| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 1, February 2021, pages 14-17
Bone Marrow Involvement by Aggressive B Cell Lymphoma Undetected by Aspirate Immunophenotyping
Habib Moshref Razavi
Fraser Health Authority, The Royal Columbian Hospital, 330 East Columbia Street New Westminster, BC, V3L 3W7, Canada
Manuscript submitted October 19, 2020, accepted November 3, 2020, published online February 6, 2021
Short title: DLBCL Not Detected by Immunophenotyping
doi: https://doi.org/10.14740/jh766
| Abstract | ▴Top |
Staging for newly diagnosed lymphoma is an essential diagnostic step aimed at not only estimating prognosis but also refining the ensuing therapeutic pathway. Bone marrow is routinely sampled for this reason. Morphological assessment of the bone marrow aspirate and biopsy remains the gold standard approach. Nonetheless, ancillary testing such as aspirate immunophenotyping is also used with the aim to increase sensitivity and add diagnostic utility, e.g., to provide proof of clonality. Both of these techniques are fraught with shortcomings and concordance is often not perfect. Cases of infiltrative lymphoma identified by morphology, and not detected by flow cytometry highlights the dangers of over reliance on aspirate immunophenotyping. Under sampling, disintegration, fibrosis and hemodilution are but some causes of a false negative flow result. Therefore, neither technique is sufficient in isolation. In this submission, a case of such a discrepancy is presented as an introduction for review of literature that highlights this phenomenon.
Keywords: Aggressive B cell lymphoma; Staging; Bone marrow biopsy; Aspirate immunophenotyping
| Introduction | ▴Top |
Staging of newly diagnosed patients with B cell lymphomas, as characterized by the Ann Arbor criteria, is a routine practice and features prominently in prognosis indices such as the international prognostic index (IPI) or the follicular lymphoma international prognostic index (FLIPI) [1-4]. The philosophy of this approach is to refine the diagnosis and identify the extent of systemic involvement, which influences the therapeutic approach. For instance, while isolated nodal involvement in stage I can presumably be locally resected with curative intent, the same case now showing bone marrow (BM) involvement requires systemic chemotherapy and is upgraded to Ann Arbor stage IV [5]. Although morphological review of BM biopsy remains the gold standard for evaluation, many sensitive ancillary techniques are used in tandem and aim to improve diagnostic accuracy. One such technique is flow cytometry (FC), which is generally a thousand fold more sensitive (0.01-0.001% versus 0.1% detection rate) [6]. Notwithstanding, discrepancies in identification of malignancy do occur where concordance of BM positive cases (BM+) and those recognized by flow (FC+) is between 80-90% [7-9]. Specifically previous studies have highlighted false negative flow results (i.e., BM+/FC-). However the reverse while less incident also does occur. Here we describe an infiltrative case of concordant aggressive large B cell lymphoma, undetected by flow cytometric immunophenotyping.
| Case Report | ▴Top |
A 76-year-old male patient presented to our hospital with abdominal distention, pain and melena stools, preceded by 4 weeks of drenching night sweats and poor appetite. Upper gastrointestinal (GI) endoscopy showed an ulcerated mass in the gastric body along with a duodenal mass. An excisional biopsy showed involvement by discohesive sheets of large cells expressing CD20, CD10, Ki67 (98%) and Epstein-Barr virus (EBV) consistent with diffuse large B cell lymphoma (DLBCL)-germinal center type. Fluorescent in situ hybridization (FISH) studies showed MYC rearrangement. A staging BM biopsy showed significant dyserythropoiesis with irregular nuclear contours/intracytoplasmic vacuolation (not shown) and nuclear budding (Fig. 1, BM aspirate, May Grunwald Giemsa stain, original magnification × 50). The trephine biopsy showed a hypercellular BM with marked abnormal localization of immature precursors (ALIP, Fig. 1, hematoxylin and eosin (H&E), original magnification × 20) unstained for e-cadherin and CD34 (not shown). CD 3 (pan T) stain showed a reactive pattern (panel showing immunohistochemical (IHC) CD3, original magnification, × 10). CD20 and CD10 highlighted an interstitial involvement by large B cells at a lower proliferative index (40-50%, Fig. 1, IHC panels marked CD20, CD10 and Ki-67 respectively, original magnification, × 20). Interestingly the aspirate flow cytometry returned no diagnostic immunophenotypic abnormalities associated with lymphoproliferative disorder (Fig. 2a-i). Sequential gating identified the lymphocytes in the side scatter versus CD45 plot. Subsequently CD19+, CD20+, CD3- populations were evaluated and showed no clonality (polytypic, Fig. 2e). Identification of the CD19+, CD20+ CD10+ cells that expressed CD38 and lacked surface immunoglobulin (Fig. 2f-i) highlighted the hematogone population accounting for the remaining events in the lymphocyte gate. Concurrent diagnosis of a myelodysplastic syndrome (MDS) was considered but not favored. Accordingly this patient had a normal karyotype without recurrent abnormalities associated with MDS. Moreover, although the diagnostic threshold for cytopenias was reached (i.e., cytopenias in at least one lineage); this patient’s anemia could easily be attributed to his comorbidities such as chronic disease, blood loss, and BM effacement, etc. The patient was treated with two (out of six) rounds of R-CHOP, and his hospitalization and treatment were complicated with tumor lysis syndrome and febrile neutropenia.
