| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 6, December 2021, pages 255-265
Monthly Continuous Erythropoietin Receptor Activator Versus Weekly Epoetin-Beta, Similar Hemoglobinization but Different Anisocytosis Degree in Hemodialysis Patients: A Randomized Controlled Trial
Miguel G. Uriol-Riveraa, e , Aina Obrador-Muleta, Sonia Jimenez-Mendozaa, Antonio Corral-Baezb, Leonor Perianez-Parragac, Angel Garcia-Alvarezc, Francisco J. de la Pradad
aNephrology Department, Hospital Son Espases, Palma de Mallorca, Balearic Islands, Spain
bNephrology Department, Policlinica Miramar-Hospital Son Espases, Palma de Mallorca, Balearic Islands, Spain
cPharmacy Department, Hospital Son Espases, Palma de Mallorca, Balearic Islands, Spain
dNephrology Department, Hospital Virgen Macarena, Sevilla, Spain
eCorresponding Author: Miguel G. Uriol-Rivera, Servicio de Nefrologia, Hospital Universitario Son Espases, C/Valldemossa 79, 07120 Palma de Mallorca, Comunidad Balear, Spain
Manuscript submitted June 9, 2021, accepted July 5, 2021, published online November 29, 2021
Short title: ESA Half-Life Hemoglobin and Anisocytosis
doi: https://doi.org/10.14740/jh862
| Abstract | ▴Top |
Background: The monthly continuous erythropoietin receptor activator (CERA) utilization maintains stable hemoglobin (Hb) after conversion from weekly epoetin-β (EB); however, how the different pharmacologic properties affect the red blood cell (RBC) size determined by RBC distribution width (RDW) has not been evaluated yet. We assess the potential differences in iron metabolism, plasma erythropoietin (EPO), hepcidin, and soluble α-Klotho (α-Klotho) levels as an emergent hematopoiesis factor.
Methods: Thirty-seven chronic hemodialysis patients were included from January 2010 to November 2011 and randomized (1:1) to continue with EB or to convert to monthly CERA. Primary outcome was the mean change in Hb between groups at months 0, 3 and 6, and the percentage of patients who maintained stable Hb (Hb ± 1 g/dL from baseline level to month 6). Secondary outcomes were the influence on the erythropoietic process and iron metabolism markers. Thirty-one patients completed the study (CERA: n = 15, EB: n = 16).
Results: The mean (95% confidence interval (CI)) Hb difference between groups was 0.28 g/dL (-0.36 to 0.93). There was no difference between the percentages of patients with stable Hb levels. In the CERA group RDW values increased progressively (interaction erythropoietin-stimulating agent (ESA) type and time on RDW values, F (1.57, 45.60) = 17.17, P < 0.01, partial η2 = 0.37) and the mean corpuscular volume changed at the different time points, (F (2, 28) = 29.12, P = 0.03, partial η2 = 0.23). During the evaluation period, in the CERA group, EPO was higher, and hepcidin and ferritin decreased significantly. α-Klotho decreased in both groups and correlated negatively with the changes on the RDW and positively with transferrin and serum iron. The number of serious adverse events was higher at the CERA group.
Conclusions: Monthly CERA maintained Hb concentrations; however, it showed a significant effect on RDW, probably due to its impact on the EPO and hepcidin levels. α-Klotho decreased significantly in both groups, and its changes correlated with the changes in iron metabolism. Whether the RDW evolution was associated with the serious adverse events (SAEs) is a feasible hypothesis that needs to be confirmed in large studies.
Keywords: ESA half-life; Hemoglobin; Anisocytosis; Hepcidin; Erythropoietin; Klotho
| Introduction | ▴Top |
Hemoglobin variability is a physiologic event, but it is influenced by the use of erythropoietin-stimulating agents (ESAs) [1]. There are three generations of ESAs: 1) short half-life (6 - 9 h) recombinant human erythropoietin, such as epoetin-α and epoetin-β (EB) [2]; 2) darbepoetin-α [3], with a long half-life (25 h); and 3) the third-generation ESA, methoxy polyethylene glycol-epoetin-β, a continuous erythropoietin receptor activator (CERA) with a longer half-life (about 130 h) that facilitates its administration at broader time intervals [4].
The effectiveness of CERA for the treatment of anemia and maintaining stable Hb levels in hemodialysis patients has been largely confirmed in non-inferiority randomized studies versus EB [5, 6] and darbepoetin-α [7], as well as in non-dialysis [8], peritoneal dialysis [9] and post-renal transplantation patients [10, 11]. However, CERA showed higher erythropoietic activity in comparison with epoetin [12], which may associate different effects on the red blood cell (RBC) assembling, increasing the anisocytosis degree.
Although iron and erythropoietic disturbances are deeply evaluated in renal anemia, new factors are gained interest in its pathogenesis. An important influence of anti-ageing hormone: α-soluble Klotho (α-Klotho) on the erythropoietic process [13] and with the accelerated RBC death (eryptosis) [14] have been described [14, 15]. The α-Klotho protein is produced mainly in the kidneys, and the synthesis is affected in early stage of chronic kidney disease (CKD) and correlated with its complications. In CKD patients, short RBC life span and lower α-Klotho levels are well described [16]; however, little is known about the influence of the α-Klotho in iron metabolism parameters in hemodialysis patients and the effect of the different ESA’s half-life on α-Klotho levels.
To provide new insights into this crucial issue, we designed a randomized clinical trial to address the effectiveness of once-monthly CERA after conversion from EB (1 - 3 times weekly) in chronic hemodialysis patients assessing the degree of Hb stability and the potential impact on the RBC form. The secondary objective was to evaluate the potential differences in iron metabolism, hepcidin, erythropoietin (EPO), and α-Klotho. The safety and tolerability of ESA administration were also evaluated.
| Material and Methods | ▴Top |
Patients
The clinical records of 189 consecutive chronic dialysis patients (age ≥ 18 years) were reviewed in our clinic from January 2010 to November 2011. The inclusion criteria were: 1) same hemodialysis filter during the 12 weeks prior to their inclusion; 2) Kt/V ≥ 1.2 (Daugirdas second generation); 3) Hb of 10.5 to 12.0 g/dL during the 12 weeks prior to inclusion; 4) stable EB doses: ± 1,000 IU/week, for 3 months before inclusion; and 5) transferrin saturation (TSAT) ≥ 20% and/or serum ferritin > 100 ng/mL. Exclusion criteria were: 1) New York Heart Association class IV congestive heart failure; 2) active bleeding or transfusion within 8 weeks prior to inclusion; 3) other causes of anemia (malignancies, folic acid or vitamin B12 deficiencies, hemoglobinopathies or hemolysis); 4) acute or chronic infectious; 5) uncontrolled inflammatory disease; 6) poorly controlled hypertension; 7) immunosuppressive therapy; 8) thrombocytopathies; 9) pregnancy or desire for pregnancy.
