| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 3, June 2021, pages 136-138
Early Onset Neutropenia due to Rituximab Therapy in Mantle Cell Lymphoma: A Case Report
Blessie Elizabeth Nelsona, d, Shelby Tiptonb, Rohit Venkatesanc
aDepartment of Hematology & Oncology, University of Texas Medical Branch, Galveston, TX, USA
bDepartment of Internal Medicine, University of Texas Medical Branch, Galveston, TX, USA
cDepartment of Hematology & Oncology, MD Anderson Cancer Center, Houston, TX, USA
dCorresponding Author: Blessie Elizabeth Nelson, Department of Hematology & Oncology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77550, USA
Manuscript submitted February 28, 2021, accepted March 19, 2021, published online June 16, 2021
Short title: EON due to Rituximab in Mantle Cell Lymphoma
doi: https://doi.org/10.14740/jh814
| Abstract | ▴Top |
Cases of late onset neutropenia (LON) after rituximab therapy have been documented, but few cases have been documented of early onset neutropenia (EON). We present a case report of a patient with mantle cell lymphoma who presented with EON, only 6 days after initiation of rituximab therapy, notable for the shortest duration to EON ever reported in literature. Throughout this paper, we explore the potential pathogenesis and incidence of EON with the help of our unique case.
Keywords: Neutropenia; Rituximab; Lymphoma
| Introduction | ▴Top |
Rituximab is a chimeric monoclonal antibody that targets the CD20 antigen found on B-cell lymphocytes, both normal and malignant [1]. Originally, rituximab was administered only for treatment of non-Hodgkin’s lymphomas [2], but now its use encompasses other cancers and rheumatic diseases, with a common one being refractory systemic lupus erythematosus (SLE) [2]. Generally, rituximab has safe side effect profile when compared to conventional cytotoxic chemotherapy [1, 2]. Infusion reactions, infections, fever, and hypotension are the most common side effects [1, 2]. Many recent reports of late onset neutropenia (LON) have been documented; however, early onset neutropenia (EON) is very rare and has only been documented in approximately seven patients [1-3]. EON is defined as neutropenia occurring within 4 weeks of starting treatment, and LON is anything later than 4 weeks [2, 3]. In this report, we detail a patient with mantle cell lymphoma who developed grade 4 EON after treatment with just one dose of rituximab in 6 days.
| Case Report | ▴Top |
A 65-year-old man was admitted to our hospital due to neutropenic fever. He was initially diagnosed with high-risk stage IV mantle cell lymphoma involving the bone marrow and right pleural effusion and was treated in January 2020 at an outside institution with bendamustine and rituximab after his initial diagnosis; subsequently, he developed grade 3 neutropenia with anasarca. He was later seen at our institution, and the regimen was then transitioned to single agent ibrutinib which he tolerated well for 5 months with no significant cytopenias. On progression, rituximab was added to the regimen to improve therapeutic response. In July 2020, he had received his first dose of rituximab 375 mg/m2 while continuing ibrutinib. A repeat complete blood count (CBC) drawn 6 days after administration showed an undetectable absolute neutrophil count (ANC) level, and ibrutinib was held. He presented to the emergency department (ED) with new onset neutropenic fever with temperature of 38.3 °C, white blood cell (WBC) count of 3.06 × 109/L, absolute neutrophil count (ANC) of < 0.03 × 103/L, hemoglobin of 10.9 g/dL, and platelet count of 197 × 109/L. Urine cultures, head computed tomography (CT), chest X-ray, and port cultures were negative during his hospital stay. Physical examination was benign for any pertinent findings.
He received granulocyte colony-stimulating factor (G-CSF) and broad-spectrum antibiotics which was stopped after blood cultures remained negative for 48 h. Over the next 5 days, his ANC improved to 4.12 × 103/L and he became afebrile. No infectious cause was found. He was restarted on ibrutinib with no further neutropenia (Fig. 1).
