| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 11, Number 2, April 2022, pages 55-61
Iron Deficiency Anemia: An Overlooked Complication of Crohn’s Disease
Ahmed Abomhyaa, c , Waqqas Taia, Salman Ayaza, Farrah Khana, Waleed Saadedeena, Olufunmilola Ajalaa, Rana Mohamedb
aDepartment of Internal Medicine, The Brooklyn Hospital Center, Brooklyn, NY, USA
bDigestive Diseases and Nutrition Department, University of Kentucky, Lexington, KY, USA
cCorresponding Author: Ahmed Abomhya, Department of Internal Medicine, The Brooklyn Hospital Center, Brooklyn, NY 11201, USA
Manuscript submitted February 15, 2022, accepted April 5, 2022, published online April 12, 2022
Short title: Iron Deficiency Anemia in Patients With CD
doi: https://doi.org/10.14740/jh989
| Abstract | ▴Top |
Background: There are few studies to evaluate the association between iron deficiency anemia (IDA) and Crohn’s disease (CD). We examined this association in a USA-based cohort of patients with CD.
Methods: We queried the Nationwide Readmission Databases 2018 using the International Classification of Disease, 10th Revision, and Clinical Modification (ICD-10-CM) codes to identify all adult patients admitted with a diagnosis of CD. Primary outcomes were the prevalence of IDA among patients with CD. Secondary outcomes included inpatient mortality, the length of stay, all-cause 30-day non-elective readmission rate, and total cost of hospitalization. Multivariate regression analysis was performed to study the impact of IDA on inpatient mortality and non-elective readmissions.
Results: Of the 72,076 patients discharged from an index hospitalization for CD, 8.1% had IDA. CD patients with IDA had increased length of stays in days (4, interquartile range (IQR): 2 - 6 vs. 3, IQR: 2 - 5; P < 0.001), increased median total charges ($35,160, IQR: $19,786 - $64,126 vs. $31,299, IQR: $17,226 - $59,561; P < 0.001), and were more common to require blood transfusion during hospitalization (13.6% vs. 3.4%, P < 0.001) compared to CD patients without IDA, respectively. IDA was independently associated with increased odds of all-cause 30-day non-elective readmission (odds ratio (OR): 1.254, 95% confidence interval (CI): 1.154 - 1.363, P < 0.001) and increased odds of all-cause 90-day non-elective readmission (OR: 1.396, 95% CI: 1.302 - 1.498, P < 0.001).
Conclusions: In a large nationwide cohort of patients hospitalized for CD, we observed a significant burden of IDA. Additionally, we found a significant association between IDA and worse hospitalization outcomes.
Keywords: Iron deficiency anemia; Crohn’s disease; Anemia; Anemia of chronic disease
| Introduction | ▴Top |
The World Health Organization (WHO) defines anemia as a blood hemoglobin concentration below 13 g/dL in males or below 12 g/dL in females. Iron deficiency anemia (IDA) is the most common form of anemia in the world [1].
Crohn’s disease (CD) is a chronic inflammatory disease characterized by transmural inflammation that can affect the entire gastrointestinal tract with relapsing and remitting symptoms. The prevalence of CD in the USA is one of the highest worldwide and is estimated to be 214 per 100,000 [2]. In half of the CD patients, inflammation can lead to complications over time such as strictures, fistulas, abscesses, or lower gastrointestinal bleeding [2]. A variety of extraintestinal manifestations (EIMs) have been associated with CD. Yet, anemia is the most common systemic complication in patients with CD [3, 4]. A study by Akhuemonkhan et al showed that more than 30% of patients with CD in the USA did not receive appropriate screening for anemia during a 2-year period. Among those screened, the prevalence of anemia was more than 32% [5]. The reported rates of anemia in patients with inflammatory bowel disease (IBD) range from 6% to 74% [6, 7].
In the USA, there is a significant health care cost associated with anemia, with annual emergency room visits for anemia averaging around 209,000 [8].