Intervention
The principal investigator used a random number generator (SPSS 18.0 statistical software package, IBM, Chicago, IL, USA) to create the two arms: 1) control arm: to maintain weekly intravenous EB (NeoRecormon®, Roche Farma SA, Madrid, Spain); 2) experimental arm: to convert to monthly intravenous CERA (Mircera®, Roche Farma SA, Madrid, Spain) according to the datasheet. No blinding method was used. The graphic program used was Sigma Plot 10.0.
The study design (Fig. 1) was made up of 24 weeks. Clinical and laboratory data were collected on a monthly basis and ESA doses adjusted as follow: 1) 25% increased ESA doses for Hb decreases < 2 g/dL or Hb ≥ 9 and < 11 g/dL, or 50% increased ESA doses when Hb decreased ≥ 2 g/dL, or Hb < 9 g/dL; 2) ESA doses were reduced by 25% for Hb increases ≥ 1 g/dL or Hb ≥ 12 and ≤ 14 g/dL, or by 50% for Hb increases > 2 g/dL. If Hb was > 14 g/dL, we stopped ESA for 1 month and restarted with a 25% reduction of the lowest dose previously administered. Intravenous iron supplementation was prescribed to maintain the TSAT level ≥ 20% during the study.
 Click for large image | Figure 1. Study design. CERA: continuous erythropoietin receptor activator. |
Outcome evaluation
We evaluated Hb stability, including the mean differences between months 0, 3, and 6. We determined the percentage of patients who maintained stable Hb. Hb stability was defined as Hb within 1 g/dL of baseline during the study. The percentage of patients on stable Hb was calculated as follows: ((number of patients with Hb stability at each evaluation visit/number of included patients in each arm of treatment) × 100). To determine the heterogeneity of RBCs, we assessed RBC distribution width (RDW).
The safety and tolerability of ESA were assessed by analyzing the changes in office blood pressure throughout the study and reporting serious adverse events (SAEs).
Ethical issues and informed consent
The study was approved by the Ethical Committee of the Balearic Islands (IB 1180/09 PI) and the Spanish Agency for Medicines and Health Products. Informed consent was obtained from all patients. The study was performed according to the 1964 Helsinki Declaration and its later amendments (trial registration and full trial protocol: EudraCT 2009-015511-40-ES (Clinical Trials Register: 2009-015511-40)).
Assays
Hb and other hematologic indices were performed every month before midweek dialysis sessions by flow cytometry using the automatic analyzer CELL-DYN Sapphire (Abbott Diagnostics®, Abbott Laboratories, Abbott Park, IL, USA). Iron metabolism parameters were assessed by standard methods.
During the evaluation period, serum hepcidin was determined using DRG Hepcidin Prohormone enzyme-linked immunoassay (ELISA) Kit; EPO determined by the Quantikine® IVD® Human Epo ELISA, and soluble α-Klotho by immunoenzymatic method using the Human Soluble α-Klotho Assay Kit-IBL (Immuno-Biological Laboratories, Japan).
Data analysis
This was a pilot study for evaluating the influence of the two ESAs’ half-life on iron markers, hematologic indices, and on the α-Klotho levels. We decided to include 20 patients in each group rather than on the basis of a power calculation. This objective was not reached due to the possibility of a suffering shortage of CERA because of manufacturing problems (http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/calidad/2012/NI_MUH_01-2012_calidad.htm).
The baseline characteristics of both groups were compared using the t-test, Mann-Whitney U-test, χ2 test, or Fisher exact test. The changes in the frequencies were analyzed by McNemar’s test or Cochran’s Q test (Cochran, 1950) for evaluating more than two time points. The results of comparing two dichotomous variables are reported as odds ratio and 95% confidence interval (OR: 95% CI).
A two-way mixed analysis of variance (ANOVA) model was implemented to assess the changes in the variables analyzed as a function of follow-up time. We evaluated the interaction effect between inter- and intra-subject effects to assess the differences in the evolution of the Hb levels in response to each treatment. The effect size was computed as partial eta-squared values (partial η2; small: ≥ 0.01; medium: ≥ 0.06; large: ≥ 0.14). All multiple comparisons were adjusted using Bonferroni’s correction. Spearman correlation was used for evaluating the association between variables.
Thirty-seven patients were included. During 1 month, two patients (one in each group) received a higher ESA dose than what the protocol indicated. Six patients did not complete the study for different reasons (Fig. 2). For the primary objective, the results obtained when only considering the patients who completed the three visits of the protocol (PP) were also confirmed by intention-to-treat (ITT) analysis.
