| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 9, Number 3, September 2020, pages 79-83
Ofatumumab and Complement Replacement in Relapsed/Refractory Chronic Lymphocytic Leukemia
Joseph Tuscanoa, b, d, Christina Poha, c, Aaron Rosenberga, Brian Jonasa, b, Mehrdad Abedia, Gustavo Barisonea, Emily Schwaba, Kathleen Lundeberga, Paul Kaesberga
aUniversity of California Davis Comprehensive Cancer Center, Sacramento, CA, USA
bVeterans Administration, Northern California Healthcare System, Sacramento, CA, USA
cUniversity of Washington, Division of Medical Oncology, Seattle, WA, USA
dCorresponding Author: Joseph Tuscano, University of California Davis Comprehensive Cancer Center, Sacramento, CA, USA
Manuscript submitted July 12, 2020, accepted July 29, 2020, published online August 14, 2020
Short title: Ofatumumab and FFP for R/R CLL
doi: https://doi.org/10.14740/jh721
| Abstract | ▴Top |
Background: While many humanized monoclonal antibodies utilize complement-dependent cytotoxicity, the complement depleting effects of these antibodies and the impact of complement replacement on treatment response are not well-described.
Methods: We conducted a phase 2 trial involving patients with relapsed/refractory chronic lymphocytic leukemia (CLL). Patients were treated with ofatumumab with fresh frozen plasma (FFP) used as a source of complement replacement. The primary endpoint was objective response rate. Correlative endpoints included complement levels (C3 and C4) and complement activity (CH50) which was drawn at baseline and after ofatumumab with FFP administration.
Results: Among 12 enrolled patients, overall response rate was 83% with two patients (17%) achieving a complete response. While only two (17%) patients had low complement activity at baseline, eight (67%) developed low levels of complement activity after ofatumumab treatment with FFP replacement. The magnitude of complement depletion did not correlate with response. Adverse events were minimal. The combination of ofatumumab and FFP demonstrated tolerability and surprising activity in high-risk CLL patients.
Conclusions: The combination of ofatumumab and FFP demonstrated tolerability and surprising activity in high-risk CLL patients. Complement replacement should be studied further as a minimally toxic approach to improve efficacy of monoclonal antibody-based regimens.
Keywords: Chronic lymphocytic leukemia; Ofatumumab; Fresh frozen plasma
| Introduction | ▴Top |
Despite recent advances in treatment of chronic lymphocytic leukemia (CLL), this disease remains incurable and relapse is common [1]. An impaired complement system is thought to be involved in the pathophysiology of CLL and likely contributes to infectious complications [2]. Treatment with humanized antibodies that preferentially utilize complement such as ofatumumab further decreases complement levels which may impair efficacy [3-5]. Previous studies have hypothesized that complement replacement with fresh frozen plasma (FFP) may enhance the efficacy of monoclonal antibodies in patients with CLL [6-8]. A previous analysis of five patients with relapsed/refractory (R/R) CLL, three of which failed rituximab, were treated with rituximab and FFP which demonstrated favorable efficacy with minimal toxicity [9]. Therefore, we hypothesize that the addition of FFP to ofatumumab, a humanized anti-CD20 monoclonal antibody designed to enhance complement dependent cytotoxicity (CDC), will enhance its efficacy without adding appreciable toxicity. We report efficacy and safety analysis of ofatumumab treatment with FFP as complement replacement in R/R CLL.
| Patients and Methods | ▴Top |
Patient and study design
We conducted an open-label phase 2 trial involving patients at least 18 years of age with R/R CLL who have received at least one prior rituximab-containing therapy. Rituximab exposure must have been completed at least 3 months prior to study enrollment. Other inclusion criteria included a good functional status defined as an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate liver, kidney and marrow function.
