| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 1, February 2021, pages 22-24
Tamoxifen Therapy for Recurrent Mucosal Bleeding in Hereditary Hemorrhagic Telangiectasia
Stephanie Yunga, c, Julie-Anne Bellb, Jim Brookera
aDepartment of Gastroenterology, Waikato Hospital, Hamilton, New Zealand
bDepartment of Haematology, Waikato Hospital, Hamilton, New Zealand
cCorresponding Author: Stephanie Yung, Department of Gastroenterology, Waikato Hospital, Hamilton, New Zealand
Manuscript submitted December 25, 2020, accepted January 2, 2021, published online February 6, 2021
Short title: Tamoxifen for Recurrent Mucosal Bleeding in HHT
doi: https://doi.org/10.14740/jh794
| Abstract | ▴Top |
A 60-year-old Caucasian man had a 55-year history of recurrent severe epistaxis and later presented with multiple gastrointestinal (GI) bleeding from hereditary hemorrhagic telangiectasia (HHT). Bleeding was exacerbated due to coexistent mild hemophilia A. Despite repeated conventional surgical interventions, tranexamic acid and recombinant factor VIII (FVIII) prophylaxis, bleeding episodes worsened in frequency and severity, resulting in the patient becoming transfusion dependent. The introduction of tamoxifen therapy resulted in reduced transfusion requirement.
Keywords: Hereditary hemorrhagic telangiectasia; Tamoxifen; Epistaxis; GI bleeding
| Introduction | ▴Top |
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu disease, is an autosomal dominant condition. The diagnosis requires at least three out of the four Curacao criteria: 1) Recurrent epistaxis; 2) Telangiectases, typically of the lips, oral cavity, fingers and nose; 3) Telangiectases of the gastrointestinal (GI) tract, arteriovenous malformation (AVM) of lung, brain, liver or spine; 4) Family history of a first degree relative with HHT [1].
Up to 90% of patients with HHT present with recurrent spontaneous epistaxis. Epistaxis and GI bleeding may lead to repeated hospitalizations, severe iron deficiency, anemia and impaired quality of life [2]. Ablative therapy for bleeding telangiectasia provides immediate control, but adjunctive medical therapy is often required. In a recent meta-analysis of medical treatments, Hsu et al [3] concluded that tamoxifen reduced both frequency and severity of epistaxis. We report a patient with HHT complicated by mild hemophilia with severe recurrent bleeding, who became dependent on frequent clotting factors and blood products after failing to respond to conventional therapies.
| Case Report | ▴Top |
A 60-year-old Caucasian man presented with worsening recurrent GI bleeding causing collapse on a background of HHT and mild hemophilia A (baseline recombinant factor VIII (FVIII) 17%). His hemophilia DNA studies previously demonstrated a single nucleotide change in the A2 domain (c.1804C>G [Arg583>Gly], listed on FVIII mutation databases). He had suffered from recurrent severe epistaxis from age 5. The episodes of epistaxis worsened progressively over the last 15 years, including a major hemorrhage and aspiration during laser therapy requiring intubation and admission to the intensive care unit.
Numerous nasal procedures (septoplasty, buccal flaps, YAG laser and regular coblation) and GI endoscopic interventions (upper endoscopies, double balloon enteroscopy, capsule endoscopy, argon plasma coagulation and hemostatic clip placement) identified and ablated angioectasia of the nasal and upper GI mucosa (Figs. 1, 2). Despite these interventions and the administration of maintenance tranexamic acid and desmopressin (DDAVP) as required, the transfusion requirement peaked at 10 units per month.