Patients with IDA commonly present with fatigue, lightheadedness, headaches, pallor of skin and conjunctiva, decreased physical performance, and dyspnea on exertion [9]. Severe anemia can present with dizziness, syncope, or weight loss [10].
The pathogenesis of anemia in patients with CD is multifactorial, and those patients are at high risk of anemia due to chronic blood loss, reduced iron absorption, malnutrition, and chronic inflammation [11-13]. Elevated levels of hepcidin associated with CD flares decrease iron absorption and lead to iron retention in macrophages and monocytes [14]. CD patients are at significant risk for both IDA and anemia of chronic disease (ACD), and the treatment for each differs [15]. There is no single laboratory test that differentiates IDA from ACD, and multiple laboratory tests are usually required to differentiate IDA from ACD [16]. The goal of IDA treatment involves the normalization of hemoglobin levels and the repletion of iron stores. Iron supplementation should be continued for at least 6 months after normalization of hemoglobin to replenish iron stores [17]. Proper management of IDA leads to improving quality of life and might reduce hospital visits, and ultimately, lower health care costs [18].
Despite the significant association between CD and IDA, the burden and characteristics of IDA among patients with CD have not been evaluated in a large population study. We, therefore, aimed to estimate the national prevalence of IDA among patients with CD using a large USA-based population database. We also evaluated hospitalization outcomes associated with IDA, including mortality, cost, hospital length of stay, and readmission rates.
| Materials and Methods | ▴Top |
Data source
We conducted a retrospective cohort study using 2018 data from the Nationwide Readmissions Database (NRD), a resource of the Healthcare Cost and Utilization Project (HCUP) state inpatient databases. NRD is a publicly available all-payer inpatient healthcare database sponsored by the Agency for Healthcare Research and Quality (AHRQ) [19]. Diagnoses were reported via the International Classification of Disease, 10th Revision, and Clinical Modification (ICD-10-CM) codes. All procedures performed in studies involving human participants were in accordance with the 1963 Helsinki Declaration, its later amendments, and comparable ethical standards. The use of the NRD is exempt from an Institutional Review Board as there is no identifying patient information [20].
