| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 1, February 2022, pages 34-39
Severe Aplastic Anemia After Receiving SARS-CoV-2 Moderna mRNA Vaccination
Srilekha Sridharaa, c, Resmi Naira, Michael Stanekb
aBanner Baywood Medical Center, Mesa, AZ 85206, USA
bBanner MD Anderson Cancer Center at Banner Gateway Medical Center, Gilbert, AZ 85234, USA
cCorresponding Author: Srilekha Sridhara, Banner Baywood Medical Center, Mesa, AZ 85206, USA
Manuscript submitted November 26, 2021, accepted January 6, 2022, published online February 26, 2022
Short title: Aplastic Anemia After SARS-CoV-2 Vaccination
doi: https://doi.org/10.14740/jh954
| Abstract | ▴Top |
A 60-year-old male patient presented to the emergency department with complaints of easy bruising and worsening epistaxis after receiving severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Moderna mRNA vaccination. He had no personal or family history of hematological conditions. He had bruises in various stages involving the upper and lower extremities. Laboratory data revealed white blood cell count of 1.2 ×103/mm3, hemoglobin of 8.0 g/dL, platelet count of 1 ×103/mm3, immature platelet fraction of 0.7%, absolute neutrophil count of 0 ×103/µL, lymphocytes of 1.1 ×103/µL, neutrophils of 3% and lymphocytes of 93%. He had normal liver and renal function tests. Bone marrow biopsy confirmed very severe aplastic anemia with severely hypocellular bone marrow. His platelets continued to downtrend despite platelet transfusions and steroids. He was treated with immunosuppressive therapy with cyclosporine, anti-thymocyte globulin, eltrombopag and prednisone. The patient was discharged but was readmitted to the hospital secondary to recurrent neutropenic fever and pneumonia. He had high-grade vancomycin-resistant enterococcal infection and Clostridium difficile infection leading to septic shock and succumbing to cardiac arrest. This case demonstrates the possibility of very severe aplastic anemia following SARS-CoV-2 mRNA vaccination and clinicians need to be aware of this rare but serious side effect.
Keywords: SARS-CoV-2 vaccination; Aplastic anemia; Sepsis
| Introduction | ▴Top |
Aplastic anemia (AA) is a rare hematologic disorder that affects 2 - 4 persons per million per year [1-5]. AA is characterized by pancytopenia with loss of hematopoietic stem cells (HSCs), progenitor cells, and precursor cells in the bone marrow and bone marrow biopsy is essential for diagnosis of AA [4]. For risk stratification and therapy selection, AA is classified as severe AA (SAA), very severe AA (vSAA) and non-severe AA according to the following criteria [6].
Diagnosis of SAA requires both of the following criteria: 1) Bone marrow cellularity < 25% (or 25-50% if < 30% of residual cells are hematopoietic); and 2) At least two of the following: a) Peripheral blood absolute neutrophil count (ANC) of < 500/µL (< 0.5 × 109/L); b) Peripheral blood platelet count of < 20,000/µL; and c) Peripheral blood reticulocyte count of < 20,000/µL. Diagnosis of vSAA includes the criteria for SAA (above) and ANC of < 200/µL. Criteria for non-severe AA are: 1) Hypocellular bone marrow (as described for SAA); and 2) Peripheral blood cytopenias not fulfilling criteria for SAA or vSAA. AA has been reported after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [7]. A causative association between the SARS-CoV-2 mRNA vaccinations and AA is possible, and clinicians should be aware of this rare but serious adverse event.
| Case Report | ▴Top |
A 60-year-old male patient received the second dose of SARS-CoV-2 Moderna mRNA vaccination and experienced easy bruising on his arms and legs the following day after vaccination. After 2 weeks, he presented to the emergency department with worsening epistaxis but did not have a fever, chest pain, cough, shortness of breath or abdominal pain. He denied any significant past medical history such as diabetes mellitus or renal disease or autoimmune diseases and he did not routinely take any medications. He was not a smoker, and rarely consumed alcohol. There was no family history of any malignancy or hematological conditions. He worked for the city installing septic tanks and had no exposure to any chemicals or toxins. On exam, he was resting in the bed without distress, temperature was 37.3 °C, pulse rate was 80/min and blood pressure was 125/71 mm Hg. He had a nasal packing with no active bleeding and oral mucosa showed no petechiae. On neck exam, he did not have any palpable lymph nodes. The chest was clear to auscultation and the abdomen exam did not reveal any hepatosplenomegaly. On integumentary exam, he had bruises in various stages involving the upper and lower extremities with a dominant bruise in the left upper arm and left thigh without any appreciable petechiae.