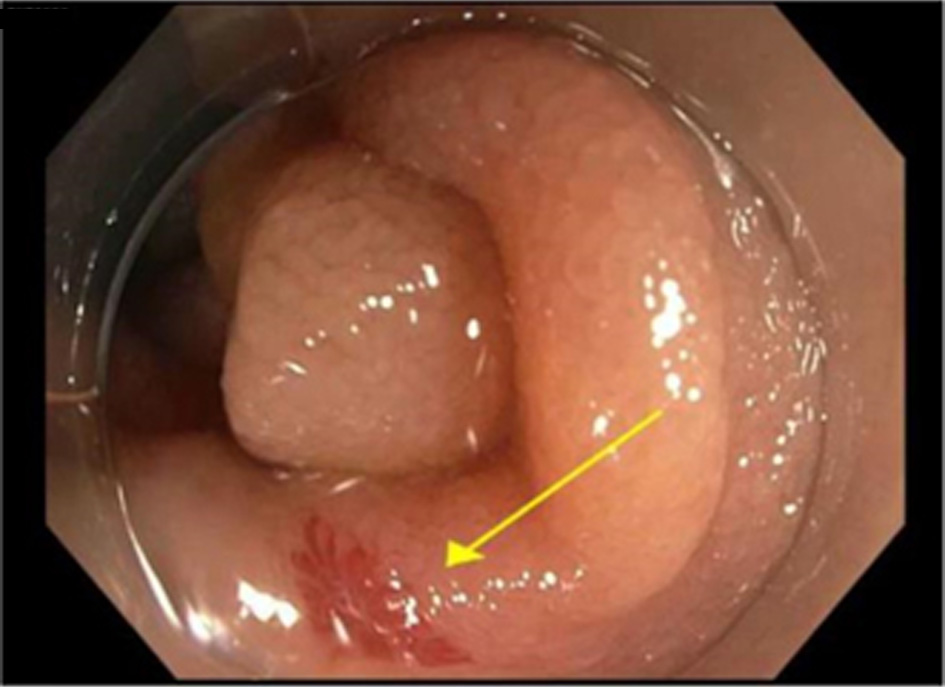 Click for large image | Figure 1. Gastroscopy revealed angiodysplasia at the second portion of the duodenum. |
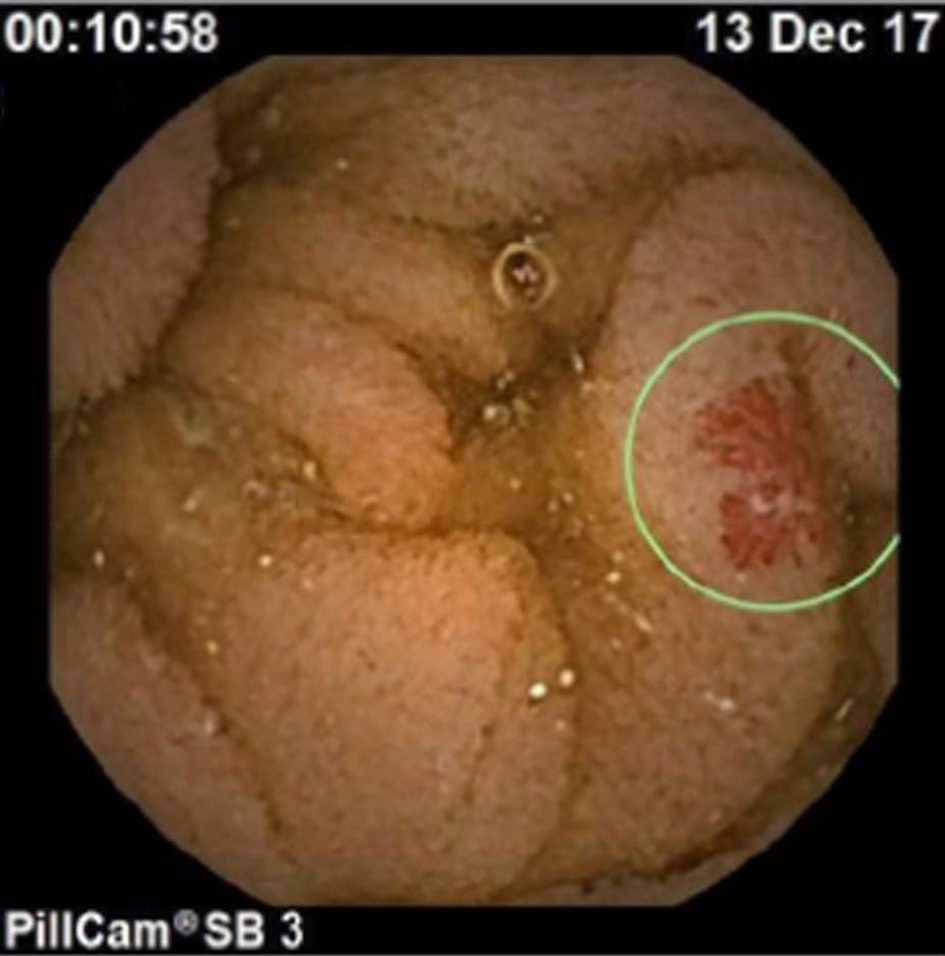 Click for large image | Figure 2. Capsule endoscopy revealed angiodysplasia at the proximal part of the small bowel. |
Other comorbidities include asthma and longstanding type 2 diabetes with associated painful neuropathy, precluding the use of thalidomide. The multitude of hospital appointments and procedures had a significant negative impact on his career and quality of life, though he managed to remain in full-time employment.
Physical examination revealed pallor and the presence of large telangiectasia of the nasal septum and smaller lesions of the lips, tongue and oral mucosa.
