| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 2, April 2022, pages 71-76
A Case of Isolated Myeloid Sarcoma Associated With Germline EGFR T790M Variant: The Importance of Recognizing Potential Germline Variants on Somatic Tumor Sequencing Panels
Margaret Walkera , Matthew Folstada, Kelcy Smith-Simmerb, Erica Reinigc, Kalyan Nadimintia, Lauren Lovriena, Jane E. Churpeka, Lauren G. Banaszaka, d
aDepartment of Medicine, University of Wisconsin-Madison, Madison, WI 53705, USA
bOncology Genetics, University of Wisconsin Carbone Cancer Center, UW Health, Madison, WI 53792, USA
cDepartment of Pathology and Laboratory Medicine, University of Wisconsin-Madison, Madison, WI 53792, USA
dCorresponding Author: Lauren G. Banaszak, Department of Medicine, University of Wisconsin-Madison, Clinical Science Center, Madison, WI 53792, USA
Manuscript submitted February 1, 2022, accepted March 9, 2022, published online April 12, 2022
Short title: MS Associated With Germline EGFR T790M Variant
doi: https://doi.org/10.14740/jh983
| Abstract | ▴Top |
Isolated myeloid sarcoma is an uncommon subtype of acute myeloid leukemia associated with variable prognosis. We present the case of a previously healthy 30-year-old man presenting with chest pain and weight loss who was found to have a large mediastinal mass. Biopsy of the mass was consistent with isolated myeloid sarcoma. A somatic tumor sequencing panel revealed an EGFR T790M variant, which was later confirmed to be of germline origin. Germline EGFR T790M variants are associated with a hereditary predisposition to lung cancer, though myeloid malignancies have not yet been described. To our knowledge, this is the first reported case of myeloid sarcoma in a patient with an underlying germline EGFR T790M mutation. As somatic tumor sequencing panels become more commonplace, it is important to recognize potential germline variants in order to facilitate appropriate referral for genetic counseling, perform confirmatory genetic testing, and to develop a personalized treatment and surveillance plan for patients and their families.
Keywords: Myeloid sarcoma; EGFR T790M; Hereditary cancer
| Introduction | ▴Top |
Myeloid sarcoma (MS) is an uncommon malignant tumor consisting of myeloid lineage cells in any tissue other than bone marrow [1]. MS may present de novo, either as an isolated form or concurrently with acute myeloid leukemia (AML), or as progression from myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), or MDS/MPN [2]. This solid tumor-like manifestation of a hematologic malignancy was first described in 1811 and was originally referred to as a chloroma due to the green color of the tumor, which is a result of myeloperoxidase production [3, 4]. Today, MS is classified as a unique subtype of AML [2]. Due to the rarity of MS, particularly in its isolated form, several questions remain regarding its pathogenesis, clinical and molecular features, and other genetic associations. Here, we present a case of isolated MS associated with a germline EGFR T790M variant.
| Case Report | ▴Top |
Investigations
A 30-year-old male presented to the emergency department with a 1-week history of chest pain. The pain was described as constant and sharp, located near the lower right ribs, and was exacerbated by movement. He also reported mild shortness of breath, fatigue, and an unintentional 50-pound weight loss over the past year. His medical history was significant for depression controlled without medication. He had no prior surgeries. The patient was enlisted in the Army on reserve duty. He reported a 4 pack-years smoking history but denied alcohol or illicit drug use. Family history (Fig. 1) revealed lung cancer in a maternal uncle without tobacco exposure and in his maternal grandfather who had an extensive history of tobacco and alcohol use. The patient also had a family history of early onset esophageal cancer on his paternal side including his father, a paternal uncle, and paternal grandfather. There was no family history of hematological disorders.