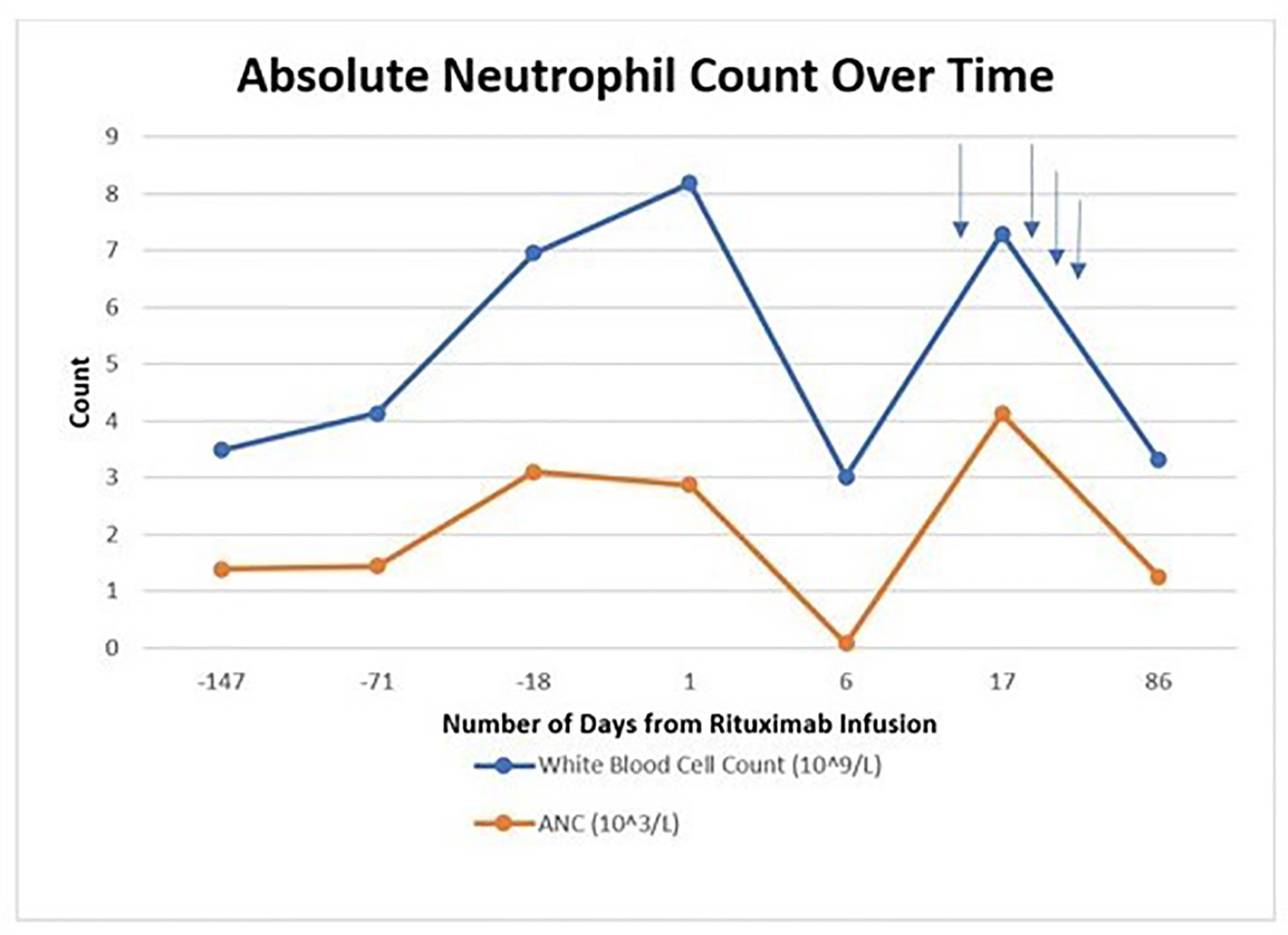 Click for large image | Figure 1. Absolute neutrophil count trend over time. Blue arrows indicate growth factor support administration. |
| Discussion | ▴Top |
Rituximab-associated neutropenia (RAN) is uncommon and has been reported in about 0.02-6% of cases, with most cases being LON rather than EON [1]. This makes EON extremely rare, only reported in eight total cases, including our own [3].
EON is defined by neutropenia occurring within 4 weeks of starting treatment [2, 3]. The noted average time to onset of neutropenia in the seven noted cases was about 15 days [2, 3]. In our case, the patient developed neutropenia at an earlier onset than the average, at just 6 days after initiation. In five out of the seven cases, the patients that developed EON were treated with rituximab at 375 mg/m2 weekly, including our patient [1-3]. The other two cases were treated with a higher dose, 1,000 mg/m2 weekly, and the time to neutropenia onset was 7 and 28 days [3]. However, our case does present an extremely rare instance of an early onset grade 4 neutropenia after first infusion of rituximab within onset of 6 days when compared to the average reported case in those receiving weekly standard dose rituximab.
To illustrate the rarity of this phenomenon, in one of the largest studies of RAN by Nitta et al, 24 out of 107 patients had RAN, although in this study the focus was on LON [1, 4]. Of note in this study, CBCs were regularly checked every 2 - 8 weeks, based on providers’ preference, after rituximab infusion was started [1, 4]. There was no comment of any noted cases of EON. This supports that EON may truly be rare, rather than just under-documented.
The pathogenesis of EON and LON due to rituximab remains unclear, and many of the proposed hypotheses seek to explain the more common phenomena of LON, which may be precipitated by a different mechanism from EON [2]. Neutrophils do not express CD20; therefore, rituximab cannot be directly toxic to neutrophils. However, some theories propose a CD20 antigen in the blood causing immune-mediated lysis [1, 5]. Studies such as Voog et al and Weissmann-Brenner et al supported the theory of antibody-mediated toxicity [1, 2]. Fukuno et al did not find evidence supporting either of these theories during his studies of RAN [1, 6]. Terrier et al supported an entirely different mechanism altogether suggesting an increase in BAFF (a stimulator of B-cell recovery) causing a rebound B-cell lymphopoiesis outcompeting neutrophil production in the bone marrow [1, 7]. Most studies favor antibody-mediated toxicity [1, 2, 5].
