| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 9, Number 4, December 2020, pages 116-122
Prognostic Role of Lymphocyte/Monocyte Ratio in Chronic Lymphocytic Leukemia
Osman Yokusa, Esma Nur Saglamb, Hasan Gozea, Fettah Sametoglub, Istemi Serina, c
aDepartment of Hematology, University of Health Sciences, Istanbul Training and Research Hospital, Fatih, Istanbul, Turkey
bDepartment of Internal Medicine, University of Health Sciences, Istanbul Training and Research Hospital, Fatih, Istanbul, Turkey
cCorresponding Author: Istemi Serin, Department of Hematology, University of Health Sciences, Istanbul Training and Research Hospital, Fatih, Istanbul 34098, Turkey
Manuscript submitted July 21, 2020, accepted August 18, 2020, published online October 1, 2020
Short title: Prognostic Role of LMR in CLL
doi: https://doi.org/10.14740/jh730
| Abstract | ▴Top |
Background: Chronic lymphocytic leukemia (CLL) is a B-lymphoproliferative disease with varying clinical characteristics, which occurs mostly in older ages. In studies from literature, we see that different parameters are examined to determine the prognosis of CLL. The main purpose of our study is to determine the relationship of lymphocyte/monocyte ratio (LMR) value in CLL, which has been previously shown to be a prognostic factor in various solid organ tumors and some hematological malignancies.
Methods: A total of 173 patients who were followed up between 2005 and 2019 were retrospectively analyzed. The diagnostic age, gender, laboratory, absolute lymphocyte and monocyte count, LMR and overall survival (OS), treatment and responses, recurrence, cytogenetic subtype and mortality rates were examined.
Results: The median LMR was 26.7 and it was considered as cut-off value of 26. A positive correlation was found between LMR and Rai Stage. LMR was significantly higher in patients who have an indication for treatment or who died.
Conclusions: In our study, in CLL, LMR has been shown to be over 26 in advanced stages, in relapse or with indication of a treatment. With the increase of LMR, it was found that survival and disease-free gap decreased.
Keywords: Chronic lymphocytic leukemia; Lymphocyte/monocyte ratio; Prognosis; Overall survival; Progression-free survival
| Introduction | ▴Top |
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative malignancy characterized by monoclonal proliferation of mature CD5 (+) B lymphocytes in peripheral blood, bone marrow, lymph node or spleen. CLL is a type of leukemia that is common in Western societies, especially in the ages of 60 - 70. It has the highest genetic predisposition in neoplasias [1-4].
Most of the patients are asymptomatic at the time of diagnosis; absolute lymphocytosis (5,000/mm3 and above) or painless lymphadenopathies are detected, and definitive diagnosis is made by advanced examination methods such as flow cytometry. Staging is done with two similar clinical staging systems called Rai and Binet; however, these are inadequate in predicting prognosis [5-7]. Although many factors such as high expression of CD38, CD49, CD305 and ZAP-70 in flow cytometry are used as poor prognostic factors, lactate dehydrogenase (LDH) and β2-microglobulin elevation are frequently used in clinical practice [8]. In addition, another factor associated with poor prognosis is the detection of 17p deletion by cytogenetic analysis [9, 10]. The P53 mutation was detected in 4-37% of CLL patients and has been associated also with poor prognosis [11, 12]. These cases have an aggressive clinical course and should be evaluated early in terms of allogeneic stem cell transplantation or initiation of new treatment agents [13, 14].
The lymphocyte/monocyte ratio (LMR), calculated by the ratio of lymphocyte count to monocyte count, is an inflammatory biomarker and indicates the balance between the host immune system and tumor microenvironment. LMR is obtained by using initial absolute lymphocyte count (ALC) and absolute monocyte count (AMC). It is also an easy method for detection, which provides prognostic information in malignancies. The contribution of LMR to stage and prognosis is unknown in CLL. With this study, it was aimed to determine the LMR cut-off value in CLL and to show whether the detected LMR value is related to some prognosis indicators such as disease stage and cytogenetic anomalies.
| Materials and Methods | ▴Top |
The study was conducted in patients diagnosed with CLL according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria, between 2005 and 2019 by our hospital’s hematology clinic, whose follow-up and treatment are still ongoing. The data were obtained by scanning the hospital electronic information system and patient files.