Study sample
We examined discharge data from patients aged 18 and older. Any patient with an ICD-10-CM diagnosis code indicating CD (K50.xx) was eligible for inclusion in this study. We excluded patients < 18 years old, those with missing information, and repeat readmission records. Patients with a comorbid diagnosis of IDA during hospitalization were identified. Included patients were then divided into two groups. The first group consisted of CD patients who did not have IDA. The second group consisted of CD patients with a comorbid diagnosis of IDA. Before comparing the two groups, we excluded CD patients with a comorbid diagnosis of ACD. The case selection process is summarized in Figure 1.
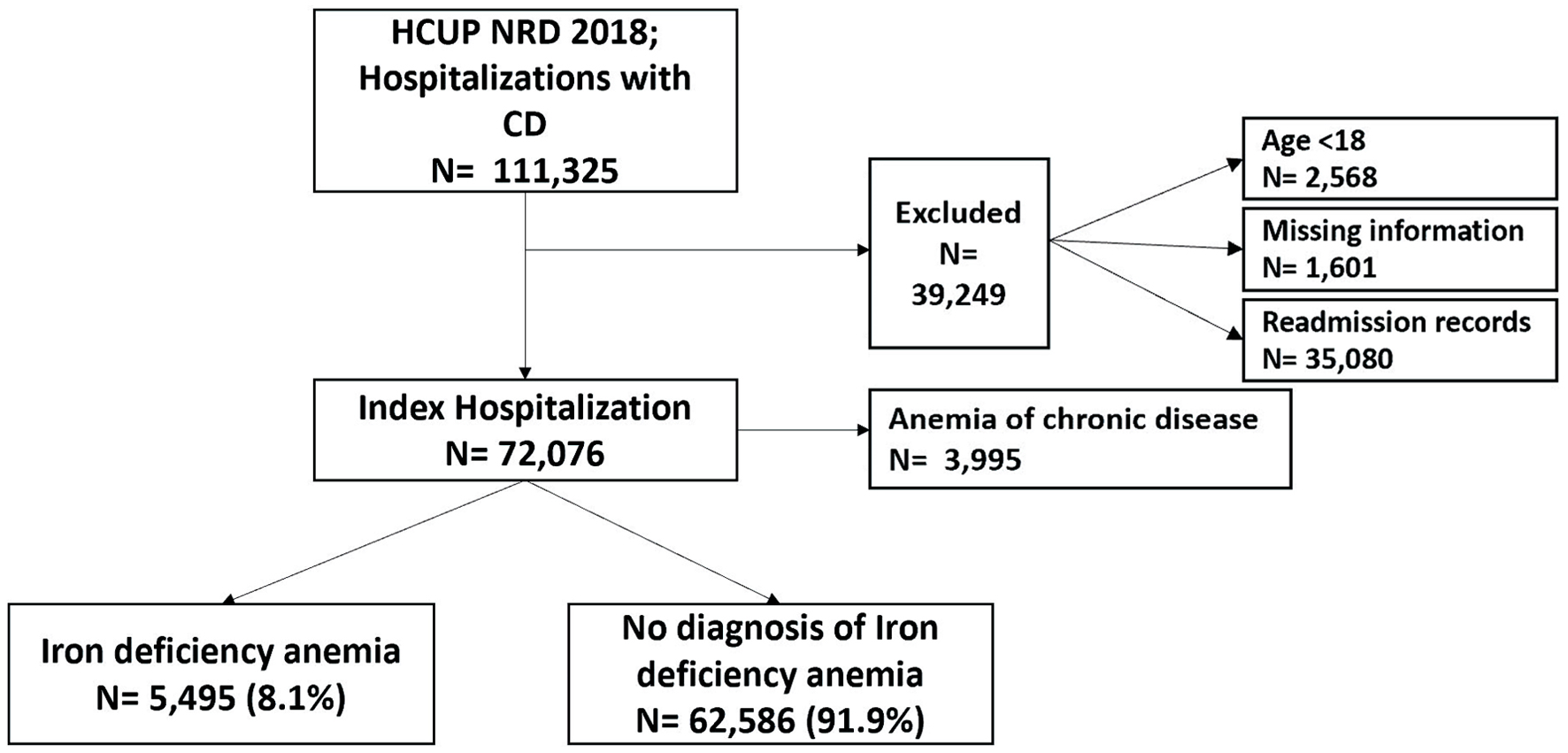 Click for large image | Figure 1. Case selection flowchart. HCUP: Healthcare Cost and Utilization Project; NRD: Nationwide Readmissions Database; CD: Crohn’s disease. |
We extracted demographic data, including age, gender, income status, elective admission, hospital bed size, and type of insurance. Additionally, relevant comorbidities were identified using ICD-10-CM codes. Income status was categorized into four groups according to the median household income quartile of the zip code where the patient lives.
Since the database captured admission purely on a calendar year without a linkage to the previous or following year, index hospitalization discharges occurring in December were excluded from the 30-day readmission analysis. For the 90-day readmission analysis, index hospitalization discharges occurring in October, November, or December were excluded. For any readmission analysis, we excluded patients who had elective readmission, or patients who died in the index hospitalization.
Outcomes of interest
Our primary outcome was to estimate the prevalence of IDA in patients hospitalized with CD. Secondary outcomes included comparing inpatient mortality, length of stay, total charges, 30-day non-elective readmission rates, and 90-day non-elective readmission rates between the two groups. We calculated the rate of the most common causes of readmissions among CD patients with IDA per 1,000 readmission records.