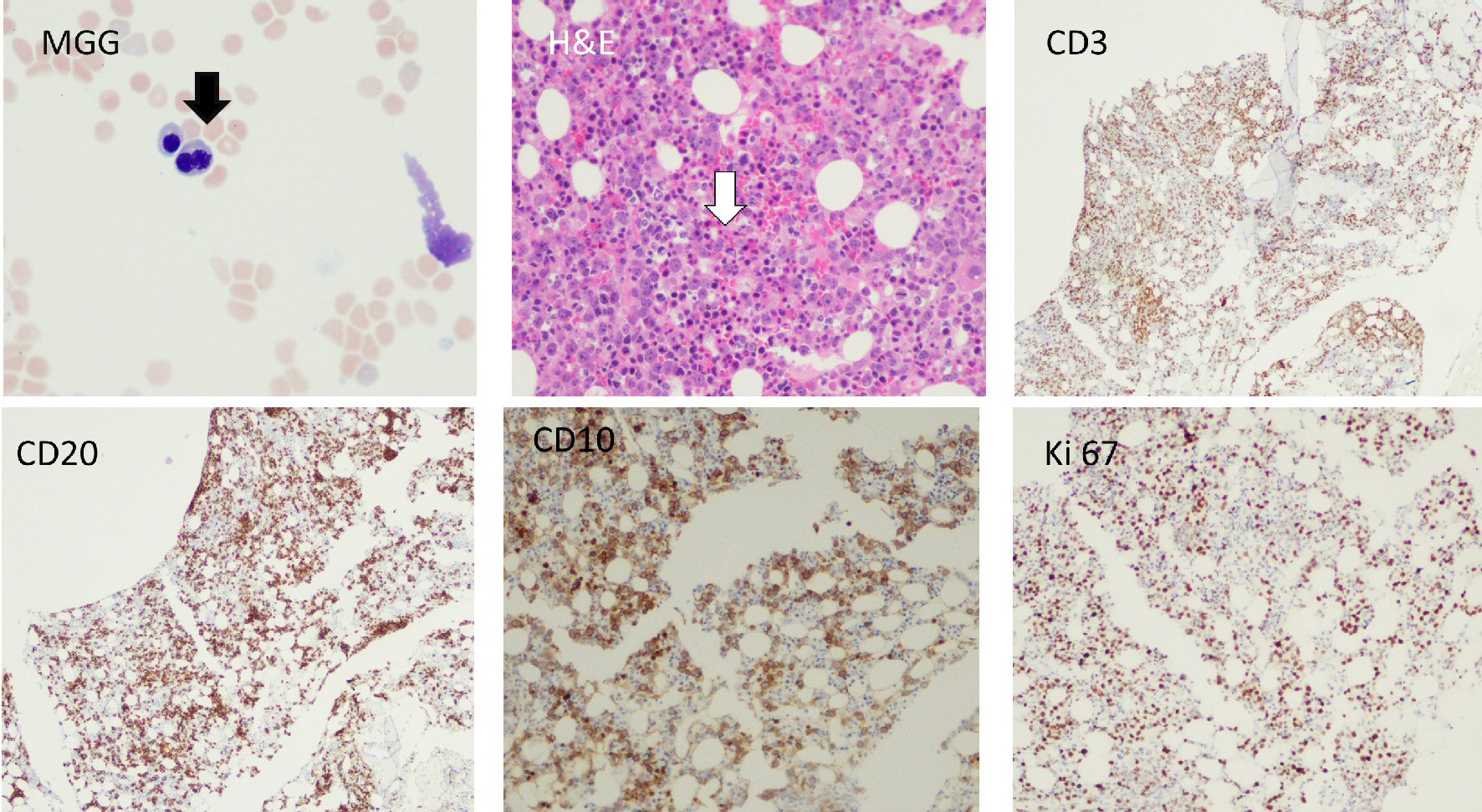 Click for large image | Figure 1. Bone marrow aspirate, trephine biopsy and immunohistochemical stains are shown. Black arrow shows a bilobed normoblast. White arrow shows presence of abnormal localization of immature precursors (ALIP). CD10, CD20 and Ki67 stains show infiltration of clonal large B-lymphocytes with a moderately high proliferative index (Ki67, 40-50%). H&E: hematoxylin and eosin. |
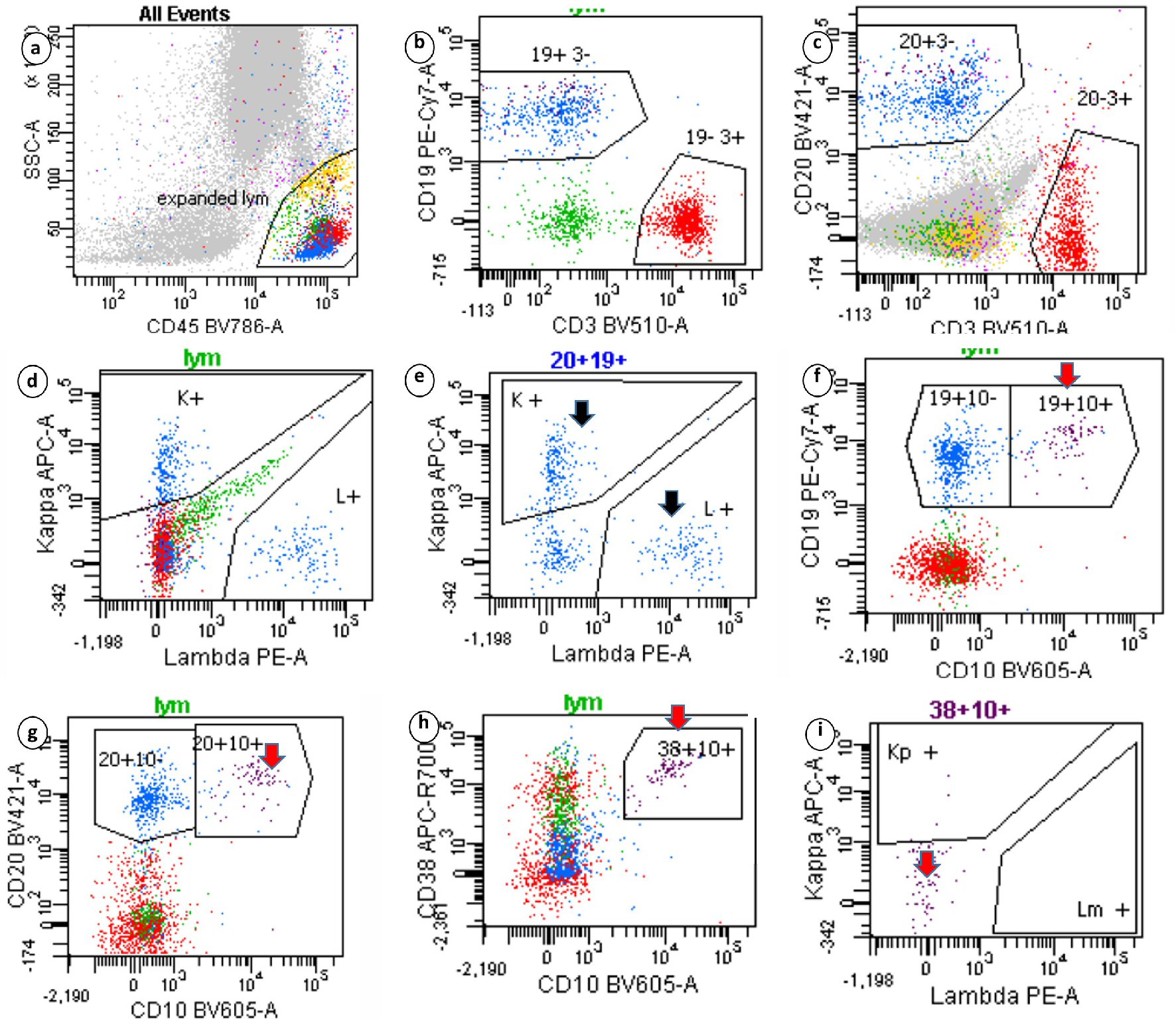 Click for large image | Figure 2. Sequential gating strategy identified the lymphocytes in the side scatter versus CD45 plot. The isolated lymphocytes (20+19+ gates) showed polytypic expression of surface immunoglobulins (black arrow). Events that expressed CD19, CD10 and CD38 highlighted the hematogone population, which lacked surface immunoglobulins (red arrows). Clonal B-lymphocytes seen on immunohistochemistry were not captured. |
| Discussion | ▴Top |
The introduction of flow cytometric analysis of BM aspirate and its diagnostic utility in staging is equivocal. Although more sensitive than morphology alone, falsely negative cases do occur and some groups have questioned the added benefit of aspirate immunophenotyping [10, 11]. Nevertheless, morphological and immunophenotypic concordance does occur in majority of the cases and is invaluable in identification of the clone, stage assignment and prognosis. For instance, in aspirates, identification of large B cells by morphology showing a high forward scatter on FC can corroborate the so-called concordant BM infiltration, which portends worse prognosis. In the same vein, in patients with aggressive B cell lymphoma observance of smaller cells in the aspirate that show an associated CLL immunophenotype is consistent with a discordant infiltration, often associated with a better outcome [12]. The presence of discrepant