| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 6, December 2021, pages 266-273
Pulmonary Toxicity of Hodgkin Lymphoma Treatment: A Prospective Single-Center Study
Adam Jonaa, d, Zsofia Miltenyia, Laszlo Pinczesa, Patricia Kereka, Nora Bittnerb, Maria Szilasib, Sandor Barnac, Arpad Illesa
aDepartment of Hematology, Faculty of Medicine, Medical School of Clinical Medicine, University of Debrecen, Hungary
bDepartment of Pulmonology, Faculty of Medicine, University of Debrecen, Hungary
cScanomed Ltd, Debrecen, Hungary
dCorresponding Author: Adam Jona, Department of Hematology, Faculty of Medicine, University of Debrecen, Nagyerdei krt. 98, Debrecen, Hungary
Manuscript submitted October 12, 2021, accepted November 17, 2021, published online December 21, 2021
Short title: Pulmonary Toxicity of HL Treatment
doi: https://doi.org/10.14740/jh929
| Abstract | ▴Top |
Background: Standard bleomycin-containing first-line therapy and/or irradiation may cause pulmonary toxicity in Hodgkin lymphoma (HL) patients. Our aim was to prospectively assess effects of chest irradiation, bleomycin administration, and other factors on lung function in the treatment of patients with HL.
Methods: Pulmonary function of newly diagnosed HL patients was assessed via a St. George Respiratory Questionnaire, dynamic inhalation lung scintigraphy, spirometry, and an assessment of the diffusion capacity of the lung for carbon monoxide (DLCO) before, during, and after treatment.
Results: This prospective study was conducted at the University of Debrecen. The study included 84 patients with classical HL. Most patients received standard doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy. Both intramuscular and intravenous administrations of bleomycin were used. Brentuximab vedotin combination chemotherapy was administered to 12 patients. Mediastinal involved-field irradiation therapy (IFRT) was used to treat 16 patients. Lung scintigraphy revealed pulmonary toxicity more sensitively than DLCO. Intravenous bleomycin administration decreased diethylenetriamine pentaacetic acid clearance. Intramuscular bleomycin had the lowest level of pulmonary toxicity among considered treatments. Currently used, mediastinal IFRT had a lower level of pulmonary toxicity than bleomycin. The current prospective evaluation confirmed previous results that determined that cumulative bleomycin dose and administration are major risk factors for pulmonary toxicity, while the currently used treatment method, mediastinal irradiation, was determined to be relatively safe for treating for HL patients.
Conclusion: We agree with decreasing bleomycin dosage and number of cycles administered and we do not recommend avoiding mediastinal IFRT, unless multiple pulmonary risk factors are present.
Keywords: Hodgkin lymphoma; Pulmonary toxicity; Bleomycin; Irradiation; Brentuximab vedotin; Scintigraphy; Pulmonary fibrosis; Combination therapy
| Introduction | ▴Top |
Hodgkin lymphoma (HL) is a malignancy of the lymphatic system, which accounts for 12-18% of all lymphomas [1]. HL is a success story in the hematology field, with more than 80% of those initially suffering from the fatal disease now becoming long-term survivors. The first-line treatment of the disease is typically bleomycin-containing polychemotherapy. In Hungary, we prefer the use of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) protocol in a number of stage- and prognosis-dependent cycles, which may be supplemented by involved-field irradiation therapy (IFRT).
Previously, the primary goal of treatment was to improve patient survival. Currently, 80-85% of patients are expected to survive in long term. Thus, long-term side effects of treatments have become increasingly important. Therefore, it is essential to avoid under- or overtreatment. In this study, we prospectively evaluated pulmonary side effects observed in HL patients.
Our aim was to prospectively assess effects of chest irradiation, bleomycin dosage and administration route, and other factors on lung function in the treatment of patients with HL.
| Materials and Methods | ▴Top |
Pulmonary complications of HL patients treated at the Department of Hematology, University of Debrecen (UD) were evaluated between February 2013 and April 2019. Patients were informed of examinations required for the surveys, and approval of the research ethics committee of the University of Debrecen was given (DE KK RKEB/IKEB 5192-2021). After obtaining informed consent from patients, we collected data on sex, age at diagnosis, the Lugano classification stage, WHO 2016 histological subtype, presence of B-type symptoms (fever, sweating, and weight loss), treatment modality (chemotherapy, combined chemoradiotherapy), dose of bleomycin received, irradiation dose, and region treated via radiotherapy [2]. In the early stage, HL patients with a favorable prognosis received 2 - 4 cycles of ABVD + IFRT. In the cases involving young women or those with coronary disease, 4 - 6 cycles of ABVD without IFRT was preferred. Early-stage HL patients with an unfavorable prognosis received 4 - 6 cycles of ABVD + IFRT. Patients with advanced stage HL received 6 - 8 cycles of ABVD or brentuximab vedotin, doxorubicin, vinblastine, dacarbazine (BV-AVD). Each cycle of ABVD consisted of 20 mg/m2 bleomycin. If the patient had an initial bulky disease or positron emission tomography (PET)-positive residual disease, IFRT was administered. One elderly patient received epirubicin, vinblastine, and dacarbazine combination therapy [3].
