| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 3, June 2022, pages 113-120
Spectrum of Immune Checkpoint Inhibitor Anemias: Results From a Single Center, Early-Phase Clinical Trials Case Series Experience
Blessie Elizabeth Nelsona, f , Chinenye Lynette Ejeziea, Bettzy A. Stephena, Mirella Nardoa, Erick Campbella, Jing Gonga, David S. Honga, Siqing Fua, Timothy A. Yapa, Mariela Blum Murphyb, Sarina Piha-Paula, Naval G. Daverc, Cristhiam M. Rojas-Hernandezd, e, Aung Nainga, e
aDepartment of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
bDepartment of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA
cDepartment of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX, USA
dDepartment of Benign Hematology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
eThese authors contributed equally to this article.
fCorresponding Author: Blessie Elizabeth Nelson, Department of Investigational Cancer Therapeutics (a Phase I Clinical Trials Program), The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Manuscript submitted April 15, 2022, accepted May 6, 2022, published online June 2, 2022
Short title: Immune Checkpoint Inhibitors-Related Anemias
doi: https://doi.org/10.14740/jh1006
| Abstract | ▴Top |
Immune checkpoint inhibitor anemias (ICI-A) are a rare entity which can be potentially life-threatening without prompt identification. The goal of the study is to characterize the presentation, evaluation, and outcomes of ICI therapy in early phase clinical trial setting to guide future research and to develop standardized care guidelines. Retrospective chart review of 333 patients who participated in early phase clinical trials at the University of Texas MD Anderson Cancer Center revealed four cases with ICI-A between 2016 and 2020. We identified a spectrum of four cases which included ICI-related autoimmune hemolytic anemias, hemophagocytic lymphohistiocytosis and thrombotic microangiopathy as a result of combinatory investigational therapies involving ICI. Patient presentation, evaluation, bone marrow pathology, interventions, and clinical course were reviewed. The median time to onset of hematological immune-related adverse events (heme-irAEs) in this retrospective series was 3.5 weeks (2 - 6 weeks). One patient had pre-existing untreated chronic lymphocytic leukemia. Glucocorticoids are an effective first-line treatment in most patients although most patients were not rechallenged but successfully had complete recovery and pursued further non-immunotherapy-based therapies. Cognizance of ICI-A in clinical trial setting is paramount to early recognition of heme-irAEs. Further research is needed to identify and stratify risk factors during clinical trial enrollment and optimal management strategies for immune-mediated hematologic toxicities.
Keywords: Immunotherapy; Anemia; Phase 1; Hematological immune adverse events
| Introduction | ▴Top |
Advent of immunotherapy has changed the treatment landscape in oncology and improved efficacy and durability of responses. The use of agents that block co-inhibitory immune checkpoint molecules, such as anticytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) amplify the adaptive T cell response. However, this can lead to disequilibrium in immunologic tolerance leading to an unexpected off-target immune response against innate organs due to lack of antigen specificity. This may clinically manifest with a myriad of autoimmune-like side-effects, such as dermatological, gastrointestinal, hepatic, pulmonary, endocrine and hematological toxicities. Such adverse events, named “immune-related adverse events (irAEs)” are principally linked to a dysregulated T-cell effect to the homeostasis of the human system [1, 2].
Traditionally, hematological toxicities are observed with chemotherapy and targeted therapies secondary to direct myelosuppression from cytotoxic or cytostatic response. Compared to chemotherapeutic or targeted agents, immunotherapies have a distinct toxicity profile by inducing infiltration of immune cells into the hematopoietic system [3]. In literature, the incidence of hematological irAEs (heme-irAEs) is less than 1%. The spectrum of hematological toxicities reported with immunotherapy include hemolytic anemia, pure red cell aplasia (PRCA), thrombocytopenia, neutropenia, lymphopenia, hemophagocytic lymphohistiocytosis (HLH), aplastic anemia, other bone marrow failure syndromes, thrombotic thrombocytopenic purpura (TTP) and other thrombotic syndromes [4, 5]. However, there is dearth of knowledge of its occurrence and presentation in the realm of investigational cancer therapeutics where heightened acuity to immune-mediated hematological adverse events is needed. Hence, we aim to examine the incidence and provide descriptive review of the spectrum of immune checkpoint inhibitor anemias (ICI-A) seen with investigational agents. To the best of our knowledge, this is the first case report series of its kind in the early phase clinical trial setting.
This was a retrospective observational study focused on heme-irAEs limited to various types of anemia induced by anti-PD-1 or anti-PD-L1 investigational agents between March 15, 2016, and February 5, 2020. This retrospective study was approved by the Institutional Review Board (IRB) at The University of Texas MD Anderson Cancer Center, and informed consent was waived due to retrospective nature of the study. All patients included in this study however, provided written informed consent before enrollment on an IRB-approved ICI-based, early phase clinical trial. We defined ICI-associated autoimmune hemolytic anemia (ICI-AIHA) based on the criteria developed by Leaf et al where a temporal correlation with ICI use was noted [6]. We screened the electronic medical record of 333 participants enrolled on ICI-based early phase clinical trials between March 15, 2016, and February 5, 2020 to identify patients as represented in consort diagram (Fig. 1), who developed an abrupt decrease in hemoglobin (Hb) ≥ 2 g/dL from baseline (defined as cycle 1 day 1). We excluded patients who did not receive ICI or developed acute bleeding from any sites, direct temporal myelosuppressive effects, infections or renal disease. We manually reviewed the electronic medical records of all 333 patients to assess the causes of anemia, temporal relationship with ICI administration, and obtain clinical and laboratory data. Four patients were identified to have developed ICI-A secondary to their direct attribution to development of the respective adverse event. Respective ClinicalTrials.gov Identifiers are NCT02009449; NCT02904226; NCT02983578 and NCT03307785. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 [7]. Tumor responses were assessed based on Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and/or Immune Response Evaluation Criteria in Solid Tumors (iRECIST) [8].