results exposes the dangers of over reliance on any one technique in isolation. False negativity of aspirate immunophenotyping has been attributed to technical insensitivity (4 - 6 versus 8 - 10 color multiparameter flow), under sampling/hemodilution, fibrosis and tumor histotypes [13]. For instance, tumors associated with paratrabecular or nodular placement, or those associated with fibrosis are less aspirable and may result in negative results. In contrast tumors that peripheralize, have a distinct biology that allows for less architectural adhesion permitting for representative sampling. Specifically specimen cellular under representation and hemodilution in follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL) and DLBCL is associated with discrepant BM+/FC- results, while good overlap is evident in mantle cell, nodal marginal and chronic lymphocytic lymphomas [14]. A variety of mechanisms have been proposed where in FL, tight adhesion to trabecular bone requires en bloc removal of adhered cells [15]. On the other hand and in case of LPL, cellular heterogeneity where small mature lymphocytes, plasmacytoid lymphocytes and plasma cells coexist may confound FC analysis. As well in this particular tumor, associated cytokine induced fibrosis may have a role in under sampling and negative results [16]. Overall, there is a direct relationship between percent biopsy cellular infiltration and congruent FC results. For instance, although the sensitivity of FC is higher than morphology, tandem FC immunophenotyping on histologically positive disaggregated biopsy samples much improves a positive hit compared to geographically unrelated aspirate samples. One possible remedy to falsely negative FC may be sampling by a bilateral BM biopsy, which may increase the yield but is cumbersome. Some studies have shown as much as 20% increase in positive sampling with this approach. Other studies have cast doubt on the utility of this approach where histological morphology and immunohistochemistry of a unilateral biopsy showed comparable results to that of bilateral biopsies [17-20].