Laboratory data showed a white blood cell count of 1.2 × 103/mm3, hemoglobin of 8.0 g/dL, platelet count of 1 × 103/mm3, immature platelet fraction (IPF) of 0.7%, reticulocyte count (RETC) of 4 × 103/µL, absolute neutrophil count (ANC) of 0 × 103/µL, and monocytes of 0.0 × 103/µL. He had normal renal function, electrolytes, and hepatic function. Lactate dehydrogenase (LDH) was 203 U/L, haptoglobin was 242 mg/dL and fibrinogen was 478 mg/dL (Table 1).
 Click to view | Table 1. Laboratory Data at the Time of Initial Hospitalization |
Peripheral smear showed pancytopenia with a marked decrease in granulocytes, normocytic anemia with non-specific anisocytosis, thrombocytopenia with unremarkable platelets and there were no schistocytes. Lymphocytes with mature chromatin, abundant cytoplasm and occasional forms with concentric irregular cytoplasmic projections concerning an atypical population were present. On a subsequent peripheral smear pancytopenia was again noted without abnormal red or white blood cells.
He was admitted to the inpatient service and was evaluated by hematology/oncology and infectious diseases services. Initial blood cultures and urine cultures did not reveal any bacterial growth. Given neutropenic fever antibiotic prophylaxis was provided with cefepime, fluconazole and valacyclovir. Microbiology data revealed positive Epstein-Barr virus (EBV) viral capsid antigen (VCA) IgG index at 7.5 (reference index is < 0.8) with positive IgG, nuclear antigen index 7.6 (reference index is < 0.8) and IgG positive for cytomegalovirus (CMV). SARS-CoV-2 RNA was negative with a positive IgG index at greater than 20 (reference index is < 0.99) suggestive of recent vaccination. He tested negative for many microbiological tests as described: Coccidioides enzyme immunoassay IgM and IgG, parvo B19 DNA qualitative real-time polymerase chain reaction (PCR), herpes simplex virus (HSV) 1, 2 DNA real-time PCR, human immunodeficiency virus (HIV) 1/2 antigen, antibody screen-fourth-generation, EBV VCA IgM, CMV antibody IgM, IgG, hepatitis B surface antigen, core antibody, IgM, hepatitis C antibody, CMV qualitative PCR, EBV qualitative RT-PCR, adenovirus, human metapneumovirus, human rhinovirus, influenza A, influenza A/H1, influenza A/H3, influenza B, parainfluenza virus 1, 2, 3, 4, respiratory syncytial virus A, B, Streptococcus pneumoniae antigen and Legionella pneumococcal antibody.
Hematological workup results are as described. Serum protein electrophoresis demonstrated hypoalbuminemia; peripheral blood leukemia lymphoma panel showed no immunophenotypic evidence of lymphoproliferative disorder, acute leukemia, or plasma cell neoplasm. Antineutrophil cytoplasmic antibody (ANCA) screen was negative, antinuclear antibody and double-stranded DNA (dsDNA) at 42 IU/mL were detected with normal complements (dsDNA antibody reference index < 4 IU/mL). Peripheral blood paroxysmal nocturnal hemoglobinuria (PNH) evaluation with fluorescein-labeled proaerolysin (FLAER) revealed no flow cytometric evidence of PNH and no evidence of aberrant loss of glycosylphosphatidylinositol (GPI)-linked markers tested. T-cell receptor beta gene rearrangement was negative (Supplementary Material 1, www.thejh.org).