Study consisted of ofatumumab administered on a standard schedule (300 mg on day 1, followed by weekly infusions of 2,000 mg to complete eight doses, followed by monthly doses of 2,000 mg to complete a total of 12 doses in 24 weeks) in combination with FFP (two units administered prior to every dose of ofatumumab starting on week 2) (Supplementary Material 1, www.thejh.org). Blood for correlative complement studies was drawn at baseline and after two doses of ofatumumab and FFP.
Response and safety assessment
Imaging using positron emission tomography (PET) and/or computerized tomography (CT) scans was performed at baseline and after completion of therapy. Disease response was assessed according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) [8].
Adverse events and their severity, assessed prior to each dose of ofatumumab and FFP therapy and during scheduled follow-up visits, were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0, http://ctep.cancer.gov).
Statistical analysis
The primary endpoint was objective response rate (ORR), defined as the proportion of patients with complete response (CR) or partial response (PR), which was analyzed using Simon’s two-stage design [10]. It was hypothesized that CLL patients that were previously treated with a rituximab-containing regimen would have an ORR of 25%. A 20% increase in the ORR with the addition of FFP to 45% would justify evaluation of this regimen in larger, more definitive trials.
Secondary endpoints included progression-free survival (PFS) and safety. Exploratory endpoints, which included complement levels (C3 and C4) and complement activity (CH50) obtained before and after treatment initiation, were analyzed using Wilcoxon signed-rank tests. PFS was estimated with the Kaplan Meier method. All analysis was based on the intention-to-treat population.
This study was carried out in compliance with the protocol and Good Clinical Practice, as described in the International Council for Harmonisation Harmonized Tripartite Guidelines for Good Clinical Practice 1996 and the Declaration of Helsinki, concerning medical research in humans. This study was reviewed and approved by a properly constituted Institutional Review Board of the University of California, Davis. IRB Number is 333961.
| Results | ▴Top |
Patient characteristics
Between June 2013 and March 2017, 12 patients were enrolled. Baseline patient characteristics are shown in Table 1. Median age was 64 years (range 50 - 76 years) and the median number of prior regimens was 1 (range 1 - 5). Seven of 12 patients (58%) had high-risk disease based on standard fluorescence in situ hybridization (FISH) analysis (del 17p or 11q/ATM).
 Click to view | Table 1. Patient Baseline Characteristics |
Efficacy and complement activity
At a median follow-up time of 37 months, the ORR rate was 83% (10 of 12 patients); CR in 16.6% (2 of 12 patients) and PR in eight patients. Two patients had progressive disease. The two patients who achieved a CR remained in continuous remission for up to 25 months thereafter. Median PFS was 12.5 months (95% confidence interval (CI): 8 - 14.6 months) (Fig. 1).
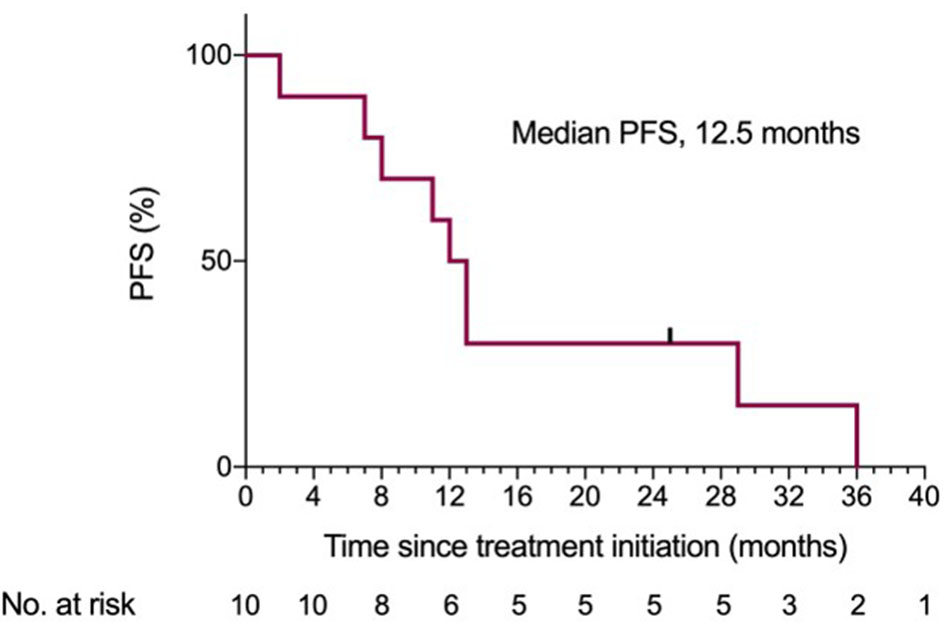 Click for large image | Figure 1. Kaplan-Meier estimates of PFS. PFS: progression-free survival. |
At baseline, 17% (two patients) had low complement activity with low C3, C4 and CH50 levels noted in 17%, 17% and 8% of patients, respectively (Table 2). After 2 weeks of ofatumumab treatment with FFP replacement, 67% (eight patients) developed low levels of complement activity with low C3, C4 and CH50 levels noted in 30%, 75% and 67% of patients, respectively. The mean reduction for C3, C4 and CH50 was significant at 14% (P < 0.001), 58% (P < 0.004) and 54% (P = 0.005), respectively. The magnitude of complement reduction did not correlate with response.