The current patient cohort consisted of patients from two departments. One department administered bleomycin intramuscularly, while the other administered the therapy intravenously. Symptoms were assessed using the St. George Respiratory Questionnaire (SGRQ), spirometry, DLCO, and dynamic inhalation pulmonary scintigraphy results before treatment (staging time point), after two cycles of treatment (interim time point), and at the time of the restaging PET/computed tomography (CT). Evaluation was done 8 weeks after completion of irradiation if applicable. The timeframe of the toxicity investigation was designed to fit the standard PET/CT imaging workup. Follow-up of signs of pulmonary toxicity was not performed after survey completion.
The SGRQ for lung disease consists of three groups of questions: symptom, activity, mobility, and impact scores, which can be used to calculate a weighted score (total score). Weight values range from 0 to 100, with higher values indicating lower quality of life, and values above 10 considered pathological. Dynamic inhalation pulmonary scintigraphy was performed at Scanomed Ltd, and spirometry, and DLCO (Piston PDT-111/p) were performed at the Department of Pulmonology, UD, to investigate functional changes in lung health. The main static (total lung capacity (TLC) and dynamic forced vital capacity (FVC), forced expiratory volume (FEV1), and Tiffeneau index (FEV1/VC)) measurements were collected. From diffusion capacity data, the transfer factor of the lung for CO and the transfer coefficient for CO were determined. Spirometric results were expressed as percentages of those of age-matched healthy controls. Alterations in 80% of patients assessed were mild, 50-79% moderate, 30-49% severe, and < 30% very severe. Severity levels were determined according to guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [4]. The severity of DLCO reduction is classified as follows: normal DLCO, > 75% of predicted, up to 140%; mild, 60% of the lower limit of normal (LLN); moderate, 40-60%; and severe, < 40% [5].
Dynamic inhalation pulmonary scintigraphy was used to reveal membrane lesions. It was selected because it was shown to be a sensitive tool in the early identification of pulmonary alterations [6]. The elimination of diethylenetriamine pentaacetic acid (DTPA) was used to reveal elimination time. Results obtained were compared with those of age and lung surface-matched healthy controls, and test results that were lower than normal were considered positive. The lower this result, the more severe the membrane lesion.
The relationship between bleomycin use and chest irradiation was evaluated. We investigated the role of previously published pulmonary risk factors (age, renal impairment, smoking, route of bleomycin administration (intravenous (IV) or intramuscular (IM)), and granulocyte colony-stimulating factor (G-CSF)). Statistical calculations were performed using SPSS version 22.0, and figures were created using GraphPad Prism version 7.0. Mann-Whitney, Wilcoxon, Kruskal-Wallis tests, linear regression analysis and repeated-measures analysis of variance (ANOVA) were used. Values of P < 0.05 were considered significant.
| Results | ▴Top |
A total of 84 patients with classical HL were enrolled in the study. The majority of patients received bleomycin-containing chemotherapy (ABVD regimen). The median bleomycin dose administered was 120 mg/m2 (range: 40 - 140 mg), which determined as described in the “Materials and Methods” section according to disease stage. Distributions of patients of age, each disease stage, bleomycin dose, renal function, smoking status, and pulmonary involvement level were comparable among the subgroups considered; however, significantly more patients received mediastinal IFRT and had bulky disease in the IM group, and significantly more patients of the bleomycin group received G-CSF support. Patient characteristics are described in Table 1. Kruskal-Wallis test was used to compare subgroups.
 Click to view | Table 1. Patient Characteristics |
Lung scintigraphy most sensitively revealed pulmonary toxicity in above-described HL patients. IV bleomycin administration decreased DTPA clearance versus baseline restaging results (right and left side: P = 0.0001*). IM bleomycin administration decreased DTPA clearance versus baseline restaging results (right side: P = 0.0425*, left side: P = 0.0962). Patients who received no bleomycin (BV-AVD) experienced a significant decrease in DTPA clearance compared to baseline (Fig. 1). Wilcoxon test was used to compare timepoints, and Kruskal-Wallis test was used to compare treatment methods. As cumulative bleomycin dose increased, scintigraphy results worsened (Fig. 2). A linear regression analysis was used to compare bleomycin dose and DTPA clearance change.