Bone marrow biopsy showed less than 5% overall cellularity. A few scattered lymphocytes, plasma cells and a rare red cell were identified. Immunohistochemical stains were performed to assist in the evaluation of residual marrow elements which identified scattered B cells, T cells, and plasma cells. Kappa and lambda were both present. The features were consistent with residual polyclonal lymphocytes and plasma cells. The clot section was composed entirely of blood without marrow elements. Bone marrow flow cytometry showed no immunophenotypic evidence of lymphoproliferative disorder, acute leukemia, or plasma cell neoplasm. Fluorescence in situ hybridization (FISH) for myelodysplastic syndrome (MDS) panel was normal and chromosome analysis showed normal male karyotype at the band level 350 as determined by the trypsin-Giemsa method. Next-generation sequencing was performed, and no relevant DNA mutation was detected. He met the criteria for vSAA with bone marrow cellularity of less than 5%, ANC of 0 × 103/µL, RETC of 4 × 103/µL (absolute) and platelet count of 1 × 103/mm3 (Supplementary Material 1, www.thejh.org).
He received platelet transfusions and based on the above workup was initiated on methylprednisolone with an initial improvement of platelet count to 89 × 103/mm3; however, platelet count down trended to 30 × 103/mm3 and white count remained at 1.3 × 103/mm3. He was transferred to oncology service at a tertiary care center for further evaluation and here he was initiated on immunosuppressive therapy with cyclosporine at 250 mg orally twice daily, lymphocyte immune globulin, anti-thy (equine) at 40 mg/kg/day (total daily dose of 3,200 mg intravenously every 24 h) for 4 days, and eltrombopag 150 mg PO daily. He was maintained on methylprednisolone and antibiotic prophylaxis with levofloxacin, fluconazole and valacyclovir. After completion of in-hospital therapies, he was discharged home on cyclosporine, eltrombopag and prednisone. His blood counts at the time of discharge were white cell count 0.1 × 103/mm3, hemoglobin 7.8 g/dL, platelet count 26 × 103/mm3, and IPF 0.1%.
Two days after discharge, the patient was evaluated in the clinic by the oncology team, and he was noted to be febrile with a temperature of 39.5 °C. He complained of left-sided chest pain and hemoptysis. Blood counts revealed white cell count of 0.1 × 103/mm3, hemoglobin of 8.4 g/dL, platelet count of 13 × 103/mm3, and IPF of 0.1%. He was readmitted to the hospital oncology service for management of neutropenic fever and pneumonia (Table 2).
 Click to view | Table 2. Comparative Laboratory Test Results |
Other investigations performed are as described. Computed tomography scans of the chest and abdomen revealed mass-like soft tissue densities in the right suprahilar region, right lower lobe, left basilar atelectasis, small left pleural effusion and diffuse wall thickening of the cecum as well as terminal ileum. Multiple sets of blood cultures continued to reveal high-grade Enterococcus bacteremia (seven sets of blood cultures). Based on radiological data and infectious workup, he was treated with cefepime, vancomycin, fluconazole, and azithromycin. On susceptibility testing, Enterococcus was vancomycin-resistant and hence vancomycin was discontinued, and he was commenced on daptomycin. The PICC line which was placed in recent hospitalization was removed and PICC line catheter tip cultures did not reveal any infection and a 2D echocardiogram showed no evidence of endocarditis. Mycoplasma pneumoniae IgG was positive, Aspergillus antigen was detected on enzyme immunoassay with an antigen index of 2.2 and Fungitell B-D glucan assay was positive with more than 500 pg/mL (< 80 pg/mL). He was continued on daptomycin along with micafungin, ceftaroline and tigecycline. Cyclosporine level was high, and therefore was discontinued. He remained on prednisone. He developed diarrhea and stool testing was positive for Clostridium difficile toxin and glutamate dehydrogenase (GDH) antigen. Stool tests were unyielding for Campylobacter, Salmonella, Vibrio cholera, enterotoxigenic E. coli (ETC), Cryptosporidium species, Entamoeba histolytica, Giardia lamblia, adenovirus EF 40/41, norovirus GI/GII, rotavirus A, Shiga-like toxin E. coli (SDC) E. coli 0157 and Shigella. Oral vancomycin was initiated for clostridial infection. He developed hypotension and remained unresponsive to intravenous hydration and laboratory data at this point showed serum bicarbonate of 15 mmol/L (bicarbonate range 19 - 31 mmol/L), anion gap of 17 (anion gap range 4 - 16), bilirubin of 5.2 mg/dL (bilirubin range 0.2 - 1.3 mg/dL), aspartate aminotransferase (AST) 1,642 U/L (AST range 12 - 47 U/L), ALT 779 U/L (ALT range 5 - 60 U/L), alkaline phosphatase 413 U/L (alkaline phosphatase range 40 - 140 U/L) and lactic acid 18.4 mmol/L (lactic acid range 0.5 - 2.2 mmol/L). Shock worsened, and he sustained pulseless electrical activity (PEA) cardiac arrest. Despite multiple rounds of cardiopulmonary resuscitation, epinephrine and bicarbonate, the medical team was unable to obtain return of spontaneous circulation and the patient expired (Fig. 1).