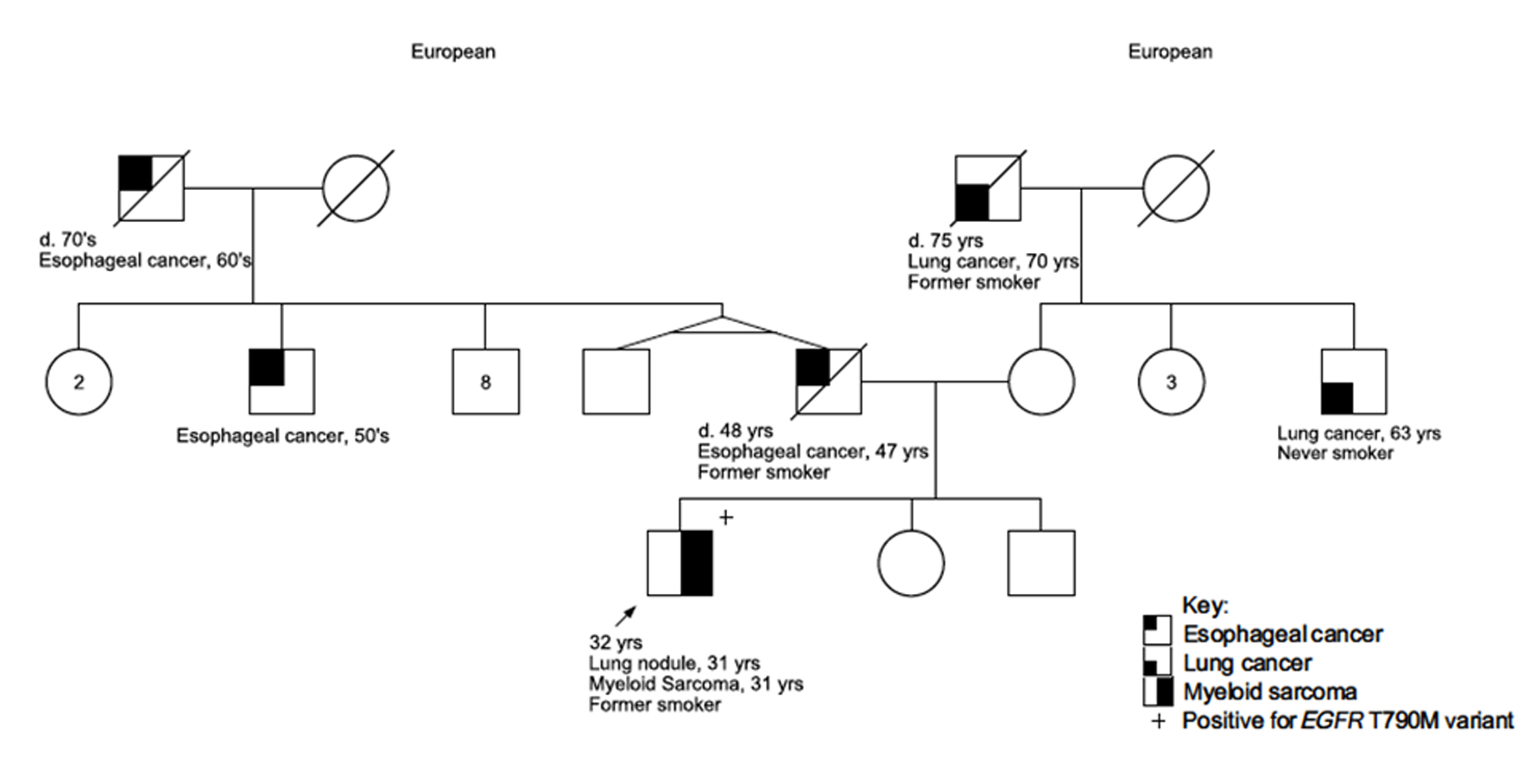 Click for large image | Figure 1. Family pedigree of the patient. The patient is indicated as the proband with an arrow. Assigned females at birth are represented as circles; assigned males at birth are represented as squares. Lines through a symbol indicate that the individual is deceased. d: deceased at age. |
Diagnosis
Presenting chemistries and blood counts were normal including white blood cell (WBC) count of 5.7 × 109/L with normal differential, hemoglobin 17.3 g/dL, and platelet count of 312 × 109/L. Computed tomography (CT) scan of the chest revealed a right posterior mediastinal mass with two right lung nodules measuring up to 2 cm and 1 cm, respectively (Fig. 2a). Pathology from a biopsy of the mass showed sheets of large neoplastic cells with blast-like morphology, including open chromatin and variably prominent nuclei, with the following immunophenotype by immunohistochemistry: CD117+, myeloperoxidase (MPO)+, CD68+, CD34 partial, CD20-, CD56-, paired box 5 (PAX5)-, and terminal deoxynucleotidyl transferase (TdT)-. The morphology and immunophenotype were diagnostic of MS. CD33 expression by flow cytometry and molecular studies were unable to be performed due to insufficient material. While in the process of establishing care with a hematologist, he re-presented to the emergency department with acute onset bilateral lower extremity numbness and weakness. An emergent magnetic resonance imaging (MRI) of the spine revealed a 10-cm posterior mediastinal mass extending into the thoracic spinal canal from T2 to T9 resulting in moderate-to-severe spinal stenosis (Fig. 2b).