Statistical analysis
The NRD data were extracted and analyzed using SPSS Version 25 (IBM Corporation, Armonk, NY, USA). Numeric variables were described with median and interquartile range (IQR) and compared with the Mann-Whitney test. Categorical variables were described with percentages and compared using the Pearson χ2 test. We conducted a multivariate regression analysis for the association between IDA and inpatient mortality, 30-day non-elective readmission, and 90-day non-elective readmission. Associations were described using odds ratio (OR) at a 95% confidence interval (CI). A two-tailed P value < 0.05 was considered statistically significant.
| Results | ▴Top |
Baseline characteristics of CD patients with and without IDA
We extracted 111,325 hospital discharge records with a diagnosis of CD. We excluded records with missing information, age < 18, and readmission records. We analyzed 72,076 index hospitalizations with CD, of whom 3,995 had ACD, and 5,495 (8.1%) had IDA. CD patients with IDA were younger (47, IQR: 32 - 65 vs. 54, IQR: 37 - 68; P < 0.001) compared to CD patients without IDA, respectively. In our CD cohort, 57.9% of the patients were females. Sixty-two percent of CD patients with IDA were females while 38% were males. Baseline characteristics of CD patients with and without IDA are summarized in Table 1.
 Click to view | Table 1. Baseline Characteristics of CD Patients With and Without IDA |
Comorbid diseases among CD patients with and without IDA
Hospitalized CD patients with IDA had statistically significant higher prevalence of thrombocytopenia (3.9% vs. 3.3%, P = 0.029), cirrhosis (2.3% vs. 1.8%, P = 0.003), chronic kidney disease (CKD) (10.5% vs. 9.3%, P = 0.003) and active lower gastrointestinal bleeding (18% vs. 7.2%, P < 0.001) compared to those without IDA.
CD patients with IDA were more common to have Crohn’s complications on admission, including fistula (5.5% vs. 3.3%, P < 0.001) and abscess (6.2% vs. 3.1%, P < 0.001) compared to CD patients without IDA, respectively. Major depressive disorder was more common among CD patients with IDA (18.9% vs. 17.6%, P = 0.007) compared to CD patients without a comorbid IDA, respectively. Comorbid diseases among CD patients with IDA are summarized in Table 2.
 Click to view | Table 2. Comorbid Diseases Among CD Patients With IDA |
Hospitalization outcomes for CD patients with and without IDA
CD patients with IDA had increased length of stays in days (4, IQR: 2 - 6 vs. 3, IQR: 2 - 5; P < 0.001), increased median total charges ($35,160, IQR: $19,786 - $64,126 vs. $31,299, IQR: $17,226 - $59,561; P < 0.001), and were more common to require blood transfusion during hospitalization (13.6% vs. 3.4%, P < 0.001) compared to CD patients without IDA respectively. CD patients with IDA had higher all-cause 30-day non-elective readmission rate (11.9% vs. 9.5%, P < 0.001) and higher all-cause 90-day non-elective readmission rate (22.9% vs. 17%, P < 0.001) compared to CD patients without IDA respectively.
In multivariate analysis, after adjusting for sex, age, CD complications, and comorbid diseases there was no statistically significant difference in mortality between the two groups (OR: 0.805, 95% CI: 0.606 - 1.07, P = 0.135) but having IDA was independently associated with increased odds of all-cause 30-day non-elective readmission (OR: 1.254, 95% CI: 1.154 - 1.363, P < 0.001) and increased odds of all-cause 90-day non-elective readmission (OR: 1.396, 95% CI: 1.302 - 1.498, P < 0.001). While the most common cause of readmissions was a CD flare (207 per 1,000 readmission records), IDA was the primary readmission cause in two per 1,000 readmission records, and patients with IDA in index hospitalization were more common to require a blood transfusion during their subsequent readmission (11.1% vs. 5.8%, P < 0.001) compared to CD patients without comorbid IDA, respectively. A subgroup analysis of hospitalization outcomes among patients with ACD showed that they had a longer length of stays (5, IQR: 3 - 9 vs. 3, IQR: 2 - 5; P < 0.001) and higher costs of hospitalizations ($43,969, IQR: $23,866 - $86,934 vs. $31,299, IQR: $17,226 - $59,561; P < 0.001) compared to CD patients without ACD respectively.
| Discussion | ▴Top |
EIMs of CD are important predictors of morbidity and quality of life. Patients with CD are usually managed by a collaborative clinical care team that involves multiple subspecialties. Few studies evaluated the prevalence, diagnosis, and management of IDA among patients with CD. Reports from Europe showed a high prevalence of anemia in hospitalized CD patients [21, 22]. Our study is significant as we used a database that is nationally representative of the US population to study the prevalence of IDA in patients with CD.