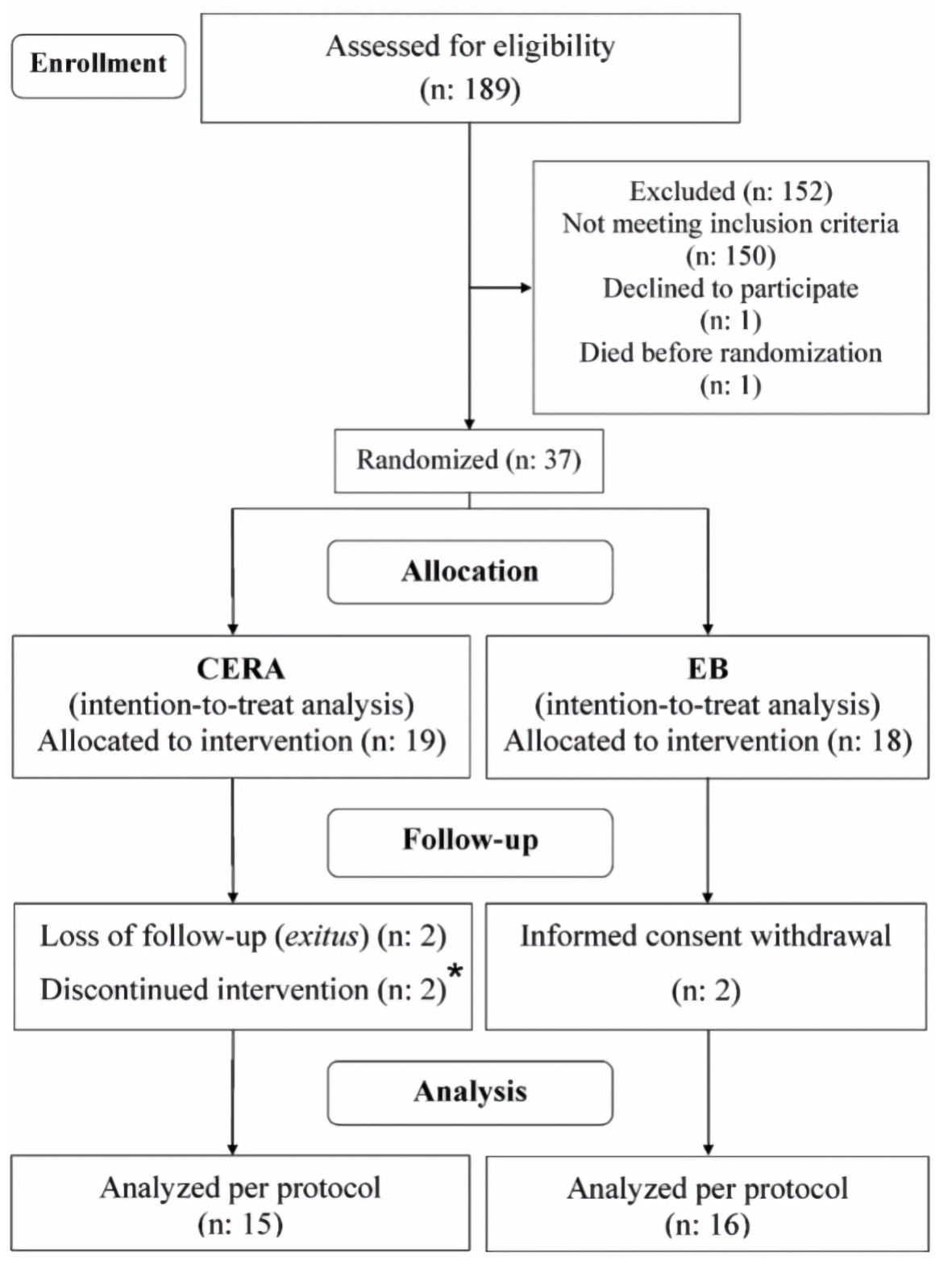 Click for large image | Figure 2. Flow of the study. CERA: continuous erythropoietin receptor activator; EB: epoetin-β. |
| Results | ▴Top |
The baseline features are given in Table 1.
 Click to view | Table 1. Baseline Characteristics of the Patients Who Completed the Study Protocol in Both Treatment Arms |
The patients allocated to the CERA group showed higher basal serum iron and office systolic blood pressure and a lower percentage of patients under intravenous iron supplementation (Table 2).
 Click to view | Table 2. Baseline Hematological Parameters and Iron Status of the Patients Who Completed the Study Protocol in Both Arms of Treatment |
Hematologic parameters
Hemoglobin variability
No interaction was found between the arm of treatment and follow-up visits ((PP, F (2, 58) = 0.03, P = 0.96; ITT, F (2, 70): 0.08, P = 0.92); Fig. 3). The difference between EB and CERA in the mean change (95% CI) in Hb concentration from baseline to evaluation was 0.28 g/dL (-0.36 to 0.93) and 0.19 g/dL (-0.37 to 0.76) for the PP and ITT analyses, respectively. After adjusting for iron supplementation, the mean change was -0.01 g/dL (-0.76 to 0.74, Fig. 3).
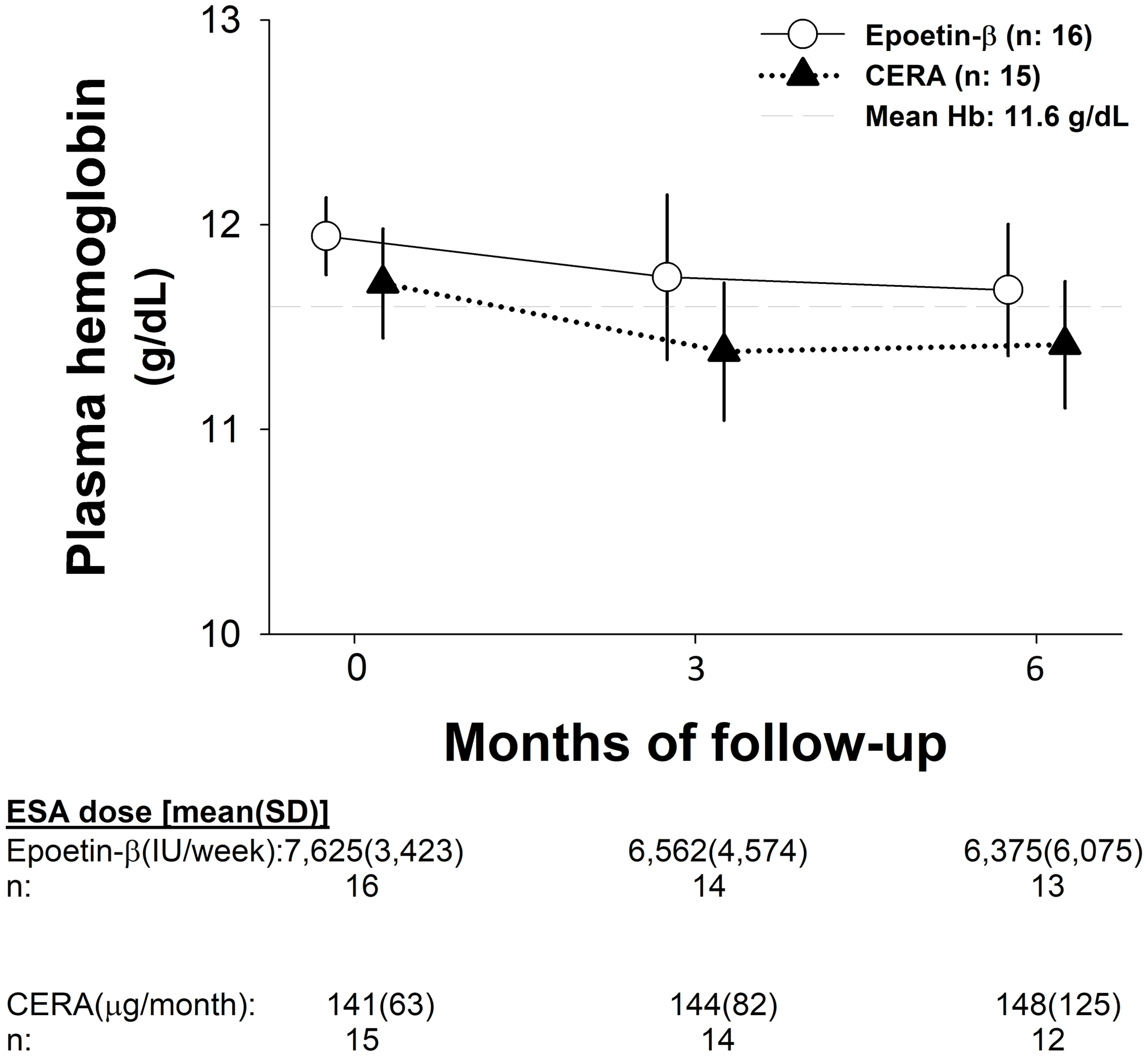 Click for large image | Figure 3. Hemoglobin evolution and ESA dosage during the study. CERA: continuous erythropoietin receptor activator; Hb: hemoglobin; SD: standard deviation. |
The percentage of patients maintaining stable Hb level was similar in both arms of treatment at month 3 (EB vs. CERA: 9 (56%) vs. 10 (66%), χ2: 0.4, P = 0.55; OR: 1.55 (95% CI: 0.36 to 6.69)) and at month 6 (EB vs. CERA: 11 (69%) vs. 8 (53%), χ2: 0.77, P = 0.37; OR: 0.51 (95% CI: 0.12 to 2.24)).