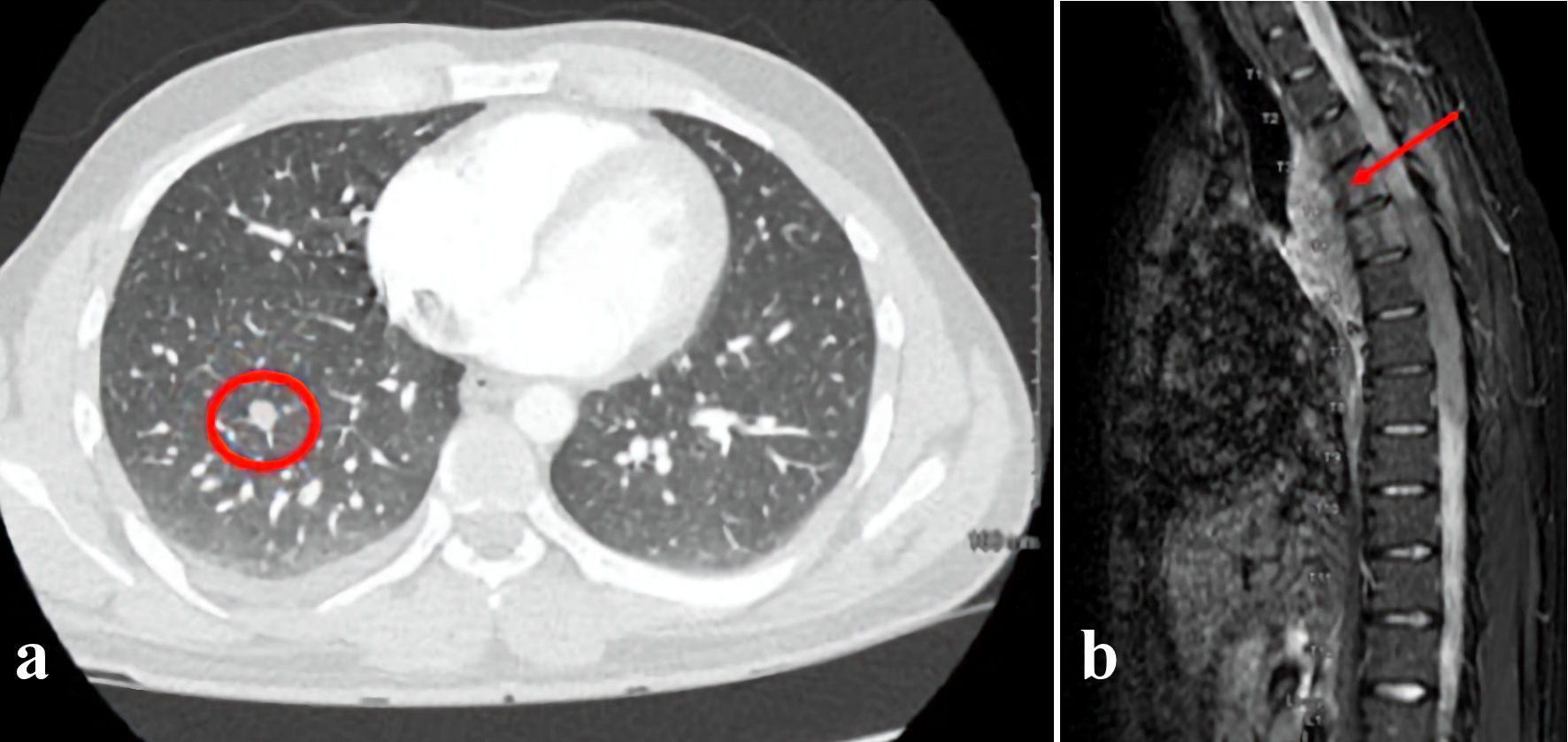 Click for large image | Figure 2. (a) CT scan of the chest obtained at diagnosis revealed a right posterior mediastinal mass with scattered right lung nodules. A representative pulmonary nodule is indicated by the red circle. (b) An MRI of the spine was performed to evaluate acute bilateral lower extremity weakness. This revealed a 10-cm posterior mediastinal mass (arrow) extending into the thoracic spinal canal from T2 to T9 resulting in moderate-to-severe spinal stenosis. CT: computed tomography; MRI: magnetic resonance imaging. |
A bone marrow biopsy did not show evidence of concurrent AML, though cytogenetics did reveal 3/16 metaphases containing inv(2)(p13q31), which was thought to represent a small myeloid clone or clonal hematopoiesis. A second biopsy of the mediastinal mass was performed for genomic studies, and cytogenetic and fluorescence in situ hybridization (FISH) analysis was remarkable for CBFB/MYH11 fusion associated with inv(16).