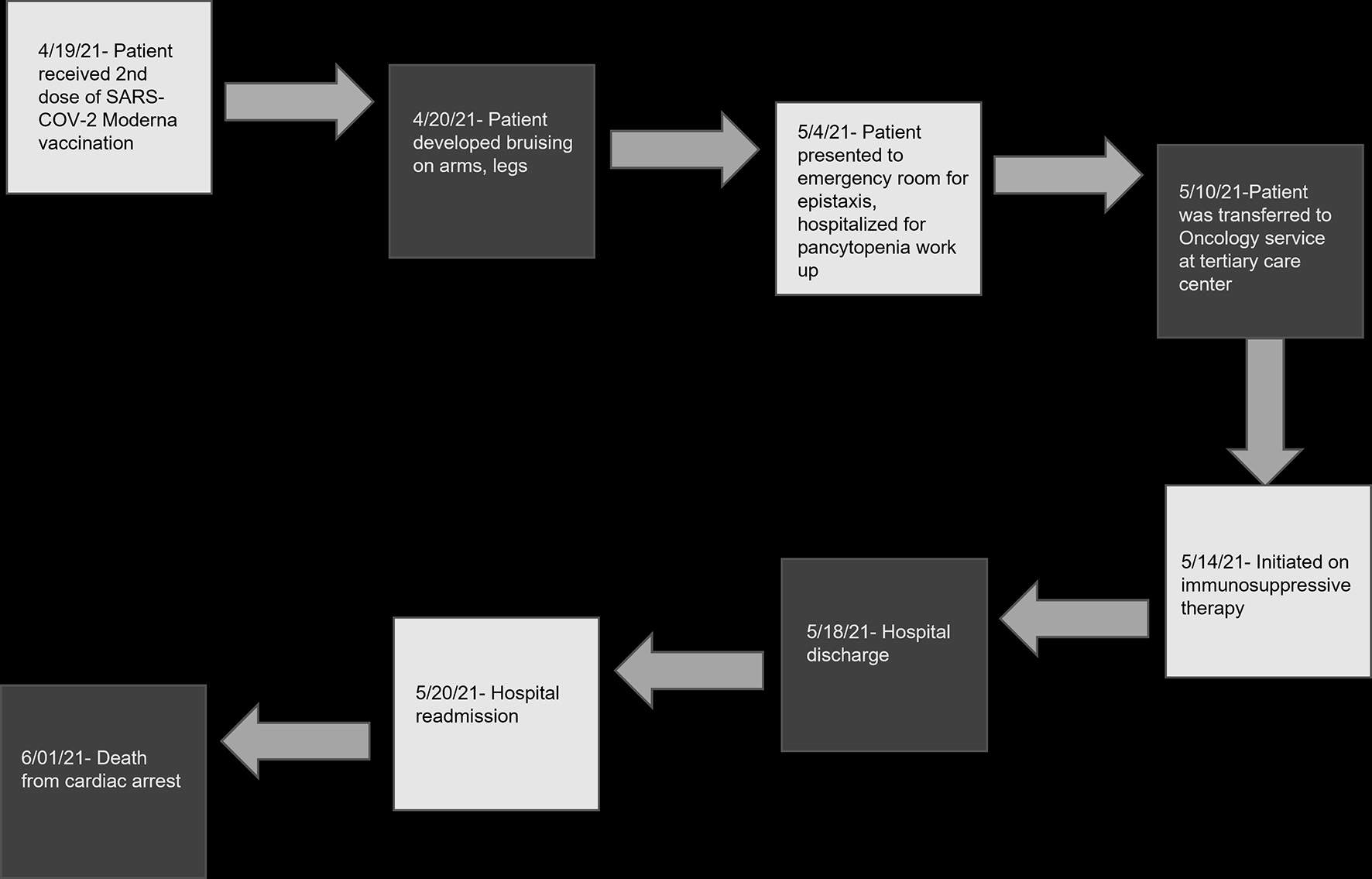 Click for large image | Figure 1. Timeline of clinical events following second SARS-CoV-2 vaccination. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. |
| Discussion | ▴Top |
AA is a life-threatening form of bone marrow failure which refers to pancytopenia in association with bone marrow hypoplasia/aplasia. Multipotent HSCs constitute a small population within CD34+/CD38- fraction of bone marrow cells. Autoimmune damage to HSCs causes or contributes to majority of cases of AA [4]. Other etiologies that can lead to AA are exposure to toxic chemicals such as benzene, solvents, drugs such as carbamazepine, chloramphenicol, cancer chemotherapy, viral infections such as EBV, HIV, immune disorders such as eosinophilic fasciitis, systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria and thymoma [4].