IBD patients with either ulcerative colitis (UC) or CD have a higher incidence and prevalence of anemia compared to matched non-IBD patients [23]. In our robust, nationally representative cohort of hospitalized CD patients, 5.54% had ACD, and 8.1% had IDA. The reported rates of anemia in patients with IBD are heterogeneous and range from 6% to 74% due to poorly standardized definitions of anemia, different study settings, and different study designs [6, 7]. Previous reports showed that IDA in IBD is often underdiagnosed and undertreated [24]. While our cohort included only hospitalized CD patients, the observed high prevalence of IDA supports previous studies that showed an increased risk of IDA in CD patients [23].
IDA negatively impacts the quality of life, physical performance levels, and cognitive functioning [25-27]. Women of reproductive age have a higher risk of developing IDA. In our cohort, CD patients with IDA were more common to be females and were younger in age compared to CD patients without IDA. In previous studies, IDA was associated with higher rates of hospitalizations, a longer length of stay, and an increased healthcare burden [28]. We showed that CD patients with IDA had an increased length of stay, higher hospitalization costs, and higher odds of readmission. ACD was also associated with worse hospitalization outcomes suggesting that proper diagnosis and management of anemia in this patient population is essential to improve hospitalization outcomes. Diagnosis of IDA in CD patients can be challenging in the context of chronic inflammatory disease. Hence, the involvement of a hematologist can be critical for appropriate diagnosis and management, and future studies should be directed toward developing diagnostic tests which can accurately diagnose IDA in patients with IBD. In our cohort of CD patients, 219 patients (0.4%) had vitamin B12 deficiency, and this reflects another aspect of the complexity of diagnosing anemia in this patient population. CD patients with IDA were less common to have diabetes, hypertension, or chronic obstructive pulmonary disease (COPD) which might be attributed to the statistically significant younger age of CD patients with IDA. IDA is associated with a substantial health and cost burden, and untreated IDA can lead to heart failure and depression [3]. We found that CD patients with IDA were more common to have a major depressive disorder compared to CD patients without a comorbid IDA; however, there was no statistically significant difference in the prevalence of heart failure between the two groups. CD patients with IDA were more common to have weight loss and less common to have obesity suggesting a potential role of malnutrition in the development of IDA in this population. More patients with IDA fell into the lowest median household income group which suggests a potential role of poor diet and malnutrition in the development of IDA in this patient population. A healthy lifestyle should always be advised, and a multidisciplinary approach might reduce disease-related costs and improve hospitalization outcomes. The involvement of IBD nutritionists in the management of these patients might help improve outcomes.
In our cohort, CD patients with IDA were younger in age compared to CD patients without IDA. Younger age at diagnosis of CD has been associated with a more aggressive clinical course and was considered a significant prognostic risk factor [29, 30]. CD patients with IDA had a higher prevalence of fistulizing CD and intestinal abscesses, indicating a potential relationship between disease severity and development of IDA. CD patients with IDA had a higher prevalence of active lower gastrointestinal bleeding compared to CD patients without a comorbid IDA. There was a higher prevalence of thrombocytopenia and cirrhosis in CD patients with IDA. Early identification and management of conditions commonly associated with increased risk of blood loss might decrease the risk of IDA in this patient population. Screening CD patients for IDA is an essential part of their management due to its high prevalence and impact on quality of life and morbidity [31]. The European Crohn’s and Colitis Organization (ECCO) recommends screening IBD patients for IDA every 3 months for active disease and every 6 - 12 months for quiescent disease [18]. The impact of IDA in the IBD population is usually underestimated, with a significant delay in the initiation of treatment [3, 22]. A study by Stein et al showed that physicians frequently deviate from international guidelines for the management of IDA in patients with IBD [21]. Nationwide studies are a powerful tool to reveal the significant prevalence and impact of comorbidities and educate healthcare policymakers about the potential risks and required screening measures to improve outcomes. Future studies are needed to estimate the impact of screening CD patients for IDA in reducing hospitalization risk and adverse outcomes.