In summary, the current case report highlights a problem which can only be addressed if these complementary methods are used together. While the diagnostic accuracy of 8 - 10 color multiparameter FC is a much improvement over previous techniques, it is only as good as the sample it analyses. On the other hand, immunophenotypic information about the original tumor can greatly aid and guide the practitioner in strategic gating of the aspirate sample [21]. Cases of BM-/FC+ do exist and bring forward a dilemma. For instance, should an otherwise stage I or limited stage II tumor with minimal BM involvement detected only by FC be called stage IV disease? At least one study has advocated a no answer if the tumor burden is < 2% [22].
In conclusion, morphological and IHC review of BM biopsy is rightly the gold standard of evaluation for staging. Appropriate ancillary testing, be it FC or molecular techniques, is complimentary and increases the diagnostic accuracy. Cases that would have otherwise been missed by FC alone, as in the current patient, highlight the inadequacy of this technique in isolation whereby caution in interpretation is warranted.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
The sole author and the case pathologist is Habib Moshref Razavi.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Fend F, Kremer M. Diagnosis and classification of malignant lymphoma and related entities in the bone marrow trephine biopsy. Pathobiology. 2007;74(2):133-143.
doi pubmed - A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909-3918.
doi - Bain BJ. Bone marrow trephine biopsy. J Clin Pathol. 2001;54(10):737-742.