 Click to view | Table 2. Complement Levels and Activity |
Safety
The most common adverse event was infusion reactions which occurred in 58% (seven patients); 17% were grade 3. All infusion reactions were limited to the first two infusions and were consistent with known reactions to ofatumumab. Other adverse events were ≤ grade 2 in severity and included hypertension (16%), fatigue (8%), neutropenia (24%) and anemia (8%). Venous thromboembolism was not observed in any patients. None of the adverse events led to dose reductions, treatment delays or discontinuation.
| Discussion | ▴Top |
In this trial, treatment with ofatumumab and FFP was well-tolerated and resulted in an encouraging ORR of 83% and a CR rate of 17% among patients with R/R CLL. While only 17% patients had low complement activity at baseline, 67% developed low levels of complement activity after ofatumumab treatment with FFP replacement. The magnitude of complement depletion did not correlate with response.
While the association between complement levels or activity and monoclonal antibody treatment is well-described [6-8], there is minimal data regarding the impact of complement repletion on monoclonal antibody treatment efficacy. Klepfish et al reported favorable efficacy and minimal toxicity with rituximab and FFP treatment in five patients with CLL who had previously failed rituximab monotherapy [9]; however, no correlative studies investigating underlying complement levels were done. In our study, a minority of patients were hypocomplementemic at baseline despite prior therapies. However, after the first two doses of ofatumumab (2,300 mg total), all patients had a reduction in complement activity ranging from 20% to 100% from baseline values (mean 54% reduction). This reduction was despite replacement with two units of FFP prior to the second dose of ofatumumab. Given the small sample size of this study, the complement depleting effects of ofatumumab without FFP replacement would be considered provocative and exploratory. In addition, while not examined, longer treatment duration with ofatumumab or other antibodies may produce even greater complement depletion than what is observed in our study. Thus, the potential magnitude of monoclonal antibody-mediated complement depletion cannot be overstated.
While previous studies cannot be directly compared the ORR observed with the combination of ofatumumab and FFP in this high-risk patient population compares favorably to previously published reports using single-agent ofatumumab for R/R CLL [11-13]. A single-arm multicenter trial in 154 patients with R/R CLL refractory to fludarabine and alemtuzumab treated with ofatumumab monotherapy resulted in an ORR of 42% with no CR noted [11]. Another open-label multicenter trial which randomized patients with relapsed CLL to fludarabine and cyclophosphamide with or without ofatumumab showed an ORR of 84% and 68%, respectively [12]. However, grade ≥ 3 adverse events were reported to range from 69% to 74% between both cohorts. Although the small sample size in our study precludes a valid assessment of efficacy and comparison to other studies, it could be hypothesized that the high ORR of 83% may be related to better baseline complement levels than seen in prior studies, and that if complement levels were more effectively repleted, this might translate into improved efficacy and may be a less toxic strategy to improve efficacy of monoclonal antibody-based regimens in future trials.