In a post hoc analysis of intra-patient Hb variability, the mean within-patient standard deviation (SD) for Hb during the titration period in the once-monthly CERA and EB groups was -0.13 and 0.12 g/dL, respectively (P = 0.40). Within-patient SD for Hb during the evaluation period was also similar across treatment groups: -0.12 and 0.11 g/dL for once-monthly CERA and EB, respectively (P = 0.42).
We found no significant changes in ESA dose in any arm of treatment during the study (Fig. 3).
Red cell distribution width
An interaction effect was found between RDW and ESA type during the follow-up (F (1.57, 44.5) = 18.3, P < 0.01, partial η2 = 0.38). After iron adjustment, the statistical association remained (Fig. 4a).
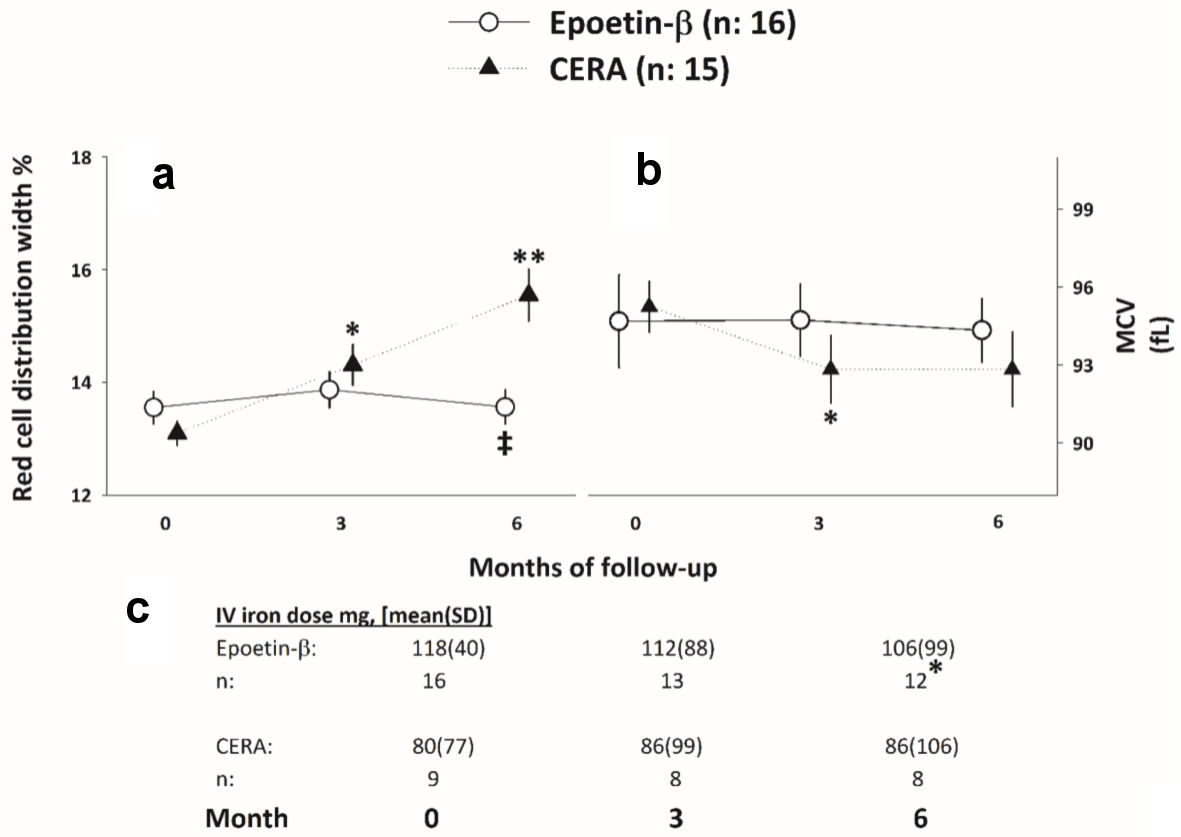 Click for large image | Figure 4. (a, b) RDW and MCV evolution throughout the study. *, **P < 0.05 respect to baseline. ‡P < 0.05 respect to the CERA arm. (c) Iron requirements evolution throughout the study. *P < 0.05, Cochran test. CERA: continuous erythropoietin receptor activator; RDW: red cell distribution width; MCV: mean corpuscular volume; SD: standard deviation. |
Mean corpuscular volume (MCV)
Differences in the CERA group were observed during the study (F (1.47, 20.7) = 4.39, P = 0.03, partial η2 = 0.23), while no changes were observed in the EB arm (F (1.45, 21.7) = 0.02, P = 0.94; Fig. 4b). After iron adjustment, the statistical association remained.