There are three available options for the treatment of IDA in patients with CD: oral iron, parenteral iron, and erythropoietin. Parenteral iron supplementation except for iron dextran is the preferred route in patients with IBD, especially since nonabsorbed oral iron can worsen IBD symptoms and aggravate intestinal inflammation [32]. Oral iron should only be used for those with quiescent CD, mild anemia (hemoglobin 11.0 - 11.9 g/dL in non-pregnant women and 11.0 - 12.9 g/L in men), and no history of intolerance to oral iron [33]. ECCO guidelines define adequate therapeutic response as a hemoglobin increase of > 2 g/dL and transferrin saturation of > 30% within 4 weeks from initiating treatment [18]. If the therapeutic response is suboptimal, oral iron supplementation should be changed to parenteral iron. Even though parenteral iron is efficacious, it remains underused in the IBD population both in Europe and in the USA [21, 24]. The third treatment option is erythropoietin which can be used for anemia associated with IBD in patients who do not respond to intravenous iron therapy [34].
Our study has limitations, which should be considered when interpreting its data. The retrospective design carries inherent weaknesses. The use of administrative codes (ICD-10-CM) is subject to misclassification. Our study did not include any outpatient records. The NRD does not provide data on medications prescribed during hospitalization, and we were unable to study the impact of intravenous iron therapy on the secondary outcomes. However, we used a robust database validated for use in clinical and epidemiological research. While the use of a nationwide database does not allow us to mention the specific laboratory tests’ cut-off values used to diagnose IDA, it enables our study to reflect the prevalence of the condition nationwide and account for the different laboratory kits used in different hospitals. Our study is the first large-scale study attempting to study the burden of IDA among hospitalized CD patients in the USA, which brings new insights into our understanding of IDA in this population.
In conclusion, IDA is frequently encountered in patients with CD, and a significant association between IDA and worse hospitalization outcomes was observed in our nationwide cohort of CD patients. Therefore, early recognition and management of IDA among patients with CD might be important to improve hospitalization outcomes. Highlighting the significant burden of IDA in patients with CD, we aim to draw attention to the significance of IDA in this patient population and hope this will incite further research in the field to improve screening, diagnosis, and management of IDA.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
As the NRD database lacks patient-specific identifiers, no consent was required for the analysis.
Author Contributions
Substantial contributions to the conception and design of the study: Ahmed Abomhya. Acquisition, analysis, interpretation, and statistical analysis of data: all authors. Literature review: all authors. Writing and revising the manuscript critically for intellectual content: all authors. Approval of the submitted version: all authors. Agreement to be accountable for all aspects of the study: all authors.
Data Availability
The NRD is available at: https://www.hcup-us.ahrq.gov/nrdoverview.jsp.
Abbreviations
CD: Crohn’s disease; IDA: iron deficiency anemia; UC: ulcerative colitis; HCUP: Healthcare Cost and Utilization Project; NRD: Nationwide Readmissions Database; ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; IQR: interquartile range; OR: odds ratio; CI: confidence interval
| References | ▴Top |
- Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832-1843.
doi pubmed - Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741-1755.
doi - Gisbert JP, Gomollon F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103(5):1299-1307.
doi pubmed - Gasche C. Anemia in IBD: the overlooked villain. Inflamm Bowel Dis. 2000;6(2):142-150; discussion 151.
doi pubmed - Akhuemonkhan E, Parian A, Miller K, Hanauer S, Hutfless S. Prevalence and screening for anaemia in mild to moderate Crohn's disease and ulcerative colitis in the United States, 2010-2014. BMJ Open Gastroenterol. 2017;4(1):e000155.