Demographic, clinical, and laboratory values/data, presence of chronic disease or malignancy, basal complete blood count, ALC and AMC were recorded. The ALC and AMC of the patients before the initiation of treatment were recorded and included in the study. Diagnosis values of all patients were in accordance with the World Health Organization (WHO) CLL diagnostic criteria.
Staging the patients was done according to Rai and Binet Staging Systems and risk groups were determined. The diagnosis was confirmed by flow cytometry (immunophenotyping) analysis for all patients; according to the results of cytogenetic examination (chromosome analysis and fluorescence in situ hybridization (FISH) analysis) in some patients, the cases were divided into risk groups as low, medium and high. In addition, as the clinic approach, patients were grouped based on the therapeutic indication. The number and responses of the patients who received more than one treatment were classified and recorded as complete remission (CR), partial remission (PR) and progressive disease (PD) according to IWCLL criteria.
Statistical method
Average, standard deviation, median lowest and highest, frequency and ratio values were used in the descriptive statistics of the data. The distribution of variables was measured by the Kolmogorov-Smirnov test. Kruskal-Wallis and Mann-Whitney U tests were used in the analysis of quantitative independent data. Spearman correlation analysis was used in the correlation analysis. Kaplan-Meier (log-rank) method was used in survival analysis. SPSS 22.0 program was used in the analysis. P < 0.05 was considered statistically significant.
Ethics approval
Our study was approved by the ethics committee of our hospital on February 15, 2019 with the decision number 1706.
| Results | ▴Top |
One hundred and seventy-three patients were included in the study; 63% were male and 37% were female. The male/female ratio was calculated as 1.7/1. The median age of the patients was 66 (range: 35 - 85). A total of 46% of the patients were diagnosed at the age of 65 and before. Percentage distribution of patients according to Rai and Binet Staging is shown in Table 1. When the risk groups were examined according to the modified Rai Staging of the patients; 25.4% were considered as low-risk, 48.6% as intermediate- and 26% as high-risk groups. The patients were divided into two groups as those received the treatment (chemotherapy) (42.2%) and those who did not (57.8%). The patients who received treatment were classified according to the number of treatment steps they received. Accordingly, 76.7% of the patients received one- or two-line treatments, while 23.3% received three or more treatments. Among the patients receiving treatment, the ratio of those who gave a CR to the treatment was 57.8%, while the ratio of those who gave a CR to the first treatment was 28.8%. The rate of progression among all patients (with or without treatment) was 25.4% (Table 1).
 Click to view | Table 1. Demographic and Clinical Features of Patients (Laboratory and Genetic Results) |
The median peripheral blood absolute leukocyte count of the patients included in the study was 27,600/mm3 (3,930 - 315,000/mm3). The median ALC was 21,540/mm3 (1,500 - 282,000/mm3), and the median AMC was 790/mm3 (10 - 19,000/mm3). The median value of LMR was 26.7 (0.66 - 2,466.5) and the cut-off value of our study was determined as 26 (Table 1). In 71.1% of our patients, cytogenetic analysis was performed with the FISH method and the results obtained are indicated in Table 1.
Patients were followed for a median of 35.5 months (0.03 - 166.2); in this process, 27 (26%) of patients died.
Relationship of LMR with clinical and laboratory findings
In our study, the median cut-off value of LMR was 26, while LMR was ≤ 26 in 86 patients, and LMR > 26 in 87 patients. When patient groups were compared by gender, the LMR value did not differ significantly between men and women (P > 0.05). As a result of correlation analysis, no significant (P > 0.05) correlation was observed between LMR and age, whereas a significant positive correlation was observed between leukocyte and lymphocyte counts (P < 0.05). A significant (P < 0.05) negative correlation was observed between the LMR and the number of monocytes. A significant (P < 0.05) correlation was also observed between LMR and Rai Stages (Table 2). When the relationship between LMR and Rai Stages was examined, it was seen that LMR increased significantly as the Rai Stage increased (P < 0.05) (Table 3, Fig. 1).