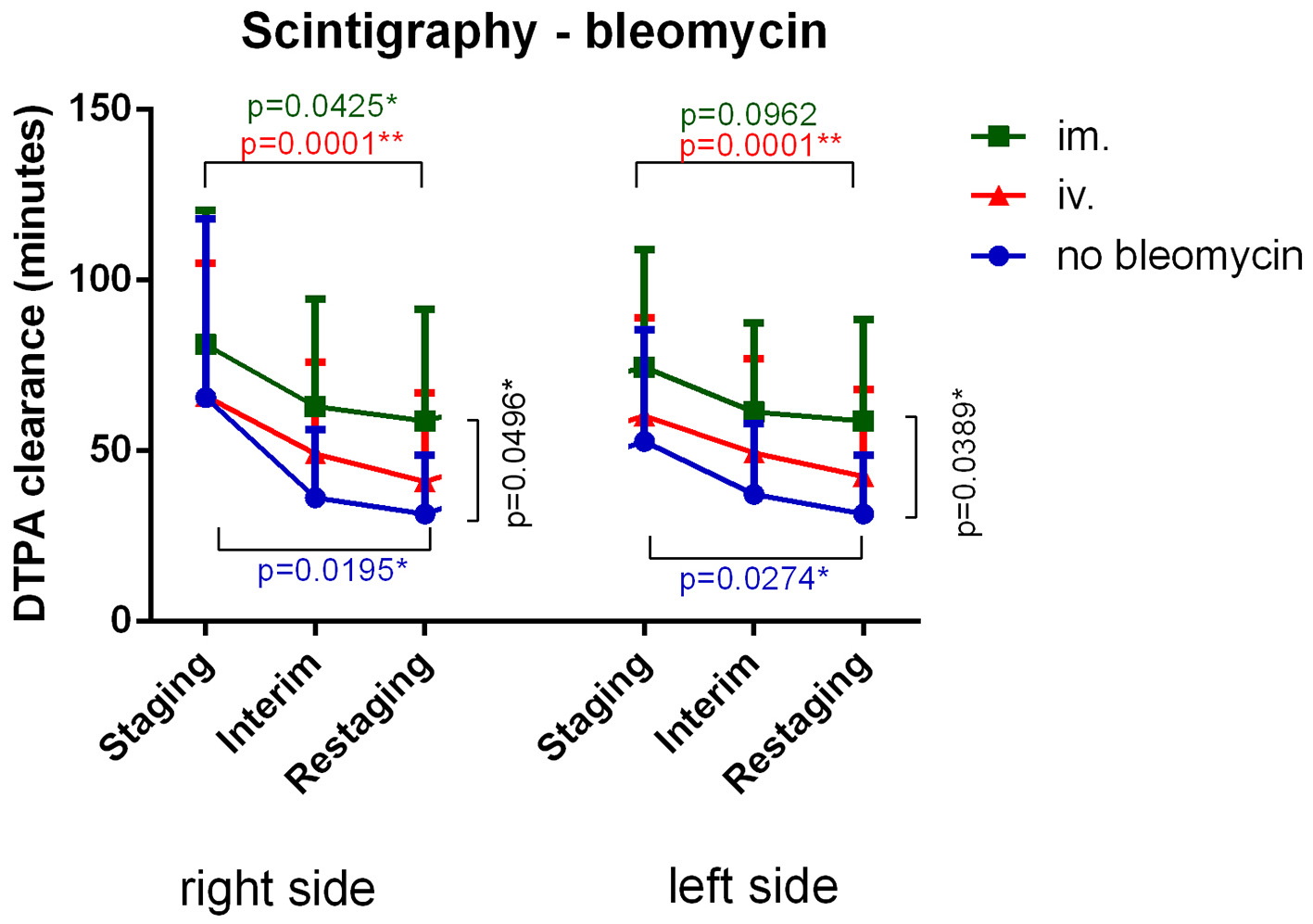 Click for large image | Figure 1. Intravenous bleomycin administration most significantly decreased DTPA clearance versus baseline. Intramuscular bleomycin administration lessened DTPA clearance decline. Patients who were not given bleomycin still experienced a significant decrease in DTPA clearance. DTPA: diethylenetriamine pentaacetic acid; IM: intramuscular; IV: intravenous. |
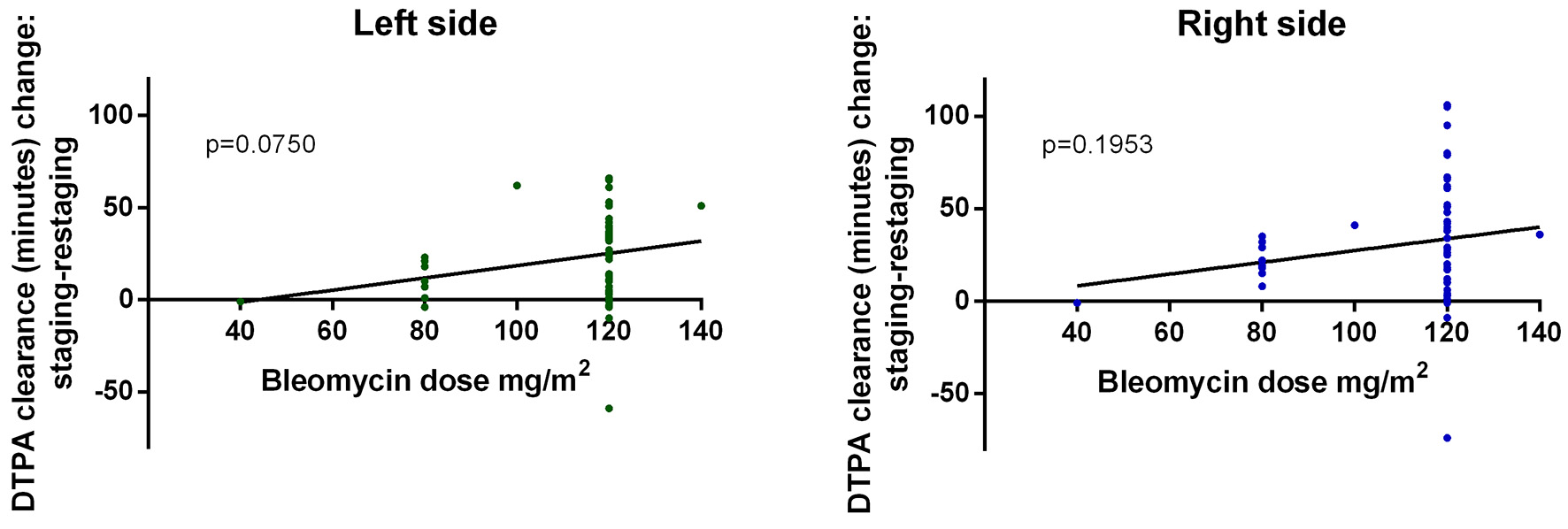 Click for large image | Figure 2. Scintigraphy results continuously worsened as cumulative bleomycin dose increased. DTPA: diethylenetriamine pentaacetic acid. |
Gold standard DLCO worsened significantly between staging and interim time points in all patients except those receiving IM administration; however, pulmonary function normalized by the restaging time point (Fig. 3a). Wilcoxon test was used to compare timepoints. SGRQ scores decreased over time. Accordingly, subjective patient symptoms generally improved, but differences were not significant over time (Fig. 3b). Wilcoxon test was used to compare timepoints. Clinically apparent bleomycin-induced toxicity occurred in three cases. In these cases, bleomycin use was canceled, and 1 mg/kg/day methyl prednisone was administered for 3 days and then tapered, and clinical symptoms fully improved.