Due to the biopsy sample being formalin-fixed rather than fresh, the MS specimen was sent to a laboratory accustomed to somatic testing for formalin-fixed paraffin-embedded (FFPE) specimens. This laboratory utilized a broad pan-cancer gene panel, including both solid tumor and myeloid malignancy genes, and identified the following pathogenic variants: EGFR T790M (variant allele frequency (VAF) 48%), KIT D816V (VAF 49%), SMARCB1 T357fs*1 (VAF 49%), SH2B3 R566W (VAF 52%), USH2A G3291S (VAF 50%), and BIRC6 I1817V (VAF 48%). The patient was referred to a genetic counselor given the finding of an EGFR T790M variant, which has been associated with a hereditary predisposition to lung cancer [5, 6]. Genetic counseling was completed and, due to his family history, informed consent for germline genetic testing via a multi-gene cancer panel rather than single-site testing for the EGFR variant alone was obtained. Germline testing was performed on DNA extracted from cultured skin fibroblasts, which demonstrated heterozygosity of the EGFR T790M variant, confirming its germline origin. The SMARCB1 and KIT variants were not found in the skin DNA, confirming their somatic origin; the SH2B3, USH2A, and BIRC6 variants were not tested in the skin DNA. His germline testing identified four additional heterozygous germline variants of uncertain significance (VUS) in the RET (c.2556C>G, p.I852M), KIF1B (c.1817C>T, p.T606I), MSH3 (c.1027+4T>C, intronic variant), and RAD51D (c.34C>G, p.L12V) genes.
Treatment
The patient received one 2-Gy fraction of radiation to the mass and was started on dexamethasone with significant improvement in his paresthesia and lower extremity weakness. In the setting of cord compromise, an intensive chemotherapy regimen was felt to be necessary in order to prevent further progression of neurologic deficits. Thus, he underwent induction chemotherapy with CLAG-M (cladribine 5 mg/m2 day 1 - 5, cytarabine 2,000 mg/m2 day 1 - 5, filgrastim 300 µg day 1 - 6, mitoxantrone 10 mg/m2 day 2 - 4) and intrathecal cytarabine (70 mg × 1). A repeat MRI 1 week after induction showed a favorable response to treatment with interval decrease in size of the posterior chest wall mass and decreased enhancement of the thoracic spinal cord. At this time, cytogenetics returned showing the presence of inv(16), so the treatment algorithm for favorable risk AML per European Leukemia Network (ELN) 2017 and AML National Comprehensive Cancer Network (NCCN) guidelines was followed [7, 8]. Therefore, he received two cycles of consolidative high-dose cytarabine (3,000 mg/m2 day 1 - 3) plus gemtuzumab ozogamicin (3 mg/m2 day 1). Cycle two was complicated by severe septic shock, so a third cycle was omitted.