 Click to view | Table 2. LMR-Age and LMR-Monocyte Correlations |
 Click to view | Table 3. Relationship Between LMR and Rai or Binet Stages |
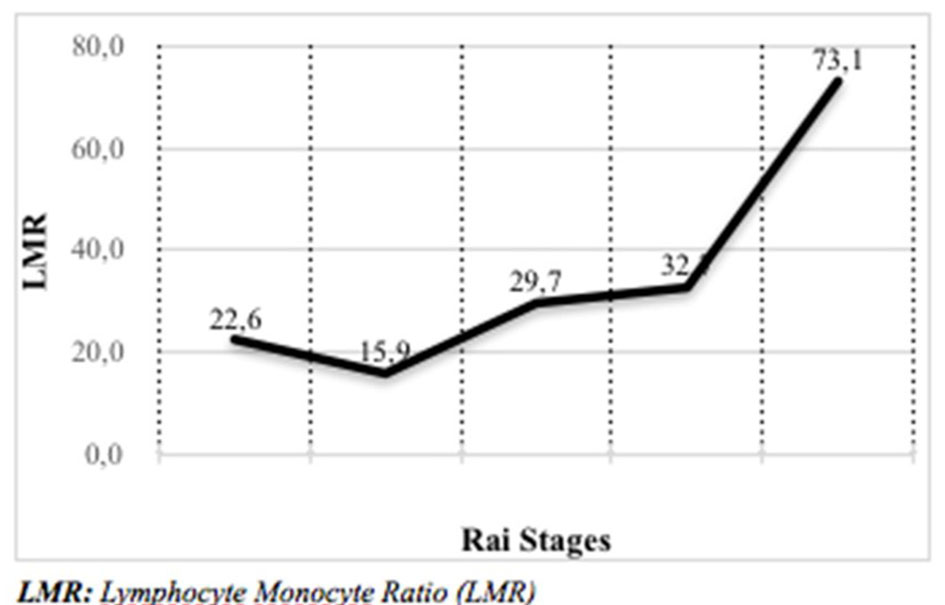 Click for large image | Figure 1. Relationship between LMR and Rai Stages. LMR: lymphocyte/monocyte ratio. |
When the relationship between LMR and Binet Stages is examined, the median LMR was 24.4 in stage A, 31 in stage B and 41.1 in stage C. In Binet Stage C, LMR was seen to be significantly higher than stage A (P < 0.05). In Binet Stage B, LMR was not significantly different from stages A and C (P > 0.05) (Table 3).
Treatment response relationship with LMR
Generally, when all patient groups were evaluated, the LMR value in the treatment indicated that patient group was significantly higher than that in the non-treated group (P < 0.05). During follow-up, at any time, the group with disease progression had a significantly higher LMR value than the group without progression (P < 0.05) (receiver operating characteristic (ROC) analysis: area under the curve (AUC): 0.749, specificity 83%, sensitivity 79.2%). In addition, the LMR value was significantly higher in patients who died than others (P < 0.05) (ROC analysis: AUC: 0.786, specificity 76%, sensitivity 76.3 %) (Table 4).
 Click to view | Table 4. Relationship of LMR With Progression |
LMR and survival or progression-free survival (PFS) relationship
When the overall survival (OS) relationship with LMR is examined, OS was 131.8 months in patient group with LMR ≤ 26 (95% confidence interval (CI): 110.9 - 152.7, P > 0.05) and 98.1 months in patient group with LMR > 26 (95% CI: 81 - 115.2, P > 0.05). No statistically significant relationship was found. In our study, the OS was calculated as 120.6 months in all patients.
When PFS relationship with LMR is examined, PFS was 107.2 months in patient group with LMR ≤ 26 (95% CI: 81.6 - 132.8, P > 0.05) and 80.6 months in patient group with LMR > 26 (95% CI: 64.2 - 97, P > 0.05). No statistically significant relationship was detected. The median PFS value of our study was calculated as 96.5 months (Table 5).