Relevant laboratory investigations demonstrated normocytic anemia, elevated reticulocytes, and raised ferritin (following iron infusion). Historically there had been microcytosis. Other laboratory tests were normal including renal, liver, thyroid function, von Willebrand factor and hemolytic screen.
Due to worsening GI bleeding and epistaxis, tamoxifen 20 mg daily was commenced in August 2019. Hemoglobin and transfusion requirement initially improved, with only occasional, minor self-limiting epistaxis thereafter for the following 6 months. The red blood cell (RBC) transfusion requirement reduced from a peak of 63 units in 6 months, to 16 units in the subsequent 6 months after commencing tamoxifen (Fig. 3). Additionally the requirement for recombinant FVIII reduced from a maximum of 231,000 IU in 6 months to 188,000 IU (19% reduction). Subjective quality of life improved. No side effects of tamoxifen were reported.
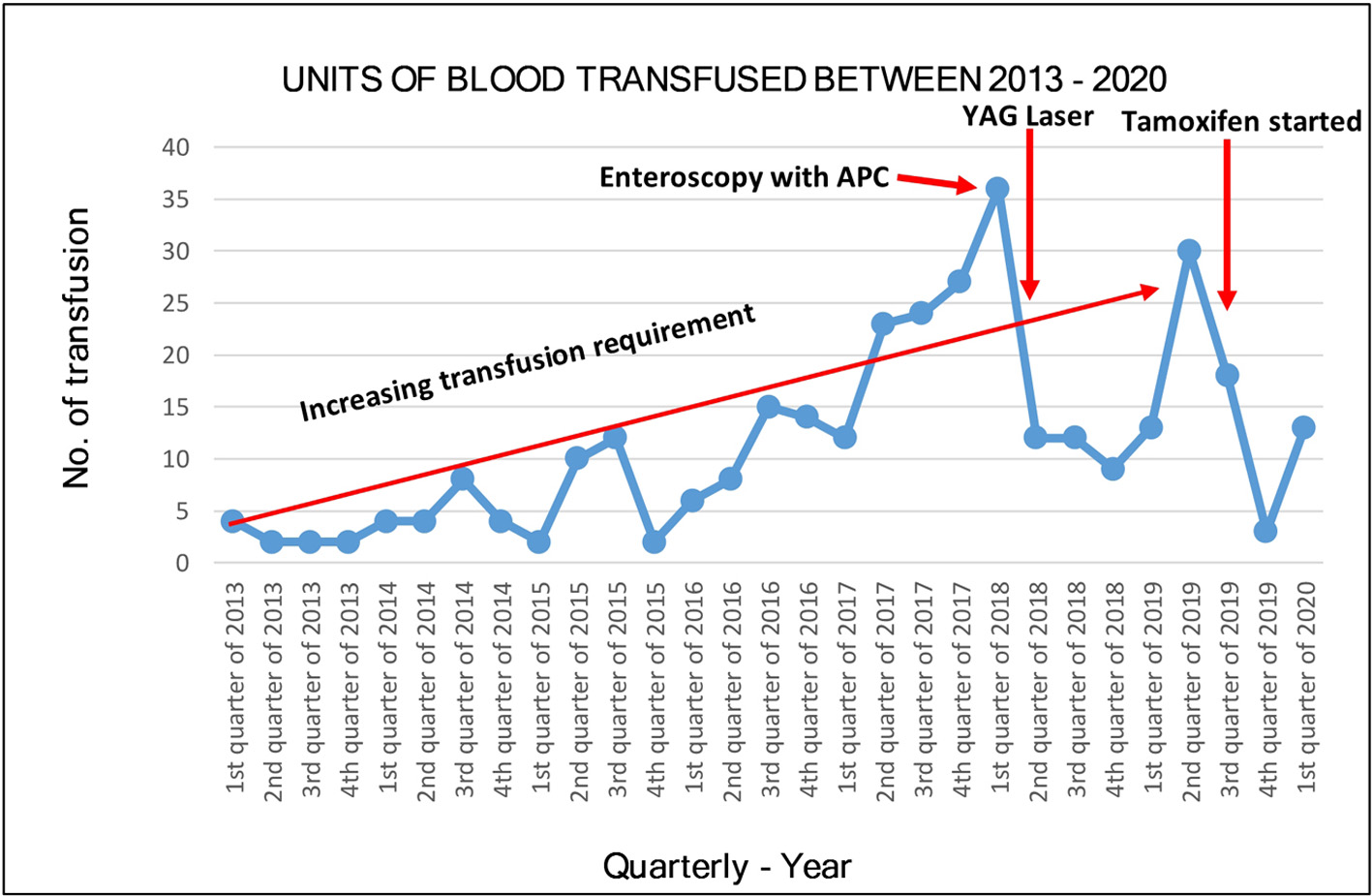 Click for large image | Figure 3. Red blood cell (RBC) transfusion trend before and after endoscopy with argon plasma coagulation (APC), YAG laser treatment and initiation of tamoxifen 20 mg daily on August 20, 2019. |
After 8 months of tamoxifen therapy this patient developed further severe bleeding from gastric angioectasia, and following successful endoscopic therapy has commenced bevacizumab [4, 5] 5 mg/kg alternate weeks for six doses then monthly. The monthly cost for tamoxifen is $15 and IV bevacizumab is $4,025 ($1,150 for 100 mg/4 mL vital).