RBCs count
The RBC count in the CERA group increased progressively, but not significantly (3.63 (0.3), 3.76 (0.4) and 3.70 (0.4), for the month 0, 3 and 6, respectively (F (2, 28) = 0.12, P = 0.88), while the evolution in the EB group were 3.69 (0.3), 3.73 (0.6), 3.65 (0.4) for the month 0, 3 and 6, respectively (F (2, 30) = 0.18, P = 0.79).
The changes overserved from month 0 to 6 in the RBC correlated with the changes on RDW in the CERA arm (ρ: 0.53, P = 0.04), while no significant association was observed in the EB group (ρ: 0.28, P = 0.29).
Iron supplements and ESA dose requirements
As a whole, there was an interaction between iron utilization at the time of the study and ESA doses (F (1.65, 48.07) = 4.79, P = 0.01, partial η2 = 0.14]; the mean difference in ESA doses throughout the study between those patients with and without iron supplements at the time of the inclusion was 36% (95% CI: 6 to 66).
The percentage of patients who started the study under iron therapy and needed a decrease in ESA dose were similar between the EB and CERA groups at month 3 (8 (50%) vs. 4 (44%), χ2: 0.07, P = 0.79; OR: 0.80 (95% CI: 0.15 to 4.12) and at month 6 (6 (38%) vs. 3 (33%), χ2: 1.15, P = 0.43, OR: 2.40 (95% CI: 0.47 to 12.13)).
The number of patients under iron therapy in the EB group decreased from 16 to 12 in the EB group (Q: 6.50, P = 0.03), and no changes in the CERA group was observed (Fig. 4c).
Changes in iron metabolism parameters
Circulating ferritin decreased significantly only in the CERA group that remained after iron adjustment (Table 3). Transferrin decreased progressively in the CERA group; however, the statistical significance disappeared after iron adjustment. The other iron markers evolution is given in Table 3.
 Click to view | Table 3. Iron Status Markers Evolution in Patients Who Completed the Study |
Hepcidin, EPO and α-Klotho levels during the evaluation period
Hepcidin decreased significantly in the CERA group, the median EPO increased in both groups but did not reach statistical significance; however, the levels in the CERA were higher (about double) than those observed in the EB group. The α-Klotho decreased significantly in both groups (Table 4).
 Click to view | Table 4. Hepcidin, EPO and α-Klotho Changes During the Evaluation Period |
As a whole, the changes in α-Klotho levels at the evaluation period were directly correlated with the changes in transferrin and free serum iron and indirectly with the changes observed on the RDW values (Fig. 5). At the EB arm, α-Klotho changes negatively correlated with EPO changes (ρ: -0.59, P = 0.04), and in the CERA groups strong positively correlate with transferrin changes (ρ: 0.79, P < 0.01).
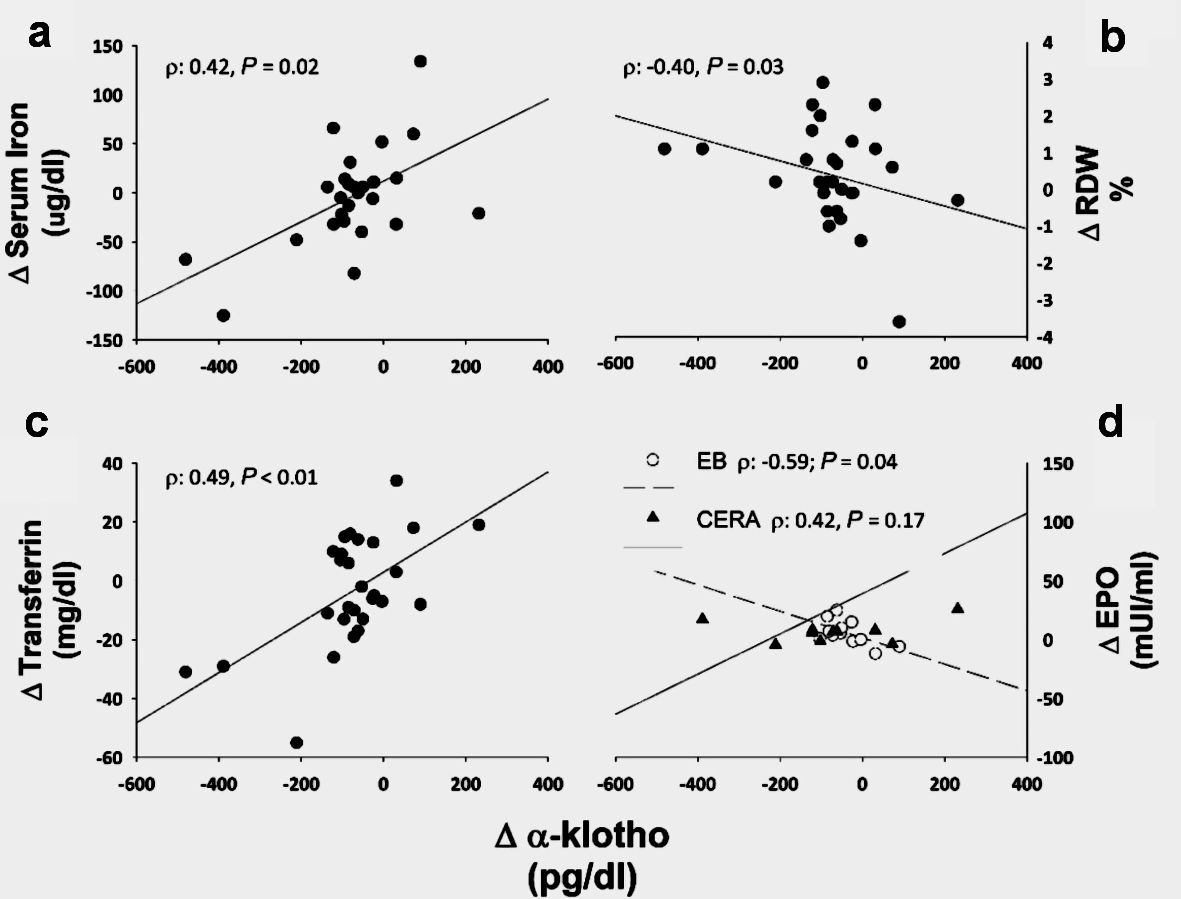 Click for large image | Figure 5. α-Klotho associations. ∆stands for the changes between month 3 and month 6. RDW: red cell distribution width; CERA: continuous erythropoietin receptor activator; EB: epoetin-β; EPO: erythropoietin. |
Safety and tolerability
The differences in systolic blood pressure between arms of treatment at baseline disappeared at the end of the study, driven by a higher increase in the EB group (9.8 ± 5.2 mm Hg) compared with the CERA group (7.1 ± 4.6 mm Hg), independent of the number of antihypertensive drugs used and without significant changes in the dry weight of patients. No other changes in the office blood pressure were observed.