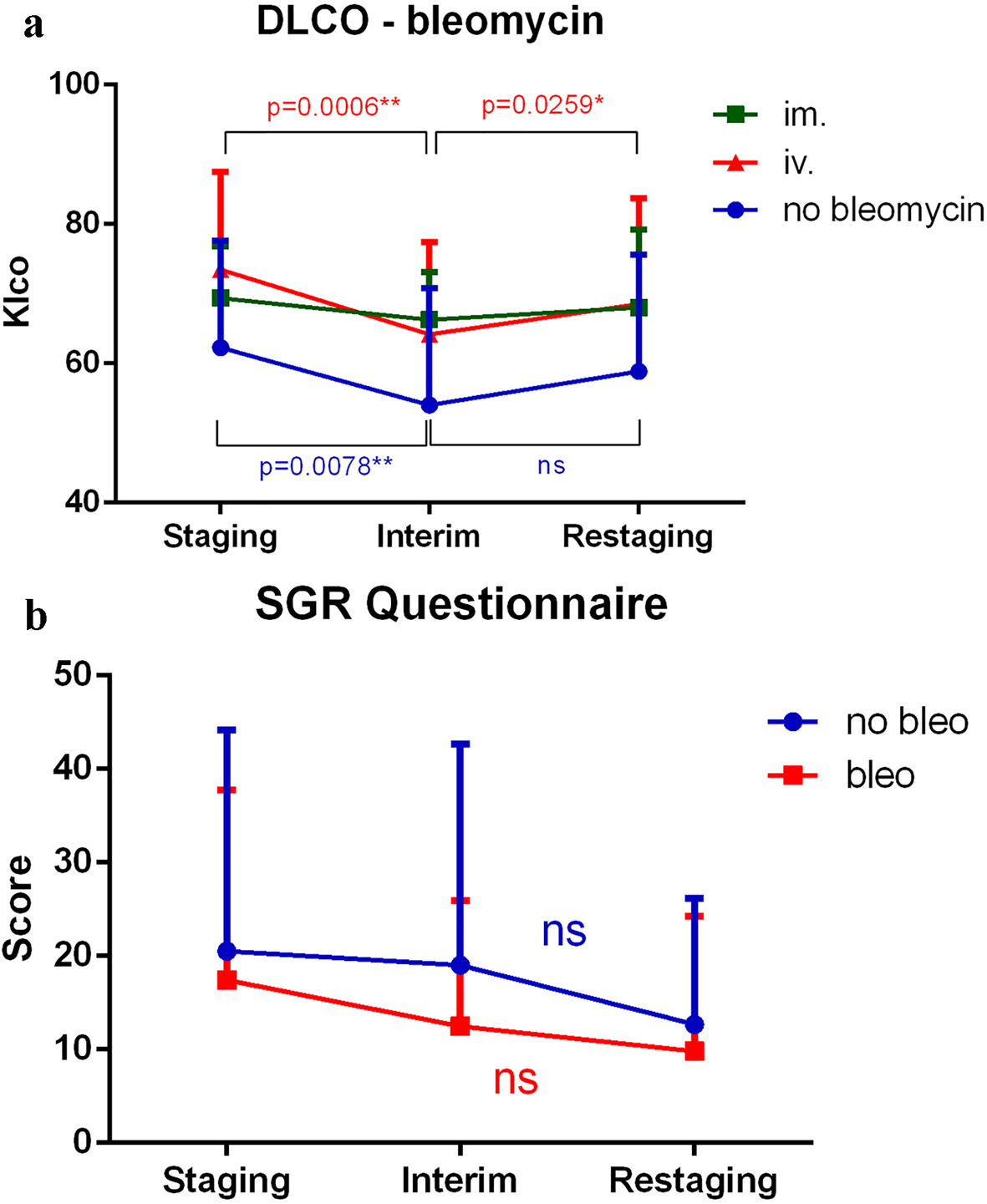 Click for large image | Figure 3. (a) DLCO significantly worsened between staging and interim time points when all treatment modalities were applied except intramuscular administration; however, pulmonary function normalized by the restaging time point. (b) SGRQ scores decreased, and therefore, subjective patient symptoms generally improved throughout treatment. However, the differences were not significant. SGRQ: St. George Respiratory Questionnaire; DTPA: diethylenetriamine pentaacetic acid; DLCO: carbon monoxide diffusion capacity of the lung; Klco: transfer coefficient of the lung for carbon monoxide; IM: intramuscular; IV: intravenous. |
Mediastinal IFRT caused a significant degree of pulmonary toxicity, which was revealed via lung scintigraphy. However, changes observed in the no-irradiation subgroup were more remarkable. Changes in DLCO were not significant. SGRQ scores decreased, and subjective patient symptoms improved during treatment. The difference between mediastinal and non-mediastinal irradiation groups was significant (Fig. 4). Wilcoxon test was used to compare timepoints, and Mann-Whitney test was used to compare treatment methods. Spirometry results did not significantly change during treatment, and no differences were observed when comparing IV, IM and no bleomycin use and the presence or absence of mediastinal irradiation (Fig. 5a, b).