| Discussion | ▴Top |
This case illustrates the management challenges in a patient with severe HHT, transfusion dependent due to significant recurrent bleeding episodes, further compounded by underlying hemophilia A.
HHT is caused by germline mutations in two genes encoding endoglin and anaplastic lymphoma kinase 1 (ALK1). These mutated proteins are expressed in endothelial cells leading to angiodysplasia [6]. The endothelial cells of the telangiectasia have shown increased fibrinolytic activity [7] with the presence of estrogen receptors [8], hence tamoxifen is thought to modify the locally exaggerated fibrinolytic activity [9]. Tamoxifen is a selective estrogen receptor modulator (SERM), typically used as treatment and chemoprevention of breast cancer [10]. Small case series have recently reported some success of tamoxifen therapy for epistaxis in HHT.
A meta-analysis of medical treatment for HHT related epistaxis summarized the available options including oral agents such as estrogen, tranexamic acid and tamoxifen, topical estriol and intranasal bevacizumab. Other potential treatment options include timolol, propranolol, thalidomide, and N-acetylcysteine; however, there have been no randomized controlled studies comparing these therapies. Only tamoxifen showed a statistically significant improvement in frequency and severity of epistaxis at 6-month follow-up when compared with placebo in randomized controlled studies. There were no serious adverse outcomes with the use of tamoxifen [3]. These findings are consistent with this case report.
Tamoxifen is a novel, cheap and widely available therapy, at a cost of NZ$15 per month, and appeared to be effective for reducing bleeding in this case.
Conclusion
This case demonstrates a promising early response to tamoxifen therapy in reducing the frequency and severity of bleeding in HHT. This has not only improved the quality of life for this patient but also reduced the burden of hospitalizations and interventions. We believe that this cheap and widely available therapy should be considered earlier in the management of this group of patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient for publication of this case report.
Authors Contributions
SY did the literature reviews and prepared the manuscript. JAB was involved in the diagnosis and management of the patient. JB was involved in correction of the manuscript.
Data Availability
The authors declare that all data concerning this case report are provided within the article.
| References | ▴Top |
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91(1):66-67.
doi - Aassar OS, Friedman CM, White RI, Jr. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 1991;101(9):977-980.
doi pubmed - Hsu YP, Hsu CW, Bai CH, Cheng SW, Chen C. Medical treatment for epistaxis in hereditary hemorrhagic telangiectasia: a meta-analysis. Otolaryngol Head Neck Surg. 2019;160(1):22-35.
doi pubmed - Iyer VN, Apala DR, Pannu BS, Kotecha A, Brinjikji W, Leise MD, Kamath PS, et al. Intravenous bevacizumab for refractory hereditary hemorrhagic telangiectasia-related epistaxis and gastrointestinal bleeding. Mayo Clin Proc. 2018;93(2):155-166.
doi pubmed - Gossage JR. The current role of bevacizumab in the treatment of hereditary hemorrhagic telangiectasia-related bleeding. Mayo Clin Proc. 2018;93(2):130-132.
doi pubmed - Berg J, Porteous M, Reinhardt D, Gallione C, Holloway S, Umasunthar T, Lux A, et al. Hereditary haemorrhagic telangiectasia: a questionnaire based study to delineate the different phenotypes caused by endoglin and ALK1 mutations. J Med Genet. 2003;40(8):585-590.
doi pubmed - Kwaan HC, Silverman S. Fibrinolytic activity in lesions of hereditary hemorrhagic telangiectasia. Arch Dermatol. 1973;107(4):571-573.
doi - Richtsmeier W, Weaver G, Streck W, Jacobson H, Dewell R, Olson J. Estrogen and progesterone receptors in hereditary hemorrhagic telangiectasia. Otolaryngol Head Neck Surg. 1984;92(5):564-570.
doi pubmed - Zacharski LR, Dunbar SD, Newsom WA, Jr. Hemostatic effects of tamoxifen in hereditary hemorrhagic telangiectasia. Thromb Haemost. 2001;85(2):371-372.
doi pubmed - Shagufta, Ahmad I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur J Med Chem. 2018;143:515-531.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