The SAEs requiring hospitalization or emergency room care was more common in the CERA arm; however, the suspected SAEs showed no differences (Table 5). Two patients in the CERA arm died during the trial, one due to intradialytic cardiac arrhythmia and another due to urinary sepsis. Three patients in each group discontinued ESA per protocol, and one patient in the CERA group required a blood transfusion after undergoing surgery for a diabetic ulcer.
 Click to view | Table 5. Adverse Events Requiring Hospital Admission or Care in the Emergency Department |
| Discussion | ▴Top |
In this study, we found that monthly CERA was as effective as EB in maintaining Hb; nevertheless, a different effect on the anisocytosis degree was observed; probably related to the different effect on EPO, hepcidin, and iron metabolism. This is a novel finding with significant clinical impact.
The efficacies of monthly CERA in maintaining Hb level and the percentage of patients with stable Hb after conversion from weekly EB in hemodialysis patients were observed in our study in line with previous reports [5, 17, 18]. The method used (general linear model) to evaluate changes in Hb indicated that intra-individual changes were not significant, which informs not only that the recommended CERA doses for switching from EB were appropriated but also reflects the high Hb stability of the patients included in the study.
Although the patients at baseline had optimal iron stores, over 30% of the ESA dose was needed in those who started the trial without iron supplementation, which corresponded to a CERA sub-group of patients. Four patients (25%) in the EB group stopped significantly iron administration; furthermore, the baseline difference between the percentages of patients under iron therapy disappeared at the end of the study. Altogether suggest lower iron requirements in the EB group, while no changes at the CERA arm was observed.
The most important finding was the effect of the ESA half-life on RDW evolution. The RDW value is usually provided in the blood count and reflects the degree of heterogeneity of erythrocyte volume (anisocytosis). The RDW increased progressively only in those under CERA, and this change was significantly even after iron adjusting. Since higher RDW values have been associated with iron deficiency [19], erythropoietin resistance [20], fistula dysfunction [21] and high mortality risk in hemodialysis patients [22], the effect of the monthly CERA on RDW should be rigorously evaluated in larger studies.
Classic factors associated with higher RDW in the CERA arm, such as iron supplementation, EPO resistance index, and nutritional status and dialysis dose, could be reasonably excluded in our study; and we hypothesized that the continuous erythropoiesis stimulation could be a feasible factor related to the changes in RDW values.
In order to identify the underline mechanisms involved in the RDW changes, we evaluate the MCV evolution for its inverse correlation with RDW [23]. The MCV did not change in the EB group; but decreased significantly in the CERA arm from month 0 to month 3, while unexpectedly remained stable from months 3 to 6. This condition may be explained by the co-occurrence of micro- and macrocytic RBC forms. We hypothesized that macrocytic RBC could be a consequence of immature forms induced by the over physiologic erythropoiesis stimulus [24, 25] and microcytic forms secondary to the iron blockage [26]. This scenario has been described in a hypoxia disease model by previous authors [27]. Notably, in the CERA group, the changes in RBC count correlated directly with the changes in the RDW.
Plasma ferritin and hepcidin decreased in those under CERA. Theoretically, these benefits consist of lowering the high iron stores and favoring physiologic iron absorption through the intestine due to a potent hepcidin inhibition [28]; however, this advantage was neither accompanied by improving Hb levels nor decreased iron and ESA requirements. The slight rise in the RBC count could justify where the iron removed by the iron body stores was reutilized, which could be the consequence of the increase in EPO levels in the CERA arm and its sustained erythropoietic activity [12].
The EPO levels were higher in the CERA group and increased during the evaluation period, probably determined by the slower binding of CERA to the EPO receptor [12]. Conversely, the EB dosage slightly decreased, and surprisingly EPO levels increased. We have no explanation for this phenomenon, and it needs to be evaluated in future studies.
We observed a significant decrease in α-Klotho in both groups; however, the median change decreased at the CERA group by about 25%, while in the EB, it only decreased 5%. It has been described that the genetic ablation of Klotho in mice results in a substantial increase in the erythropoiesis process mediated through EPO [13]. In our study, in the EB group, an inverse correlation was observed between EPO and α-Klotho changes, while in the CERA group, conversely, a positive but not significant association was observed. Although no significant changes in the ESA doses were observed in any group; whoever, the different influence of the different ESA half-life therapy on α-Klotho levels could be related to the sustained erythropoietic effect and the EPO plasma levels. Lower levels of α-Klotho have been associated with high mortality in dialysis patients.