 Click to view | Table 5. LMR and OS and PFS |
Relationship of LMR with cytogenetic prognostic factors
When the 13q14 deletion, 17p deletion, 11q deletion, trisomy 12 positivity and LMR relationship of the patients who underwent cytogenetic analysis with FISH were examined, no statistically significant relationship was found for any cytogenetic anomaly (P > 0.05). Similarly, no statistically significant relationship was detected between the group that was performed cytogenetic analysis and the group that was not performed it (P > 0.05).
| Discussion | ▴Top |
CLL is a disease with a highly variable prognosis. This heterogeneity in the clinical behavior of patients makes it difficult to provide prognostic information. New parameters have been developed for the early application of new treatment regimens in patients with poor prognosis; this contributes to prolonging survival.
In published studies, low LMR level has been associated with low survival and poor prognosis in other different solid cancers such as breast cancer or pancreatic adenocarcinoma [15]. There are different studies showing that low LMR is an independent prognostic factor associated with poor prognosis, low OS and PFS in patients with some hematological malignancies such as Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, extranodal natural killer/T (NK/T) cell lymphoma, anaplastic large B-cell lymphoma and multiple myeloma [16-21]. However, there is no study on LMR in CLL patients in the literature. When hematological malignancies other than CLL are examined (diffuse large B-cell and Hodgkin lymphoma), the LMR cut-off value varies between 2 and 6. In our study, the LMR cut-off value was accepted as 26 based on the median value. It seems to be high compared with other malignancies. This is an understandable result because CLL is a lymphocytosis-based malignancy (Table 1).
The low peripheral absolute lymphocyte and high monocyte counts have been reported also in a study in CLL patients. Szerafin et al analyzed the data of 223 newly-diagnosed CLL patients. Patients with low and high monocyte counts had a shorter time to treatment compared to patients who belonged to the intermediate monocyte count group. The low AMC was associated with increased mortality. They also found that the AMC may give some prognostic information in Rai Stage 0 [22].
In our study, as a result of correlation analysis, no significant correlation was observed between LMR and age, whereas a significant positive correlation was observed between LMR and leukocyte or lymphocyte count and Rai Stages. A significant negative correlation was observed between the LMR and the number of monocytes. High LMR may be a poor prognostic indicator for CLL. Additionally, in our study, the relationship between cytogenetic anomalies (del13q14, del11q, del17p and trisomy 12) used in CLL in terms of prognosis and LMR was also examined, but no significant relationship was found.
In conclusion, it is stated that CLL cases with high LMR value are more in the advanced stage and have more frequently an indication for treatment. OS and PFS are lower in CLL cases with a value of LMR above 26 without statistical significance. This value is a simple indicator that can help other laboratory and clinical staging systems. To clarify the availability of LMR in CLL, prospective additional studies are needed on more patients showing that different LMR values represent different stages of disease.
Major limitation of our study is that the study was conducted in a small number of patients. Only 71.1% of the cases had cytogenetic analysis. Moreover, different causes affecting ALC, such as the presence of proliferation and increased apoptosis, due to the nature of the disease, as in aggressive lymphomas, may be a limiting factor in the use of LMR as an important prognostic indicator in CLL. It can be also said that the results of the study were negatively affected by considering many additional factors such as low LMR in the presence of an infection, low lymphocyte counts as age increases and not being able to look at lymphocyte subgroups.
Acknowledgments
We respectfully remember all the colleagues we lost in the COVID-19 fight.
Financial Disclosure
Dem Ilac San. Tic. AS. is our sponsor for publishing fee.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
OY conceived the original idea of study. ENS collected and analyzed the data statistically. IS wrote the manuscript with support from ENS, FS and HG. OY and FS helped supervise the project. IS also made tables and last arrangements.
Data Availability
Data analyzed in this study are included in this published article and its additional file.
Abbreviations
CLL: chronic lymphocytic leukemia; LMR: lymphocyte/monocyte ratio; OS: overall survival; PFS: progression-free survival; LDH: lactate dehydrogenase; FISH: fluorescence in situ hybridization; IWCLL: International Workshop on Chronic Lymphocytic Leukemia; CR: complete remission; PR: partial remission; PD: progressive disease; ALC: absolute lymphocyte count; AMC: absolute monocyte count; NK/T: natural killer/T; WHO: World Health Organization; ROC: receiver operating characteristic; AUC: area under the curve; CI: confidence interval; WBC: white blood cell; SD: standard deviation
| References | ▴Top |
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019-5032.
doi pubmed - Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl). 2004;13(3):279-287.
doi pubmed - SEER cancer stat facts: chronic lymphocytic leukemia. National Cancer Institute. Bethesda, MD.