doi pubmed - Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-1265.
doi pubmed - Merli M, Arcaini L, Boveri E, Rattotti S, Picone C, Passamonti F, Tenore A, et al. Assessment of bone marrow involvement in non-Hodgkin's lymphomas: comparison between histology and flow cytometry. Eur J Haematol. 2010;85(5):405-415.
doi pubmed - Campana D, Coustan-Smith E. Advances in the immunological monitoring of childhood acute lymphoblastic leukaemia. Best Pract Res Clin Haematol. 2002;15(1):1-19.
doi pubmed - Naughton MJ, Hess JL, Zutter MM, Bartlett NL. Bone marrow staging in patients with non-Hodgkin's lymphoma: is flow cytometry a useful test? Cancer. 1998;82(6):1154-1159.
doi - Dunphy CH. Combining morphology and flow cytometric immunophenotyping to evaluate bone marrow specimens for B-cell malignant neoplasms. Am J Clin Pathol. 1998;109(5):625-630.
doi pubmed - Stacchini A, Demurtas A, Godio L, Martini G, Antinoro V, Palestro G. Flow cytometry in the bone marrow staging of mature B-cell neoplasms. Cytometry B Clin Cytom. 2003;54(1):10-18.
doi pubmed - Kim B, Lee ST, Kim HJ, Kim SH. Bone marrow flow cytometry in staging of patients with B-cell non-Hodgkin lymphoma. Ann Lab Med. 2015;35(2):187-193.
doi pubmed - Iancu D, Hao S, Lin P, Anderson SK, Jorgensen JL, McLaughlin P, Medeiros LJ. Follicular lymphoma in staging bone marrow specimens: correlation of histologic findings with the results of flow cytometry immunophenotypic analysis. Arch Pathol Lab Med. 2007;131(2):282-287.
- Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol. 2006;76(6):473-480.
doi pubmed - Katz BZ, Polliack A. Discrepancies in quantitative assessment of bone marrow involvement in lymphoma: do they reflect specialized micro-environmental cellular niches and cell-stromal interactions? Leuk Lymphoma. 2006;47(9):1730-1731.
doi pubmed - Perea G, Altes A, Bellido M, Aventin A, Bordes R, Ayats R, Remacha AF, et al. Clinical utility of bone marrow flow cytometry in B-cell non-Hodgkin lymphomas (B-NHL). Histopathology. 2004;45(3):268-274.
doi pubmed - Pittaluga S, Tierens A, Dodoo YL, Delabie J, De Wolf-Peeters C. How reliable is histologic examination of bone marrow trephine biopsy specimens for the staging of non-Hodgkin lymphoma? A study of hairy cell leukemia and mantle cell lymphoma involvement of the bone marrow trephine specimen by histologic, immunohistochemical, and polymerase chain reaction techniques. Am J Clin Pathol. 1999;111(2):179-184.
doi pubmed - Bassarova A, Troen G, Spetalen S, Micci F, Tierens A, Delabie J. Lymphoplasmacytic lymphoma and marginal zone lymphoma in the bone marrow: paratrabecular involvement as an important distinguishing feature. Am J Clin Pathol. 2015;143(6):797-806.
doi pubmed - Coller BS, Chabner BA, Gralnick HR. Frequencies and patterns of bone marrow involvement in non-Hodgkin lymphomas: observations on the value of bilateral biopsies. Am J Hematol. 1977;3:105-119.
doi pubmed - Luoni M, Declich P, De Paoli Ap, Fava S, Marinoni P, Montalbetti L, Sangalli G, et al. Bone marrow biopsy for the staging of non-Hodgkin's lymphoma: bilateral or unilateral trephine biopsy? Tumori. 1995;81(6):410-413.
doi pubmed - Juneja SK, Wolf MM, Cooper IA. Value of bilateral bone marrow biopsy specimens in non-Hodgkin's lymphoma. J Clin Pathol. 1990;43(8):630-632.
doi pubmed - Coad JE, Olson DJ, Christensen DR, Lander TA, Chibbar R, McGlennen RC, Brunning RD. Correlation of PCR-detected clonal gene rearrangements with bone marrow morphology in patients with B-lineage lymphomas. Am J Surg Pathol. 1997;21(9):1047-1056.
doi pubmed - Carulli G, Marini A. Diagnosis and classification of B-cell non-Hodgkin lymphomas. The role of multiparameter flow cytometry. Clin Ter. 2012;163(1):47-57.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