As a whole, an indirect correlation between the changes on α-Klotho and RDW was observed, and positively with serum iron and transferrin. Herein, we report that α-Klotho could be involved in erythropoiesis through its association with EPO and the iron metabolism control. This novel link between α-Klotho and RDW may be supported by the fact that both α-Klotho deficiency and high RDW are associated with high ESA requirements and higher cardiovascular and mortality risk CKD patients [22, 29-32].
In our study, monthly CERA treatment was associated with a higher number of AEs requiring hospitalization or care at the emergency room in comparison with EB treatment, but the incidence of suspected adverse reactions showed no differences between groups. The numbers of vascular access complications were double in the CERA group in comparison with EB. Previous reports showed a similar association, but this effect did not reach statistical significance [5, 33]. The two deaths during the study were in the CERA group but were not attributable to ESA use. Recently, controversial information was reported about the safety profile of the long-acting ESAs in comparison with short-acting ESAs has been described [34-36]. Hypothetically, according to the bibliography and our data, the expected adverse events could be related to thrombosis. Anisocytosis influences erythrocyte rheology, a factor associated with thrombosis [37]. Whether the RDW evolution had a relationship with SAE, and in particular, with the vascular access complications need to be further evaluated in large studies.
Of note, there was an increase in blood pressure values in the EB arm, and EB patients were matched at the end of the study with CERA patients, whose blood pressure was higher at baseline. While this finding does not have a straight forward explanation, it might be related to a direct hypertensinogenic effect of a higher number of drug administrations in this arm of treatment [38].
The main weakness of our study is the small number of patients included, so general conclusions should be drawn with caution, especially those related to the safety issue. The strengths derive from the following factors: the high stability of the hematologic parameters and ESA doses at baseline, the exclusion of renal anemia causes, its randomized design, and its implementation in a real-world clinical practice setting.
Conclusions
Monthly CERA maintains the Hb stability of hemodialysis patients after the switch from EB; nonetheless, its higher erythropoietic stimulus may associate a different effect on anisocytosis. This difference may be related to higher EPO levels and its strong hepcidin inhibition. The α-Klotho decreased in both groups, and their associations suggest a role in the erythropoiesis process through mechanisms that involve not only EPO but also the iron metabolism in these patients.
The effect of the short- and long-acting ESA utilization on RDW needs to be confirmed in future studies because of its important clinical implication, and also RDW should be considered when treating anemia for the high information that it brings about the erythropoietic response.
Acknowledgments
Thanks to the nursing staff, and especially to Pastor Adelaida for their assistance in the data collection. Thanks to Antonio Palomero for registering, storing and distributing the medication used in the study.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
Consultancies: Miguel G. Uriol-Rivera (Alexion Pharmaceuticals). Honoraria: Miguel G. Uriol-Rivera (Alexion Pharmaceuticals). Stock ownership or options: Miguel G. Uriol-Rivera (owner of a patent EP3104872A: “Use of paricalcitol in the treatment of inflammatory anemia”). Honoraria: Aina Obrador-Mulet (Alexion Pharmaceuticals). All the other authors have declared no conflict interest.
Informed Consent
All subjects provided written informed consent.
Author Contributions
All authors have made substantial contributions to the following: conceived and designed the study: MUR; performed the study: ACB, AGA, AOM, FPA, LPP, MUR and SJM; wrote the paper: MUR; performed the acquisition of data: ACB, SJM and MUR; read the manuscript and revised it for important intellectual content: AOM and FPA; read and approved the final manuscript: ACB, AGA, AOM, FPA, LPP, MUR and SJM.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AEs: adverse events; CERA: continuous erythropoietin receptor activator; CKD: chronic kidney disease; EB: epoetin-β; EPO: erythropoietin; ESA: erythropoietin-stimulating agent; Hb: hemoglobin; ITT: intention-to-treat; MCV: mean corpuscular volume; PP: per-protocol; RBC: red blood cell; RDW: red blood cell distribution width; SAEs: serious adverse events; SD: standard deviation; TSAT: transferrin saturation
| References | ▴Top |
- Kalantar-Zadeh K, Aronoff GR. Hemoglobin variability in anemia of chronic kidney disease. J Am Soc Nephrol. 2009;20(3):479-487.
doi pubmed - Spinowitz B. The practical aspects of therapy with rHuEPO. Am J Nephrol. 1990;10(Suppl 2):24-28.
doi pubmed - Macdougall IC, Matcham J, Gray SJ, NESP 960245/246 Study Group. Correction of anaemia with darbepoetin alfa in patients with chronic kidney disease receiving dialysis. Nephrol Dial Transplant. 2003;18(3):576-581.
doi pubmed - Ohashi N, Sakao Y, Yasuda H, Kato A, Fujigaki Y. Methoxy polyethylene glycol-epoetin beta for anemia with chronic kidney disease. Int J Nephrol Renovasc Dis. 2012;5:53-60.
doi pubmed - Sulowicz W, Locatelli F, Ryckelynck JP, Balla J, Csiky B, Harris K, Ehrhard P, et al. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007;2(4):637-646.
doi pubmed - Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, Villa G, et al. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol. 2008;28(2):280-289.
doi pubmed - Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, Villa G, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008;23(11):3654-3661.
doi pubmed - Koch M, Henrich D, Faust J, Nawka J, Rath T, Wanner C. Initial use of once-monthly administration of C.E.R.A. is effective and safe in correcting renal anemia in non-dialysis patients: the MERCUR trial. Clin Nephrol. 2012;78(3):189-197.
doi pubmed - Koch M, Treiber W, Fliser D. Effective achievement of hemoglobin stability with once-monthly C.E.R.A. in peritoneal dialysis patients: a prospective study. Clin Drug Investig. 2013;33(10):699-706.
doi pubmed - Campistol JM, Carreno A, Morales JM, Pallardo L, Franco A, Navarro D, Grinyo JM, et al. Once-monthly pegylated epoetin Beta versus darbepoetin alfa every two weeks in renal transplant recipients: a randomized trial. Transplantation. 2013;95(2):e6-e10.