There is evidence of the deficient quantity and/or function of T-regulatory cells in AA [8, 9]. Cytotoxic lymphocytes and type I cytokines appear to be proximate effectors of autoimmune aplasia in AA. Interferon (IFN) gamma, other cytokines (e.g., interleukin (IL)-17), natural killer cells, and autoantibodies are also implicated in immune destruction of HSCs in AA [10-12].
Occurrence of AA following immunizations is rare and there are few reports of vaccine-related AA described in medical literature. Viallard et al reported a patient who had developed severe pancytopenia after 3 weeks following a third vaccination boost of recombinant hepatitis B vaccine. Bone marrow biopsy of this patient demonstrated paucity of late myeloid elements and maturation arrest. IFN gamma production from peripheral blood mononuclear cells was dramatically increased and the authors hypothesized that pancytopenia was induced by a dysregulation of CD8+ cells via increased IFN gamma production [13]. Shenoy et al reported a patient who had developed severe pancytopenia and hepatitis after 10 days following a third dose of recombinant hepatitis B vaccine. Bone marrow aspiration cytology of this patient revealed hypocellular marrow with marked reduction in erythropoiesis, granulocytopoiesis and absence of precursors of granulocyte series [14]. Both the patients described by Viallard et al and Shenoy et al were teenagers. Shah et al reported development of AA in two healthy males after immunization with hepatitis B vaccine boost 7 days prior to presentation and anthrax vaccine 30 days prior to presentation. The first patient’s bone marrow revealed hypocellular marrow with 15% cellularity and markedly reduced megakaryocytes and he was treated with allogenic bone marrow transplant. The second patient’s bone marrow revealed 40% cellularity with markedly decreased megakaryocytes, and he was also treated with allogeneic bone marrow transplant. The authors hypothesized that underlying immune predisposition might have enabled the vaccines to trigger cytotoxic T-lymphocyte response possibly leading to AA [15]. Angelini et al described transient SAA following varicella-zoster vaccine in an immunocompetent 17-month-old girl after 3 weeks following immunization with VARIVAXIII. Her bone marrow revealed absence of megakaryocytes and neutrophils, although the sample was insufficient to determine the overall cellularity. She showed signs of hematologic recovery 4 weeks after initial presentation and therefore immunosuppressive therapy was not considered [16]. Donnini et al reported occurrence of acquired SAA in a 25-year-old man following administration of H1N1 influenza virus vaccine. Bone marrow biopsy of this patient revealed AA and immunophenotype demonstrated absence of CD34+ cells with relative increment in T lymphocytes. He received bone marrow human leukocyte antigen (HLA)-identical sibling transplantation and he demonstrated near complete hematological recovery 110 days following transplantation [17]. Pertaining to SARS-COV-2 mRNA vaccinations, two cases of AA following administration of SARS-CoV-2 Pfizer-BioNTech mRNA vaccine are thus far reported. Cecchi et al reported occurrence of AA in a 76-year-old man after 1 month following second dose of SARS-CoV-2 Pfizer-BioNTech vaccination. Bone marrow biopsy showed 10% cellularity with normal cytogenetics, and he was treated with cyclosporin, rabbit anti-thymocyte globulin. This patient however has persistent pancytopenia and remains transfusion-dependent. The authors hypothesized that vaccine may have induced an immune activation that disclosed a latent autoimmunity [18]. Tabata et al also reported SAA in a previously healthy 56-year-old man following administration of SARS-CoV-2 Pfizer-BioNTech mRNA vaccination and he was treated with allogeneic hematopoietic stem cell transplantation (HSCT) [19].