- Pamuk ON, Pamuk GE, Soysal T, Ongoren S, Baslar Z, Ferhanoglu B, Aydin Y, et al. Chronic lymphocytic leukemia in Turkey: experience of a single center in Istanbul. South Med J. 2004;97(3):240-245.
doi pubmed - Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W, Chanan-Khan AA, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer. 2015;121(17):2883-2891.
doi pubmed - Tsai HT, Caporaso NE, Kyle RA, Katzmann JA, Dispenzieri A, Hayes RB, Marti GE, et al. Evidence of serum immunoglobulin abnormalities up to 9.8 years before diagnosis of chronic lymphocytic leukemia: a prospective study. Blood. 2009;114(24):4928-4932.
doi pubmed - Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198-206.
doi - Shanafelt TD, Geyer SM, Kay NE. Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103(4):1202-1210.
doi pubmed - Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910-1916.
doi pubmed - Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174.
doi - Zenz T, Vollmer D, Trbusek M, Smardova J, Benner A, Soussi T, Helfrich H, et al. TP53 mutation profile in chronic lymphocytic leukemia: evidence for a disease specific profile from a comprehensive analysis of 268 mutations. Leukemia. 2010;24(12):2072-2079.
doi pubmed - Seiffert M, Dietrich S, Jethwa A, Glimm H, Lichter P, Zenz T. Exploiting biological diversity and genomic aberrations in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(6):1023-1031.
doi pubmed - Pettitt AR, Matutes E, Oscier D. Alemtuzumab in combination with high-dose methylprednisolone is a logical, feasible and highly active therapeutic regimen in chronic lymphocytic leukaemia patients with p53 defects. Leukemia. 2006;20(8):1441-1445.
doi pubmed - Pettitt AR, Jackson R, Carruthers S, Dodd J, Dodd S, Oates M, Johnson GG, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J Clin Oncol. 2012;30(14):1647-1655.
doi pubmed - Marin Hernandez C, Pinero Madrona A, Gil Vazquez PJ, Galindo Fernandez PJ, Ruiz Merino G, Alonso Romero JL, Parrilla Paricio P. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476-483.
doi pubmed - Porrata LF, Ristow KM, Habermann TM, Macon WR, Witzig TE, Colgan JP, Inwards DJ, et al. Peripheral blood absolute lymphocyte/monocyte ratio recovery during ABVD treatment cycles predicts clinical outcomes in classical Hodgkin lymphoma. Blood Cancer J. 2013;3:e110.
doi pubmed - Katoh D, Ochi Y, Yabushita T, Ono Y, Hiramoto N, Yoshioka S, Yonetani N, et al. Peripheral blood lymphocyte-to-monocyte ratio at relapse predicts outcome for patients with relapsed or refractory diffuse large B-cell lymphoma in the rituximab era. Clin Lymphoma Myeloma Leuk. 2017;17(12):e91-e97.
doi pubmed - Lee SF, Luque-Fernandez MA. Prognostic value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in follicular lymphoma: a retrospective cohort study. BMJ Open. 2017;7(11):e017904.
doi pubmed - Li N, Zhang L, Song HL, Zhang J, Weng HW, Zou LQ. Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol. 2017;39(5):1010428317705503.
doi pubmed - Jia T, Zhang R, Zhu HY, Liang JH, Wang L, Wu W, Cao L, et al. Prognostic significance of peripheral blood absolute monocyte count and lymphocyte to monocyte ratio in anaplastic large cell lymphoma. Cancer Biomark. 2018;22(4):807-813.
doi pubmed - Tian Y, Zhang Y, Zhu WQ, Chen XL, Zhou HB, Chen WM. Peripheral blood lymphocyte-to-monocyte ratio as a useful prognostic factor in newly diagnosed multiple myeloma. Biomed Res Int. 2018;2018:9434637.
doi pubmed - Szerafin L, Jako J, Risko F. [Prognostic value of absolute monocyte count in chronic lymphocytic leukaemia]. Orv Hetil. 2015;156(15):592-597.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