- Sanchez-Fructuoso A, Guirado L, Ruiz JC, Torregrosa V, Gonzalez E, Suarez ML, Gallego R, et al. Anemia control in kidney transplant patients treated with methoxy polyethylene glycol-epoetin beta (mircera): the Anemiatrans Group. Transplant Proc. 2010;42(8):2931-2934.
doi pubmed - El-Komy MH, Schmidt RL, Widness JA, Veng-Pedersen P. Differential pharmacokinetic analysis of in vivo erythropoietin receptor interaction with erythropoietin and continuous erythropoietin receptor activator in sheep. Biopharm Drug Dispos. 2011;32(5):276-288.
doi pubmed - Vadakke Madathil S, Coe LM, Casu C, Sitara D. Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis. Am J Pathol. 2014;184(3):827-841.
doi pubmed - Kempe DS, Ackermann TF, Fischer SS, Koka S, Boini KM, Mahmud H, Foller M, et al. Accelerated suicidal erythrocyte death in Klotho-deficient mice. Pflugers Arch. 2009;458(3):503-512.
doi pubmed - Lang F, Bissinger R, Abed M, Artunc F. Eryptosis - the neglected cause of anemia in end stage renal disease. Kidney Blood Press Res. 2017;42(4):749-760.
doi pubmed - Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018;19(1):285.
doi pubmed - Duman N, Uyanik A, Unsal A, Sezer S, Camsari T, Cirit M, Yilmaz ME, et al. Once-monthly continuous erythropoietin receptor activator (CERA) for haemoglobin maintenance in haemodialysis patients with chronic renal anaemia. Clin Kidney J. 2014;7(5):464-469.
doi pubmed - Levin NW, Fishbane S, Canedo FV, Zeig S, Nassar GM, Moran JE, Villa G, et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA). Lancet. 2007;370(9596):1415-1421.
doi - Morgan DL, Peck SD. The use of red cell distribution width in the detection of iron deficiency in chronic hemodialysis patients. Am J Clin Pathol. 1988;89(4):513-515.
doi pubmed - Afsar B, Saglam M, Yuceturk C, Agca E. The relationship between red cell distribution width with erythropoietin resistance in iron replete hemodialysis patients. Eur J Intern Med. 2013;24(3):e25-29.
doi pubmed - Bojakowski K, Dzabic M, Kurzejamska E, Styczynski G, Andziak P, Gaciong Z, Soderberg-Naucler C, et al. A high red blood cell distribution width predicts failure of arteriovenous fistula. PLoS One. 2012;7(5):e36482.
doi pubmed - Vashistha T, Streja E, Molnar MZ, Rhee CM, Moradi H, Soohoo M, Kovesdy CP, et al. Red cell distribution width and mortality in hemodialysis patients. Am J Kidney Dis. 2016;68(1):110-121.
doi pubmed - Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost. 2010;36(5):537-549.
doi pubmed - Forni V, Bianchi G, Ogna A, Salvade I, Vuistiner P, Burnier M, Gabutti L. Reticulocyte dynamic and hemoglobin variability in hemodialysis patients treated with Darbepoetin alfa and C.E.R.A.: a randomized controlled trial. BMC Nephrol. 2013;14:157.
doi pubmed - Onuma S, Honda H, Kobayashi Y, Yamamoto T, Michihata T, Shibagaki K, Yuza T, et al. Effects of long-term erythropoiesis-stimulating agents on iron metabolism in patients on hemodialysis. Ther Apher Dial. 2015;19(6):582-589.
doi pubmed - Jonckheere S, Dierick J, Vanhouteghem H, Devleeschouwer M, Stove V. Erythrocyte indices in the assessment of iron status in dialysis-dependent patients with end-stage renal disease on continuous erythropoietin receptor activator versus epoetin beta therapy. Acta Haematol. 2010;124(1):27-33.
doi pubmed - Ycas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: A biomarker of hypoxemia? Clin Chim Acta. 2015;448:107-117.
doi pubmed - Sasaki Y, Noguchi-Sasaki M, Matsuo-Tezuka Y, Matsumoto-Omori Y, Kurasawa M, Yorozu K, Shimonaka Y. Epoetin beta pegol (C.E.R.A.) promotes utilization of iron for erythropoiesis through intensive suppression of serum hepcidin levels in mice. Int J Hematol. 2014;99(5):561-569.
doi pubmed - Marcais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, Genet L, et al. Circulating klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. 2017;102(9):3154-3161.
doi pubmed - Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, Vasilikos V, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019;20(1):217.
doi pubmed - Fukasawa H, Ishibuchi K, Kaneko M, Niwa H, Yasuda H, Kumagai H, Furuya R. Red blood cell distribution width is associated with all-cause and cardiovascular mortality in hemodialysis patients. Ther Apher Dial. 2017;21(6):565-571.
doi pubmed - Chen X, Shen B, Zou J, Liu Z, Lv W, Cao X, Yu J, et al. The prognostic value of red blood cell distribution width in patients on maintenance hemodialysis. Blood Purif. 2016;42(4):314-321.
doi pubmed - Hahn D, Cody JD, Hodson EM. Frequency of administration of erythropoiesis-stimulating agents for the anaemia of end-stage kidney disease in dialysis patients. Cochrane Database Syst Rev. 2014;5:CD003895.
doi pubmed - Sakaguchi Y, Hamano T, Wada A, Masakane I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol. 2019;30(6):1037-1048.
doi pubmed - Locatelli F, Hannedouche T, Fishbane S, Morgan Z, Oguey D, White WB. Cardiovascular safety and all-cause mortality of methoxy polyethylene glycol-epoetin beta and other erythropoiesis-stimulating agents in anemia of CKD: a randomized noninferiority trial. Clin J Am Soc Nephrol. 2019;14(12):1701-1710.
doi pubmed - Karaboyas A, Port FK, Massy ZA, Locatelli F, Cases A, Nitta K, Liabeuf S, et al. Long-versus short-acting erythropoiesis-stimulating agent type and mortality. Kidney Int Rep. 2021;6(1):214-218.
doi pubmed - Koppensteiner R, Stockenhuber F, Jahn C, Balcke P, Minar E, Ehringer H. Changes in determinants of blood rheology during treatment with haemodialysis and recombinant human erythropoietin. BMJ. 1990;300(6740):1626-1627.
doi pubmed - Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol. 2009;4(2):470-480.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