doi pubmed - Danese S, Hoffman C, Vel S, Greco M, Szabo H, Wilson B, Avedano L. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn's and Ulcerative Colitis Association's online survey. Eur J Gastroenterol Hepatol. 2014;26(12):1385-1391.
doi pubmed - Bager P, Befrits R, Wikman O, Lindgren S, Moum B, Hjortswang H, Dahlerup JF. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand J Gastroenterol. 2011;46(3):304-309.
doi pubmed - National Hospital Ambulatory Medical Care Survey: 2010. Available from: https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf.
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907-916.
doi - Daneryd P, Svanberg E, Korner U, Lindholm E, Sandstrom R, Brevinge H, Pettersson C, et al. Protection of metabolic and exercise capacity in unselected weight-losing cancer patients following treatment with recombinant erythropoietin: a randomized prospective study. Cancer Res. 1998;58(23):5374-5379.
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011-1023.
doi pubmed - Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95(2):175-178.
doi pubmed - Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis. 2012;18(10):1961-1981.
doi pubmed - Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093.
doi pubmed - Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marx JJ. Iron and inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15(4):429-438.
doi pubmed - Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease - a practical approach. Ann Gastroenterol. 2013;26(2):104-113.
- Bayraktar UD, Bayraktar S. Treatment of iron deficiency anemia associated with gastrointestinal tract diseases. World J Gastroenterol. 2010;16(22):2720-2725.
doi pubmed - Dignass AU, Gasche C, Bettenworth D, Birgegard G, Danese S, Gisbert JP, Gomollon F, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211-222.
doi pubmed - HCUP-US NRD Overview. [Accessed September 21, 2021]. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp.
- CFR 46. [Accessed September 21, 2020]. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html.
- Stein J, Bager P, Befrits R, Gasche C, Gudehus M, Lerebours E, Magro F, et al. Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol. 2013;25(12):1456-1463.
doi pubmed - Blumenstein I, Dignass A, Vollmer S, Klemm W, Weber-Mangal S, Stein J. Current practice in the diagnosis and management of IBD-associated anaemia and iron deficiency in Germany: the German AnaemIBD Study. J Crohns Colitis. 2014;8(10):1308-1314.
doi pubmed - Patel D, Yang YX, Trivedi C, Kavani H, Xie D, Medvedeva E, Lewis J, et al. Incidence, duration, and management of anemia: a nationwide comparison between IBD and non-IBD populations. Inflamm Bowel Dis. 2020;26(6):934-940.
- Khan N, Patel D, Shah Y, Yang YX. Factors predicting testing and treatment of iron deficiency in a nationwide cohort of anemic UC patients. Inflamm Bowel Dis. 2016;22(12):2894-2901.
doi pubmed - Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. 2005;8(6):629-638.
doi pubmed - Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12(2):123-130.
doi pubmed - Konig P, Jimenez K, Saletu-Zyhlarz G, Mittlbock M, Gasche C. Iron deficiency, depression, and fatigue in inflammatory bowel diseases. Z Gastroenterol. 2020;58(12):1191-1200.
doi pubmed - Blaney H, Vu P, Mathew A, Snelling R, England J, Duong C, Hammonds K, et al. Anemia severity associated with increased healthcare utilization and costs in inflammatory bowel disease. Dig Dis Sci. 2021;66(8):2555-2563.
doi pubmed - Solberg IC, Vatn MH, Hoie O, Stray N, Sauar J, Jahnsen J, Moum B, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430-1438.
doi pubmed - Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006;130(3):650-656.
doi pubmed - Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):44S-49S.
doi pubmed - Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545-1553.
doi pubmed - Schroder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100(11):2503-2509.
doi pubmed - Moreno Lopez R, Sicilia Aladren B, Gomollon Garcia F. Use of agents stimulating erythropoiesis in digestive diseases. World J Gastroenterol. 2009;15(37):4675-4685.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