Based on the above summarized literature about vaccine-associated AA, a causative association between various vaccinations and immune-related bone marrow failure leading to AA is possible, and our patient’s presentation suggests a temporal relationship between mRNA SARS-CoV-2 vaccination and vSAA might exist. This is the first described case of vSAA following administration of SARS-CoV-2 Moderna mRNA vaccine. Clinicians should be aware of this rare but severe adverse event and perform thorough clinical assessments, investigations and provide appropriate therapies.
| Supplementary Material | ▴Top |
Suppl 1. Leukemia lymphoma panel from peripheral blood, bone marrow specimen, and FISH study.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
All authors certify that they have participated sufficiently in the intellectual content of the manuscript. They have reviewed the final version of the manuscript and approve it for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and supplementary file.
| References | ▴Top |
- Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008;93(4):489-492.
doi pubmed - Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Leaverton PE, Shapiro S, Young NS. The epidemiology of aplastic anemia in Thailand. Blood. 2006;107(4):1299-1307.
doi pubmed - Levy M, Kelly JP, Kaufman DW, Shapiro S. Risk of agranulocytosis and aplastic anemia in relation to history of infectious mononucleosis: a report from the international agranulocytosis and aplastic anemia study. Ann Hematol. 1993;67(4):187-190.
doi pubmed - Young NS. Aplastic Anemia. N Engl J Med. 2018;379(17):1643-1656.
doi pubmed - Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187-207.
doi pubmed - Davies JK, Guinan EC. An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol. 2007;136(4):549-564.
doi pubmed - Hock H, Kelly HR, Meyerowitz EA, Frigault MJ, Massoth LR. Case 31-2021: a 21-year-old man with sore throat, epistaxis, and oropharyngeal petechiae. N Engl J Med. 2021;385(16):1511-1520.
doi pubmed - Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, Kajigaya S, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603-1606.
doi pubmed - Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, Huang Z, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120(8):1624-1632.
doi pubmed - Hirano N, Butler MO, Von Bergwelt-Baildon MS, Maecker B, Schultze JL, O'Connor KC, Schur PH, et al. Autoantibodies frequently detected in patients with aplastic anemia. Blood. 2003;102(13):4567-4575.
doi pubmed - Feng X, Chuhjo T, Sugimori C, Kotani T, Lu X, Takami A, Takamatsu H, et al. Diazepam-binding inhibitor-related protein 1: a candidate autoantigen in acquired aplastic anemia patients harboring a minor population of paroxysmal nocturnal hemoglobinuria-type cells. Blood. 2004;104(8):2425-2431.
doi pubmed - Zeng W, Maciejewski JP, Chen G, Risitano AM, Kirby M, Kajigaya S, Young NS. Selective reduction of natural killer T cells in the bone marrow of aplastic anaemia. Br J Haematol. 2002;119(3):803-809.
doi pubmed - Viallard JF, Boiron JM, Parrens M, Moreau JF, Ranchin V, Reiffers J, Leng B, et al. Severe pancytopenia triggered by recombinant hepatitis B vaccine. Br J Haematol. 2000;110(1):230-233.
doi pubmed - Shenoy AK, Adhikari PMR, Chakrapani M, Shenoy D, Pillai A. Pancytopenia after recombinant hepatitis B vaccine - an Indian case report. Br J Haematol. 2001;114(4):954-962.
doi pubmed - Shah C, Lemke S, Singh V, Gentile T. Case reports of aplastic anemia after vaccine administration. Am J Hematol. 2004;77(2):204.
doi pubmed - Angelini P, Kavadas F, Sharma N, Richardson SE, Tipples G, Roifman C, Dror Y, et al. Aplastic anemia following varicella vaccine. Pediatr Infect Dis J. 2009;28(8):746-748.
doi pubmed - Donnini I, Scappini B, Guidi S, Longo G, Bosi A. Acquired severe aplastic anemia after H1N1 influenza virus vaccination successfully treated with allogeneic bone marrow transplantation. Ann Hematol. 2012;91(3):475-476.
doi pubmed - Cecchi N, Giannotta JA, Barcellini W, Fattizzo B. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. 2021.
doi pubmed - Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: Causality or coincidence? J Autoimmun. 2022;126:102782.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