Several other risk factors have been analyzed for EON/LON, such as co-treatment with other chemotherapies [1]. In the study by Adler et al, there was concern for an increased risk of neutropenia due to co-treatment of rituximab with mycophenolate mofetil (MMF), although grade 4 EON was noted 28 days after first rituximab infusion [3]. Notably, many other cases of RAN such as those by Arroyo-Avila et al, Gottenberg et al, and the LUNAR trial by Rovan et al, documented patients also treated with MMF [2, 8-10]. Of the seven cases of EON, four of these cases were administered MMF along with rituximab therapy [3]. In the study by Arroyo-Avila et al, the patient developed grade 4 EON, 5 days after the second weekly rituximab infusion [2]. Gottenberg et al noted grade 3 neutropenia 10 days after first rituximab infusion [8]. Enriquez et al treated their patient with cyclophosphamide in addition to rituximab and noted EON 5 days after second weekly infusion of rituximab [11]. In our case, the patient was also concurrently treated with ibrutinib, but it should be noted that patient has not demonstrated neutropenia while on ibrutinib monotherapy previously.
Six out of the seven cases had complete recovery [3]. Only two were spontaneous, the others were given G-CSF and then experienced full recovery [3]. In the study by Gottenberg et al, one of the patients did not recover to normal neutrophil counts [3, 8]. Some presentations of EON are more severe ranging from sepsis-like presentation to neutropenic fever to asymptomatic neutropenia [1, 3]. No deaths have been reported related to EON [3].
Other studies demonstrate that neutropenia is a rare side effect of ibrutinib treatment, with bleeding and bruising being the most common side effects [4]. In a study by Ujjani et al, ibrutinib was combined in therapy with lenalidomide and rituximab for treatment of chronic lymphocytic leukemia. The treatment combination was never pursued due to grade 3 to 4 neutropenia developing in 67% of patients [12]. In our case, rituximab was stopped and ibrutinib was resumed after resolution of the neutropenia and it resulted in no further worsening of neutropenia. So, while the synergistic effect of ibrutinib and rituximab causing EON is a possible explanation in our case, it would seem less likely given the time course of events.
Conclusions
We present this case with acute onset of grade 4 EON within 6 days of first dose of rituximab infusion to bring awareness to the inherent adverse effects of EON associated with rituximab therapy. This phenomenon is quite rare in occurrence. The pathogenesis behind EON remains largely unknown, and many proposed theories focus more on LON rather than EON. EON associated with rituximab treatment, although less common than LON, should be noted as a possible cause of neutropenia.
Learning points
RAN is an extremely rare entity that has been reported in about 0.02-6% of cases in the literature.
Identifying this entity as a viable etiology of cancer-induced myelosuppression is vital for successful cancer therapy.
Acknowledgments
The authors would like to thank the Department of Internal Medicine of University of Texas Medical Branch, Galveston, Texas in patient care.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from patient.
Author Contributions
BN and ST developed the original idea, analyzed the data, prepared the manuscript, and provided additional review. RV provided critical revision of the manuscript for important intellectual content.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Weissmann-Brenner A, Brenner B, Belyaeva I, Lahav M, Rabizadeh E. Rituximab associated neutropenia: description of three cases and an insight into the underlying pathogenesis. Med Sci Monit. 2011;17(11):CS133-137.
doi pubmed - Arroyo-Avila M, Fred-Jimenez RM, Vila LM. Early-onset neutropenia induced by rituximab in a patient with lupus nephritis and hemolytic anemia. Case Rep Rheumatol. 2015;2015:616787.
doi pubmed - Adler BL, Crew AB, Woodley DT. Early-onset neutropenia after rituximab therapy for bullous pemphigoid. Clin Exp Dermatol. 2019;44(3):334-336.
doi pubmed - Hillmen P, Rawstron AC, Brock K, Munoz-Vicente S, Yates FJ, Bishop R, Boucher R, et al. Ibrutinib plus Venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30):2722-2729.
doi pubmed - Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica. 2005;90(Suppl):ECR23.
- Voog E, Morschhauser F, Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348(26):2691-2694; discussion 2691-2694.
doi pubmed - Terrier B, Ittah M, Tourneur L, Louache F, Soumelis V, Lavie F, Casadevall N, et al. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica. 2007;92(2):e20-23.
doi pubmed - Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, Larroche C, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64(6):913-920.
doi pubmed - Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215-1226.
doi pubmed - Nitta E, Izutsu K, Sato T, Ota Y, Takeuchi K, Kamijo A, Takahashi K, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: a single-institution study. Ann Oncol. 2007;18(2):364-369.
doi pubmed - Enriquez R, Borras-Blasco J, Sirvent AE, Masia M, Amoros F. Early onset neutropenia after rituximab in lupus nephritis. Clin Exp Rheumatol. 2007;25(2):345.
- Ujjani C, Wang H, Skarbnik A, Trivedi N, Ramzi P, Khan N, Cheson BD. A phase 1 study of lenalidomide and ibrutinib in combination with rituximab in relapsed and refractory CLL. Blood Adv. 2018;2(7):762-768.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