Follow-up and outcomes
A positron emission tomography (PET) scan following completion of consolidation chemotherapy demonstrated resolution of the posterior mediastinal mass with no fluorodeoxyglucose (FDG) evidence of viable residual disease. The pulmonary nodules remained unchanged in size, were FDG non-avid, and had benign imaging characteristics per review by expert thoracic radiologists. In the context of the known germline EGFR variant, in which characteristic pulmonary nodules are common on surveillance imaging, radiology and thoracic oncology recommended continued close surveillance [9]. The patient underwent PET/CT every 3 months for the first year to assess for recurrence of his mediastinal mass and to follow the pulmonary nodules. A post treatment bone marrow biopsy was negative for AML. At 1 year of follow-up, the patient has no evidence of disease. His paresthesia has resolved, and his strength has returned to baseline levels. Because the patient was confirmed to harbor a germline EGFR T790M variant, his mother and sister were referred for genetic counseling to discuss germline sequencing. Segregation studies are currently underway to clarify mode of inheritance.
| Discussion | ▴Top |
Concurrent extramedullary involvement of AML is common and can be found in approximately 19% of patients at the time of AML diagnosis [10]. However, isolated MS presenting as de novo disease is rare with a prevalence of approximately 1% of new AML cases [11, 12]. Prognosis is variable and determined by individual clinical and genetic features [12, 13]. Research has primarily focused on identifying clinical and molecular prognostic factors based on known AML associations given their common cell lineage [14-16]. However, MS is unique from AML due to its presentation as a solid tumor. Thus, it has been hypothesized that MS may have genetic alterations more consistent with solid rather than hematologic malignancies [17]. For example, patients with MS and concurrent AML exhibit an abundance of RAS pathway somatic mutations compared to patients with isolated AML [11, 17]. Furthermore, MS has been reported in the setting of MSH6, SMARCB1, and PALB2 germline variants, which are more typically associated with non-hematologic malignancies [18-20].
In our patient, an EGFR T790M variant was detected within the tumor tissue at high VAF. The EGFR gene encodes the epidermal growth factor receptor (EGFR), a cell-surface growth factor receptor with intrinsic tyrosine kinase activity critical for normal cell growth and development [21]. Overexpression of EGFR can promote constitutive growth signaling through RAS-activated MAPK and PI3K-activated AKT pathways. These genetic alterations are well-established drivers of malignancy, particularly in non-small cell lung cancer (NSCLC). As such, tyrosine kinase inhibitors (TKIs) that target EGFR are effective therapies in EGFR-mutated NSCLC [22-24]. The EGFR T790M variant does not confer increased kinase activity, but rather alters the transmembrane protein binding pocket leading to increased affinity for adenosine triphosphate (ATP) and resistance to early-generation TKIs [25]. In fact, somatic EGFR T790M variants are most often seen in patients previously treated with anti-EGFR therapy due to selective pressure [26]. Germline EGFR T790M variants are rare and to date are most clearly associated with a hereditary predisposition to lung cancer [5, 6, 9]. In patients with germline EGFR T790M variants, a somatic “second-hit” gain-of-function mutation in the wild-type EGFR allele typically acts synergistically with the germline variant to promote malignant cell growth. A concurrent somatic mutation in KRAS has also been described as a possible contributor to lung cancer development [27].
Per NCCN guidelines, identification of the EGFR T790M variant in patients with NSCLC prior to TKI exposure should prompt genetic counseling and germline sequencing, as it is a known heritable risk factor for lung malignancy [28]. This recommendation can be expanded to any patient undergoing genetic testing found to have an EGFR T790M variant without TKI exposure. In these cases, molecular tumor boards and dedicated molecular pathologists can help to identify the EGFR T790M variant as being potentially germline in origin, particularly in the setting of a high VAF, and bring this to the attention of the treating physician.
The role of EGFR variants in the pathogenesis of myeloid malignancies is less clear. There is some evidence that EGFR is expressed in leukemic cell lines, and its expression may be associated with poor prognosis [29]. Anti-EGFR TKIs have shown potential antineoplastic effects in vitro [30]. These observations provided the basis for a phase II clinical trial of an EGFR-targeted TKI, gefitinib, in AML. Unfortunately, gefitinib did not confer any clinical benefit [31]. To our knowledge, this is the first reported case of MS with an underlying germline EGFR T790M variant, but at this time, it is unclear if this EGFR variant or others contribute to the pathogenesis of myeloid malignancies.