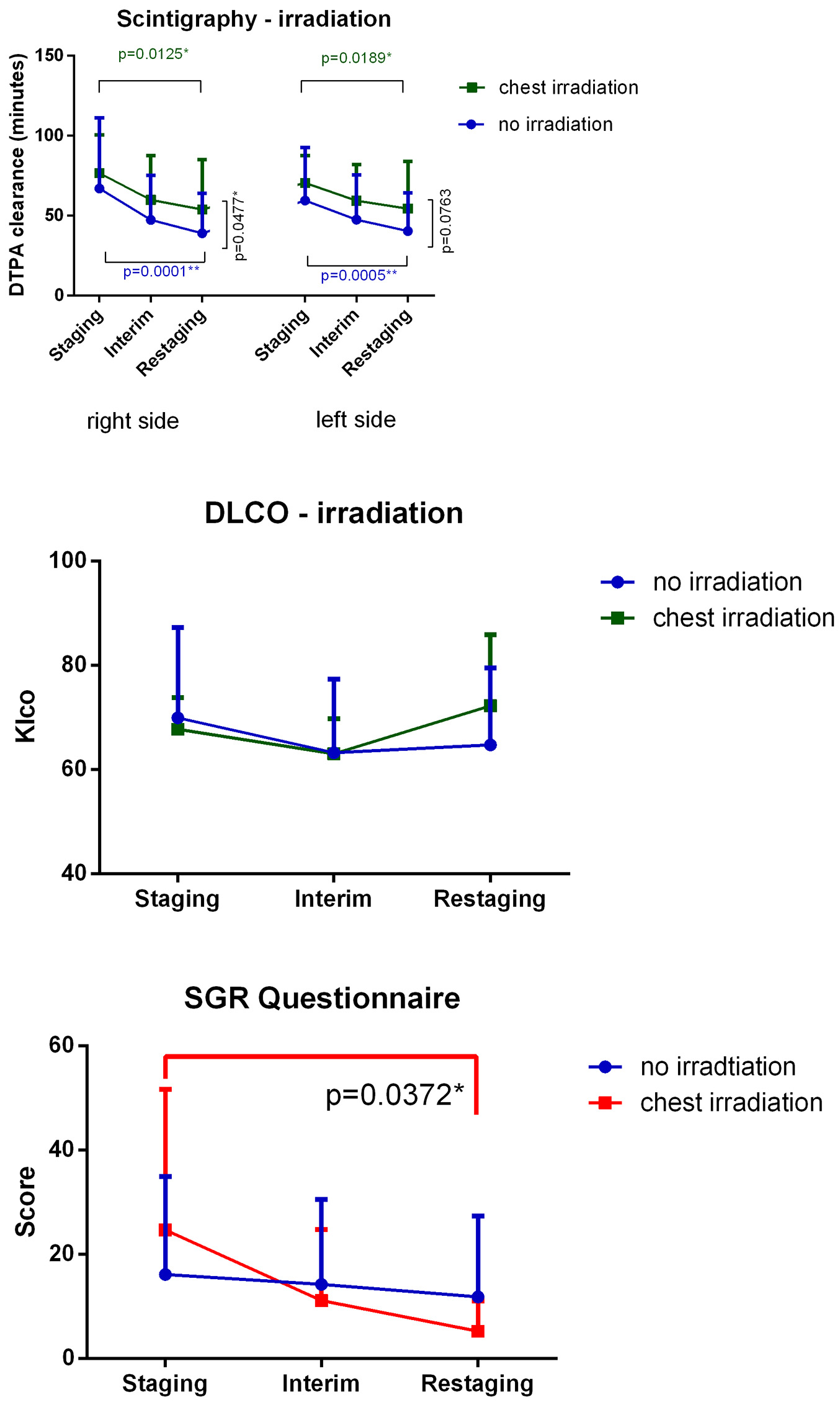 Click for large image | Figure 4. Mediastinal IFRT caused a significant degree of pulmonary toxicity. Findings evaluated via lung scintigraphy are shown. Changes observed in the no irradiation subgroup were greater than those observed in other groups. Changes in DLCO values were not significant. SGRQ scores decreased, and hence, subjective patient symptoms improved during treatment. Differences observed in the mediastinal irradiation group was significant. DTPA: diethylenetriamine pentaacetic acid; DLCO: carbon monoxide diffusion capacity of the lung; IFRT: involved-field irradiation therapy; Klco: transfer coefficient of the lung for carbon monoxide. |
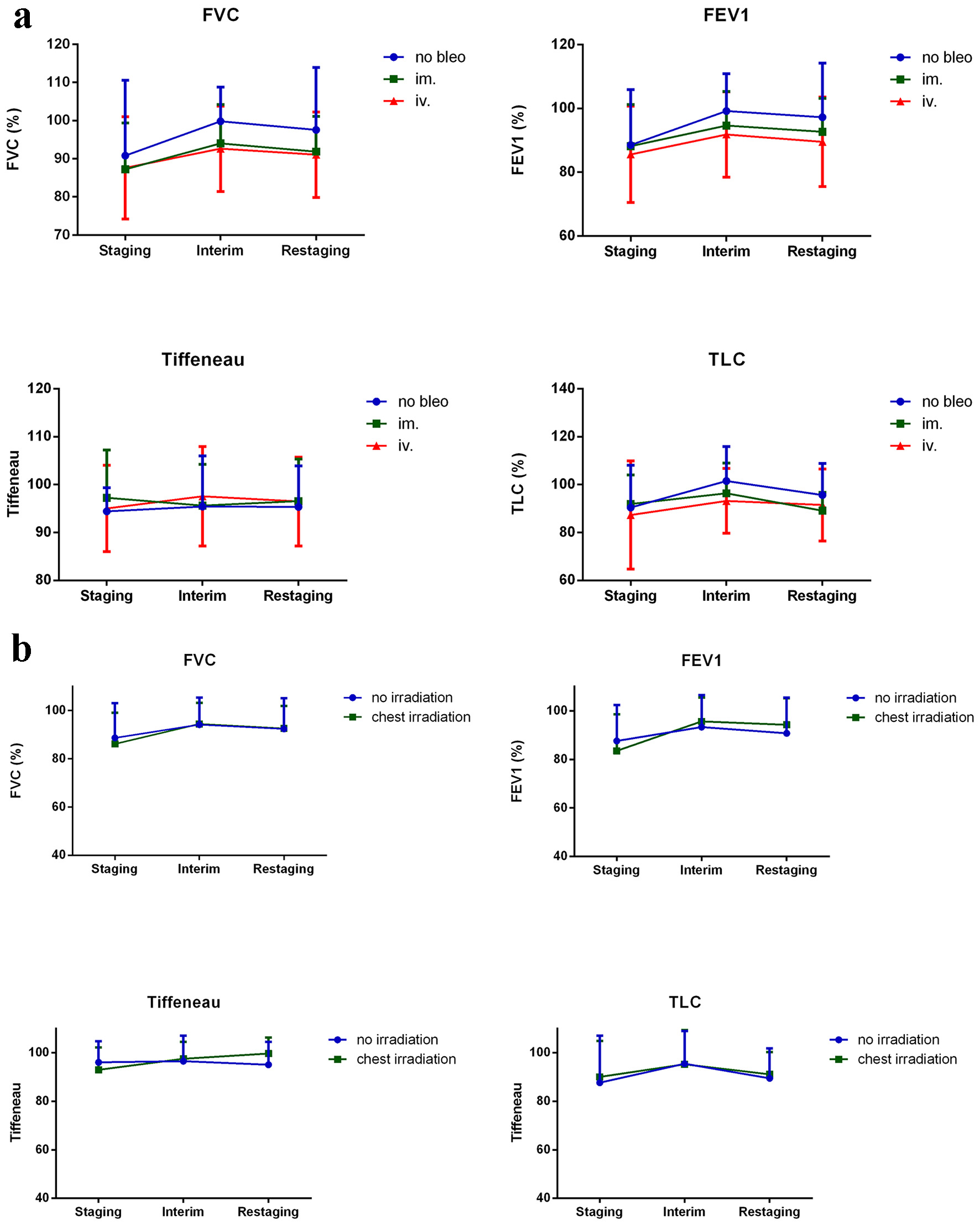 Click for large image | Figure 5. Spirometry results that did not significantly change during treatment are shown. (a) No differences in terms of bleomycin administration and (b) the presence or absence of mediastinal irradiation were observed. Results are shown as percentages of age-matched healthy controls. TLC: total lung capacity; FVC: forced vital capacity; FEV1: forced expiratory volume; Tiffeneau index: FEV1/vital capacity. |
Repeated ANOVA was used to identify additional risk factors of pulmonary toxicity. Mediastinal IFRT affected SGRQ results (P = 0.030*). Among the spirometry results considered, bleomycin use affected FVC and TLC. Pulmonary involvement affected FEV1 (P = 0.040*), whereas smoking affected Tiffeneau values (P = 0.048*). No correlation between pulmonary toxicity and other investigated factors including stage, presence of B-symptoms, or use of G-CSF was identified.
