achieved complete response (CR) and 28% partial response (PR) 76% of DLBCL (30/39) patients obtained a complete response, while between indolent NHL patients 21% (3/14 patients) achieved CR. While, among HCV positive NHL, 73.3% (11/15 patients) of DLBCL achieved CR, whereas, 33.3% (3/9 patients) of an indolent NHL achieved CR (P-value = 0.07). While, Pellicelli et al [15] showed that 54% of DLBCL achieved CR and 55% of indolent NHL patients achieved complete response. In our study, there was not any statistical significant difference found when we compared the complete response rates in the three groups.
Among the fifty seven NHL patients in our study eight patients (14%) developed hepatic enzyme flare and the highest percentage of enzymatic flare was among HCV infected patients (29.2%), while only one patient (3%) in the HCV negative group (C). There was a statistical significant difference (P = 0.007) between the two groups. This result comes in agreement with another study which reported that among the HCV-infected subjects, the incidence of hepatitis flares was 26.3% vs 2.1% among the HCV-uninfected individuals [3].
It was found that 20.8% of HCV infected NHL patients (5/24 patients) developed HCV PCR reactivation. Also, Parag et al [5] reported that 36.3% of HCV infected NHL patients proved by PCR (8/22 patients) developed HCV PCR reactivation. While, Boyle et al [16] reported that 66.6% of HCV infected NHL patients (6/9 patients) developed HCV PCR reactivation and the difference between studies regarding the percentage of hepatic enzyme flare or HCV PCR reactivation might attributed to difference in the definition of hepatic enzyme flare and HCV PCR reactivation between studies, different sample size, heterogeneity of the histopathology [1], different HCV genotype and association with other viral infection (HBV or HIV) and/or difference in duration of chronic HCV infection and the risk of developing cirrhosis.
A significant relation between HCV reactivation and hepatic enzyme flare (P < 0.001) was detected and also Parag et al [5] found the same of our result , in contrary to Marignani et al [13], who found no significant relation (P = 0.8) and this difference might attributed to difference in selection criteria.
As regarding the toxicities (apart from hepatic toxicity), they were similar in all groups; there were not any significant differences, with only one patient developed Grade 4 anemia in group A (the intervention arm who received ribavirin) but without significant difference comparing to other groups (P = 0.58). The hematologic toxicity with the use of ribavirin was to some extent accepted.
The overall survival and disease-free survival in the three patients groups were comparable, although many patients among the HCV infected NHL group showed some delay in the treatment schedule, but this follow up was short.
Conclusion
Frequency of HCV infection in patients with B cell NHL is higher than in the general population. Acute exacerbation and reactivation of chronic HCV infection occur in a sizeable subset of patients with NHL during chemotherapy and addition of ribavirin did not decrease hepatic enzyme flare or HCV PCR reactivation during chemotherapy, also ribavirin did not affect response to chemotherapy or survival rates.
| References | ▴Top |
- Arcaini L, Merli M, Passamonti F, Bruno R, Brusamolino E, Sacchi P, Rattotti S, et al. Impact of treatment-related liver toxicity on the outcome of HCV-positive non-Hodgkin's lymphomas. Am J Hematol. 2010;85(1):46-50.
pubmed - Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012;9(3):156-166.
doi pubmed - Nosotti L, D'Andrea M, Pitidis A, Pimpinelli F, Dessanti ML, Pisani F, Vignally P, et al. Hepatitis C virus infection prevalence and liver dysfunction in a cohort of B-cell non-Hodgkin's lymphoma patients treated with immunochemotherapy. Scand J Infect Dis. 2012;44(1):70-73.
doi pubmed - Pitini V, Sturniolo G, Arrigo C, Leonardi S, Pino S, Altavilla G. HCV genotype 2 as a risk factor for reactivation in patients with B-cell lymphoma undergoing rituximab combination chemotherapy. Br J Haematol. 2010;150(1):116-118.
pubmed - Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57(6):1177-1185.
doi pubmed - Yang D and Roberts L. Assessment of Liver Function and Viral Hepatitis for the Medical Oncologist. ASCO educational book. 2010;142-147.
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6(10):986-994.
doi pubmed - McGovern BH, Birch CE, Bowen MJ, Reyor LL, Nagami EH, Chung RT, Kim AY. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49(7):1051-1060.
doi pubmed - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Cowgill KD, Loffredo CA, Eissa SA, Mokhtar N, Abdel-Hamid M, Fahmy A, Strickland GT. Case-control study of non-Hodgkin's lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol. 2004;33(5):1034-1039.
doi pubmed - Coppola N, Pisaturo M, Guastafierro S, Tonziello G, Sica A, Iodice V, Sagnelli C, et al. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis. 2012;44(1):49-54.
doi pubmed - Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355(9207):887-891.
doi - Marignani M, Mangone M, Cox MC, Angeletti S, Veggia B, Ferrari A, di Fonzo M, et al. HCV-positive status and hepatitis flares in patients with B-cell non-Hodgkin's lymphoma treated with rituximab-containing regimens. Dig Liver Dis. 2011;43(2):139-142.
doi pubmed - Luppi M, Longo G, Ferrari MG, Barozzi P, Marasca R, Morselli M, Valenti C, et al. Clinico-pathological characterization of hepatitis C virus-related B-cell non-Hodgkin's lymphomas without symptomatic cryoglobulinemia. Ann Oncol. 1998;9(5):495-498.
doi pubmed - Pellicelli AM, Marignani M, Zoli V, Romano M, Morrone A, Nosotti L, Barbaro G, et al. Hepatitis C virus-related B cell subtypes in non Hodgkin's lymphoma. World J Hepatol. 2011;3(11):278-284.
doi pubmed - Boyle F and Reid E. Viral reactivation and clinical hepatitis in patients with hepatitis C who receive rituximab as part of chemotherapy for treatment of lymphoma: A case series. J Clin Oncol 2010;28(suppl):abstr e18559.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