The recognition and subsequent confirmation of a germline EGFR variant in this case led to a change in clinical management. Our patient will undergo more comprehensive lung cancer screening during his lifetime given his increased risk. Estimates of lifetime lung cancer risk in patients with a germline EGFR T790M variant have been shown to be as high as 31% in non-smokers compared to 0.2% of the general population of non-smokers and 23% of smokers [32]. As there are no data from clinical trials to guide optimal screening for patients with this rare germline variant, clinical practice is largely driven by expert opinion. The TALENT lung cancer screening study included cases of familial lung cancer, suggesting annual low-dose CT is a reasonable option for screening until EGFR-specific evidence is available [33]. Our patient was found to have multiple right-sided pulmonary nodules on initial staging imaging that have remained stable in size with absence of FDG activity for over a year. For now, he is undergoing PET/CT every 3 months for surveillance of his MS. Once these exams are no longer required for MS, he will undergo yearly low-dose chest CT for surveillance of his lung nodules and screening for lung cancer.
Conclusions
Here we report the first case of de novo isolated MS in a patient with an underlying germline EGFR T790M variant. This case highlights the importance of performing expanded cancer mutation panels from tumor tissues in patients presenting in an atypical fashion. This case also highlights the importance of recognizing potential germline variants when performing tumor sequencing, encourages appropriate pre-test counseling regarding the possibility of germline findings on tests sent for the primary purpose of identifying somatic variants, and how this can alter clinical management to optimize the health of patients and their families.
Learning points
Learning points from this case include: 1) Isolated MS is a rare subtype of AML associated with variable prognosis. 2) In addition to routine sequencing for myeloid driver mutations, an expanded cancer mutation panel should be considered in patients with myeloid malignancies presenting in an atypical fashion. 3) Family history remains a vital component in selecting patients for additional comprehensive genetic evaluation. 4) Germline EGFR T790M variants are associated with a hereditary predisposition to lung cancer. This is the first reported case of MS in a patient with an underlying germline EGFR T790M mutation. 5) The recognition of potential germline variants in somatic tumor sequencing panels is important to facilitate appropriate referral for counseling and confirmatory genetic testing, which can provide insight into the best treatment and surveillance strategies for patients and their families.
Acknowledgments
The authors wish to thank our patient and his family for participating in this research.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
MW, MF, KN, KSS, LL, ER, and JEC participated in the clinical care of the patient. MW, MF, and LGB wrote the manuscript. LL, KSS, and LGB created the accompanying figures. All authors contributed to the editing of the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
MS: myeloid sarcoma; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; WBC: white blood cell; FISH: fluorescence in situ hybridization; FFPE: formalin-fixed paraffin-embedded; VAF: variant allele frequency; VUS: variant of uncertain significance; FDG: fluorodeoxyglucose; EGFR: epidermal growth factor receptor; NSCLC: non-small cell lung cancer; TKI: tyrosine kinase inhibitor; NCCN: National Comprehensive Cancer Network; PET: positron emission tomography
| References | ▴Top |
- Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119(1):34-43.
doi pubmed - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Burns A. Observations on the surgical anatomy of the head and neck. Edinburgh, UK: Thomas Royce and Co; 1811. p. 364-366.
- King A. Case of chloroma. Monthly Journal of Medical Science. 1853;8(44):97-104.
- Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, Sharma SV, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315-1316.
doi pubmed - Tibaldi C, Giovannetti E, Vasile E, Boldrini L, Gallegos-Ruiz MI, Bernardini I, Incensati R, et al. Inherited germline T790M mutation and somatic epidermal growth factor receptor mutations in non-small cell lung cancer patients. J Thorac Oncol. 2011;6(2):395-396.
doi pubmed - Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447.
doi pubmed - Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, de Lima M, et al. NCCN guidelines insights: acute myeloid leukemia, Version 2.2021. J Natl Compr Canc Netw. 2021;19(1):16-27.
doi pubmed - Gazdar A, Robinson L, Oliver D, Xing C, Travis WD, Soh J, Toyooka S, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol. 2014;9(4):456-463.
doi pubmed - Stolzel F, Luer T, Lock S, Parmentier S, Kuithan F, Kramer M, Alakel NS, et al. The prevalence of extramedullary acute myeloid leukemia detected by (18)FDG-PET/CT: final results from the prospective PETAML trial. Haematologica. 2020;105(6):1552-1558.
doi pubmed - Abbas HA, Reville PK, Geppner A, Rausch CR, Pemmaraju N, Ohanian M, Sasaki K, et al. Clinical and molecular characterization of myeloid sarcoma without medullary leukemia. Leuk Lymphoma. 2021;62(14):3402-3410.