| Discussion | ▴Top |
Current standard first-line bleomycin-containing polychemotherapies (ABVD, BEACOPP) and chest IFRT have the potential to restrict lung capacity. Bleomycin was discovered in 1966 and was first isolated from Streptomyces verticillus. It was later used as a chemotherapeutic agent because of its capacity to generate free radicals [7]. Bleomycin is predominantly excreted by the kidneys, but may also be eliminated by a specific bleomycin deactivating enzyme, bleomycin hydrolase (BLMH) [8]. Despite its chemotherapeutic efficacy, its extensive use is limited by its potentially life-threatening capacity to damage the lungs [9]. Its toxic effects are due to its ability to induce free radical formation. Specific effects observed in the lungs have been attributed to their lack of BLMH. Bleomycin-induced lung injuries range from hypersensitive pneumonitis to interstitial pneumonia and progressive pulmonary fibrosis.
In the past, pulmonary toxicity associated with chest radiotherapy was likely the most common cause of lung damage. Irradiation pneumonitis typically occurs 1 - 3 months after irradiation. The likelihood of its development depends on the size of the irradiated lung, the type of irradiation used, and daily and total doses delivered [10].
In this prospective report, we confirmed findings of one of our previous reports [6]. The report retrospectively evaluated late pulmonary complications of HL treatment after a median follow-up of 11 years, and determined via lung scintigraphy, DLCO, or SGRQ that currently used mediastinal IFRT did not significantly contribute to pulmonary damage. However, increasing the cumulative bleomycin dose worsened pulmonary function, especially when the drug was administered intravenously.
This current prospective evaluation also confirmed prior findings that identified significant factors contributing to pulmonary injury. Intramuscular bleomycin administration was shown to cause the lowest degree of pulmonary toxicity among treatments evaluated (even when considering that patients in this subgroup had more bulky disease, and hence, underwent more mediastinal IFRT than other patients). The suggested dose of bleomycin is 10 mg/m2 administered intravenously or intramuscularly. The half-life of bleomycin when administered intravenously and intramuscularly is similar; however, the peak concentration obtained intravenously is only 10% of the peak value reached via intramuscular administration [11]. Interestingly, patients with no bleomycin use experienced significant pulmonary toxicity, which raises the possibility that BV possesses pulmonary toxicity. Lung scintigraphy showed pulmonary toxicity more sensitively and irreversibly than DLCO.