In this study which examined the relationship between monoclonal antibody treatment with FFP-based complement repletion, complement activity and efficacy, ofatumumab and FFP treatment was generally well tolerated and shown to be surprisingly effective in a high-risk CLL population. Future trials are needed to further explore and validate complement replacement as a less toxic approach to improving efficacy of monoclonal antibody-based regimens in CLL.
| Supplementary Material | ▴Top |
Suppl 1. Treatment schema.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by research funding from Novartis/GSK Pharmaceutical Company, Basel, Switzerland. Award number GSK 1841157.
Conflict of Interest
JT: Seattle Genetics, Amgen, Celgene: Honoraria; Celgene, Novartis, Spectrum, Takeda, AbbVie, Genentech, Pharmacyclics: Research Funding. AR: Amgen: Consultancy, Research Funding. BJ: AbbVie, Accelerated Medical Diagnostics, AROG, Celgene, Daiichi Sankyo, Esanex, Forma, Genentech/Roche, GlycoMimetics, Incyte, LP Therapeutics, Pharmacyclics: Research Funding; AbbVie, Amgen, Celgene, GlycoMimetics, Jazz, Pharmacyclics, Tolero: Consultancy, Membership on an entity’s Board of Directors or advisory committees; AbbVie, Amgen, GlycoMimetics: Other: Travel expenses. MA: AbbVie, Takeda, BMS, Celgene, Gilead: Speakers Bureau. CP, GB, ES, KL and PK declare no conflict of interest.
Informed Consent
Written informed consent was obtained from every study participant.
Author Contributions
JT conceived the study, contributed to its design and coordination, analyzed the data, and drafted the manuscript. CP edited and finalized the manuscript. AR, BJ, MA and PK enrolled patients and edited the manuscript. GB, KL and ES analyzed data and edited the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Sharma S, Rai KR. Chronic lymphocytic leukemia (CLL) treatment: So many choices, such great options. Cancer. 2019;125(9):1432-1440.
doi pubmed - Middleton O, Cosimo E, Dobbin E, McCaig AM, Clarke C, Brant AM, Leach MT, et al. Complement deficiencies limit CD20 monoclonal antibody treatment efficacy in CLL. Leukemia. 2015;29(1):107-114.
doi pubmed - Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793-1800.
doi pubmed - Baig NA, Taylor RP, Lindorfer MA, Church AK, LaPlant BR, Pettinger AM, Shanafelt TD, et al. Induced resistance to ofatumumab-mediated cell clearance mechanisms, including complement-dependent cytotoxicity, in chronic lymphocytic leukemia. J Immunol. 2014;192(4):1620-1629.
doi pubmed - Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJ, Parren PW, et al. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188(7):3532-3541.
doi pubmed - Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JG, Parren PW, Taylor RP. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183(1):749-758.
doi pubmed - Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172(5):3280-3288.
doi pubmed - Tempescul A, Bagacean C, Riou C, Bendaoud B, Hillion S, Debant M, Buors C, et al. Ofatumumab capacity to deplete B cells from chronic lymphocytic leukaemia is affected by C4 complement exhaustion. Eur J Haematol. 2016;96(3):229-235.
doi pubmed - Klepfish A, Rachmilewitz EA, Kotsianidis I, Patchenko P, Schattner A. Adding fresh frozen plasma to rituximab for the treatment of patients with refractory advanced CLL. QJM. 2008;101(9):737-740.
doi pubmed - Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1-10.
doi - Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A, Hx CDSI. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118(19):5126-5129.
doi pubmed - Robak T, Warzocha K, Govind Babu K, Kulyaba Y, Kuliczkowski K, Abdulkadyrov K, Loscertales J, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: results from the COMPLEMENT 2 trial. Leuk Lymphoma. 2017;58(5):1084-1093.
doi pubmed - Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, Wooldridge J, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111(3):1094-1100.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