doi pubmed - Movassaghian M, Brunner AM, Blonquist TM, Sadrzadeh H, Bhatia A, Perry AM, Attar EC, et al. Presentation and outcomes among patients with isolated myeloid sarcoma: a Surveillance, Epidemiology, and End Results database analysis. Leuk Lymphoma. 2015;56(6):1698-1703.
doi pubmed - Wilson CS, Medeiros LJ. Extramedullary manifestations of myeloid neoplasms. Am J Clin Pathol. 2015;144(2):219-239.
doi pubmed - Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, Liso A, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21(7):1566-1570.
doi pubmed - Claerhout H, Van Aelst S, Melis C, Tousseyn T, Gheysens O, Vandenberghe P, Dierickx D, et al. Clinicopathological characteristics of de novo and secondary myeloid sarcoma: A monocentric retrospective study. Eur J Haematol. 2018;100(6):603-612.
doi pubmed - Ansari-Lari MA, Yang CF, Tinawi-Aljundi R, Cooper L, Long P, Allan RH, Borowitz MJ, et al. FLT3 mutations in myeloid sarcoma. Br J Haematol. 2004;126(6):785-791.
doi pubmed - Choi M, Jeon YK, Sun CH, Yun HS, Hong J, Shin DY, Kim I, et al. RTK-RAS pathway mutation is enriched in myeloid sarcoma. Blood Cancer J. 2018;8(5):43.
doi pubmed - Liu Y, GuLiBaHa M, Yue YB, Li MW, Cao SB, Yan M. An isolated childhood myeloid sarcoma with germline MSH6 mutation-a case report. Transl Pediatr. 2021;10(8):2136-2143.
doi pubmed - Beer A, Beck R, Schedel A, von Bonin M, Meinel J, Friedrich UA, Menzel M, et al. A rare PALB2 germline variant causing G2/M cell cycle arrest is associated with isolated myelosarcoma in infancy. Mol Genet Genomic Med. 2021;9(9):e1746.
doi pubmed - Metts JL, Park SI, Soares BP, Fong C, Biegel JA, Goldsmith KC. Concurrent myeloid sarcoma, atypical teratoid/rhabdoid tumor, and hypereosinophilia in an infant with a germline SMARCB1 mutation. Pediatr Blood Cancer. 2017;64(9):e26460.
doi pubmed - Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2-16.
doi pubmed - Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-137.
doi pubmed - Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6(6):352-366.
doi pubmed - Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
doi pubmed - Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105(6):2070-2075.
doi pubmed - Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015;4(1):67-81.
- Thomas A, Xi L, Carter CA, Rajan A, Khozin S, Szabo E, Dennis PA, et al. Concurrent molecular alterations in tumors with germ line epidermal growth factor receptor T790M mutations. Clin Lung Cancer. 2013;14(4):452-456.
doi pubmed - Network NCC. Non-Small Cell Lung Cancer (Version 6.2021). September 30, 20212021.
- Sun JZ, Lu Y, Xu Y, Liu F, Li FQ, Wang QL, Wu CT, et al. Epidermal growth factor receptor expression in acute myelogenous leukaemia is associated with clinical prognosis. Hematol Oncol. 2012;30(2):89-97.
doi pubmed - Stegmaier K, Corsello SM, Ross KN, Wong JS, Deangelo DJ, Golub TR. Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood. 2005;106(8):2841-2848.
doi pubmed - Deangelo DJ, Neuberg D, Amrein PC, Berchuck J, Wadleigh M, Sirulnik LA, Galinsky I, et al. A phase II study of the EGFR inhibitor gefitinib in patients with acute myeloid leukemia. Leuk Res. 2014;38(4):430-434.
doi pubmed - Berry DK, Wang X, Michalski ST, Kang HC, Yang S, Creelan BC, McLeod HL, et al. Clinical Cohort Analysis of Germline EGFR T790M Demonstrates Penetrance Across Ethnicities and Races, Sexes, and Ages. JCO Precis Oncol. 2020;4:170-175.
doi pubmed - Yang P. National lung cancer screening program in Taiwan: the TALENT study. 2021. Presented at: International Association for the Study of Lung Cancer 2020 World Conference. January 30, 2021.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