A recent study from the Mayo Clinic reported the impact of pulmonary bleomycin toxicity on survival [12]. They reported that the incidence of bleomycin pulmonary toxicity decreased over time. Findings agree with current trends in which bleomycin dosage and number of cycles administered are decreased. Based on our results, we agree with the application of this approach. Since chest irradiation does not significantly cause pulmonary side effects, we do not recommend avoiding IFRT based on these observations, unless multiple pulmonary risk factors are present. However, increasing the cumulative bleomycin dose may cause significant pulmonary side effects. Our results are in accordance with current ideas that indicate that chemotherapy cycles should be reduced as much as possible while maintaining favorable survival rates [13]. A limitation of our study is that, from a clinical point of view, subjective patient symptoms do not support clinical findings. The relatively low number of cases considered, the lack of follow-up of our patients and using not gold standard instruments (lung scintigraphy) to evaluate pulmonary toxicity are limitations, and further studies addressing these shortcomings should be complemented in the future.
A slightly different approach was used in a recent, large, randomized, controlled non-inferiority trial, which did show that omitting bleomycin (treated with AVD between cycles 3 and 6) decreases efficacy after a negative interim PET/CT scan taken after two cycles of ABVD in newly diagnosed advanced-stage HL patients. However, a lower incidence of bleomycin pulmonary toxicity was observed in patients for whom bleomycin was omitted versus those who continued with the standard ABVD therapy [14].
In conclusion, increasing the cumulative bleomycin dose worsened pulmonary function, especially when the drug was administered intravenously. Therefore, we think our observation confirms current trends in which bleomycin dosage and number of cycles administered are decreased. Since currently used mediastinal IFRT did not significantly contribute to pulmonary damage, we do not recommend avoiding IFRT based on these observations.
Acknowledgments
The authors thank Aniko Sapi and Kalmanne Csapo for organizing and performing the examinations.
Financial Disclosure
AJ was supported by the UNKP-20-4 New National Excellence Program of the Ministry for Innovation and Technology of the National Research, Development and Innovation Fund.
Conflict of Interest
Author Sandor Barna is employed by Scanomed Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Patients provided written informed consent.
Author Contributions
AJ designed the study, analyzed data, and wrote the manuscript; ZM designed the study and analyzed data; LP analyzed data; PK collected and analyzed data; NB contributed essential examinations to the study; MS contributed essential examinations to the study; SB contributed essential examinations to the study; AI designed the study; and approved the final manuscript. All authors read and approved the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Villasboas JC, Ansell SM. Recent advances in the management of Hodgkin lymphoma. F1000Res. 2016;5(F1000 Faculty Rev):768.
doi pubmed - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Illes A, Molnar Z. State of the art treatment of hematological diseases [Hematologiai betegsegek korszeru kezelese]. In: Lehocky D, editor. State of the art treatment of hematological diseases [Hematologiai betegsegek korszeru kezelese]. Budapest: Zafir Press; 2011; p. 285-309.
- Hess MW. The 2017 global initiative for chronic obstructive lung disease report and practice implications for the respiratory therapist. Respir Care. 2017;62(11):1492-1500.
doi pubmed - Ponce MC, Sharma S. Pulmonary Function Tests. StatPearls [Internet]. 2019. https://www.ncbi.nlm.nih.gov/books/NBK482339/.
- Azambuja E, Fleck JF, Menna Barreto SS, Cunha RD, Batista RG. Bleomycin lung toxicity detected by technetium-99m diethylene triamine penta-acetic acid aerosol (99mTc-DTPA) scintigraphy. J Clin Oncol. 2004;22(14_suppl):4600-4600.
doi - Umezawa H, Maeda K, Takeuchi T, Okami Y. New antibiotics, bleomycin A and B. J Antibiot (Tokyo). 1966;19(5):200-209.
- Ferrando AA, Velasco G, Campo E, Lopez-Otin C. Cloning and expression analysis of human bleomycin hydrolase, a cysteine proteinase involved in chemotherapy resistance. Cancer Res. 1996;56(8):1746-1750.
- Froudarakis M, Hatzimichael E, Kyriazopoulou L, Lagos K, Pappas P, Tzakos AG, Karavasilis V, et al. Revisiting bleomycin from pathophysiology to safe clinical use. Crit Rev Oncol Hematol. 2013;87(1):90-100.
doi pubmed - Koh ES, Sun A, Tran TH, Tsang R, Pintilie M, Hodgson DC, Wells W, et al. Clinical dose-volume histogram analysis in predicting radiation pneumonitis in Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2006;66(1):223-228.
doi pubmed - Oken MM, Crooke ST, Elson MK, Strong JE, Shafer RB. Pharmacokinetics of bleomycin after im administration in man. Cancer Treat Rep. 1981;65(5-6):485-489.
- Taparra K, Liu H, Polley MY, Ristow K, Habermann TM, Ansell SM. Bleomycin use in the treatment of Hodgkin lymphoma (HL): toxicity and outcomes in the modern era. Leuk Lymphoma. 2019;13:1-11.
doi pubmed - Behringer K, Goergen H, Hitz F, Zijlstra JM, Greil R, Markova J, Sasse S, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385(9976):1418-1427.
doi - Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, d'Amore F, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374(25):2419-2429.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