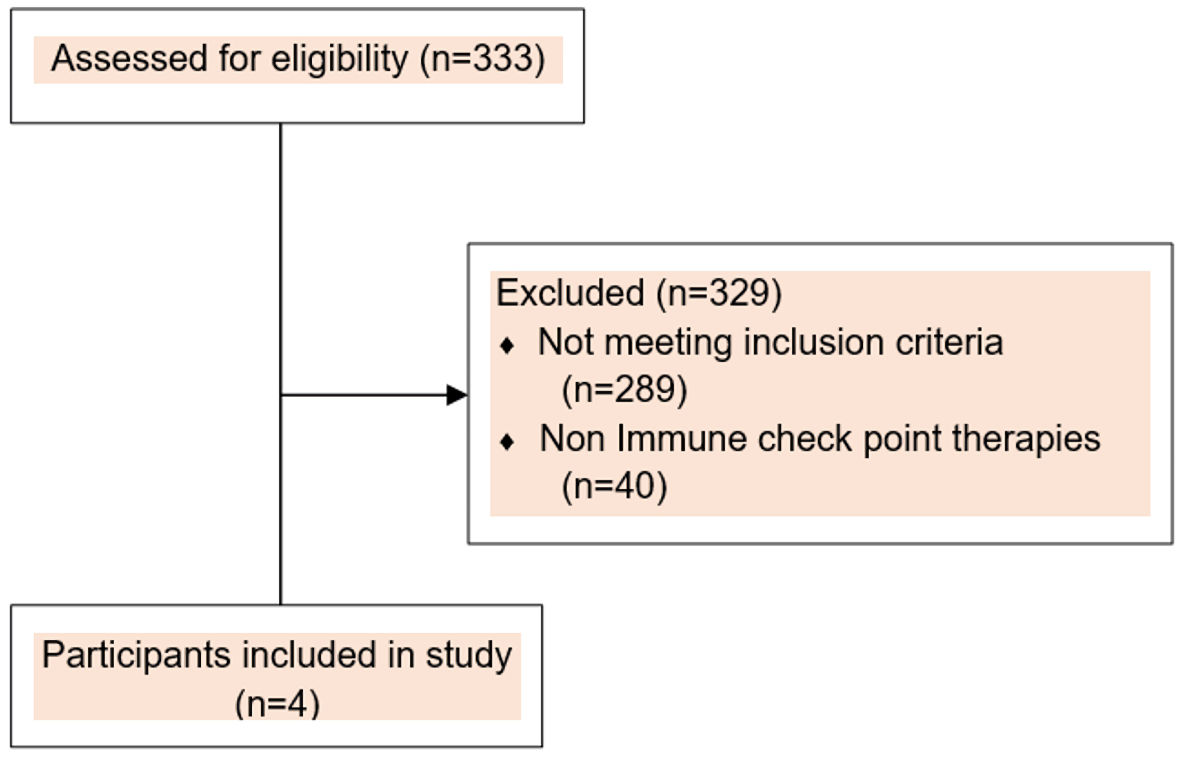 Click for large image | Figure 1. Consort flow diagram. |
| Case Reports | ▴Top |
Case 1
A 69-year-old Caucasian male with diagnosis of stage IV metastatic non-small cell lung adenocarcinoma with spread to bilateral pleurae was referred for consideration for early phase clinical trial after disease progression on three lines of therapies including cisplatin and pemetrexed, carboplatin and pemetrexed and single agent pemetrexed maintenance. Next generation sequencing of tumor specimen showed an ERBB4 and non-canonical KRAS mutation. He also had a 5-year history of untreated chronic lymphocytic leukemia (CLL) comprising 40% of bone marrow involvement with regional adenopathy with 13q deletion and unmutated IgVH status. He was enrolled into a phase 1 clinical trial employing an anti-PD1 antibody in combination with a STAT3 inhibitor and received 11 cycles uneventfully. At his 12th cycle clearance, patient developed grade 4 anemia (Hb 4.6 g/dL) and grade 3 thrombocytopenia (platelets 32,000/µL) and was admitted for further evaluation. The reticulocyte percentage was 1.2 while the serum lactate dehydrogenase (LDH) was elevated at 439 U/L (reference values: 135 - 225 U/L) and haptoglobin was undetectable although normal at baseline. Peripheral blood smear had some dysplastic changes (ovalocytes, few dysplastic neutrophils with left shift with the presence of metamyelocytes, without microspherocytes, schistocytes, or red blood cell (RBC) agglutination). A bone marrow biopsy performed 1 month prior to this event showed 10% involvement of CLL with no blasts, decreased megakaryocytes, erythrocytes with mild dysplasia. Next generation sequencing for myelodysplastic syndromes (MDS) and acute myeloid leukemia was negative for any genetic alterations. The direct antiglobulin test (DAT) was positive for C3. His iron stores were normal with no recent history of blood loss. His overall clinical picture was consistent with immune-mediated hemolytic anemia incited by ICI exposure. He was treated with cyclosporine at 200 mg daily for 1 month with mild improvement and continued need for packed red blood cell (PRBC) transfusion. He was taken off ICI trial to pursue standard of care therapy with afatinib to target his ERBB4 mutation. Hence, in order to induce remission, he was started on pulse dose steroids concomitantly with 1,000 mg of intravenous (IV) methylprednisolone weekly for 4 weeks along with rituximab at a weekly dose of 375 mg/m2 for 4 weeks. Along with monthly IV immunoglobulin at 1 g for 3 months, he was then transitioned to oral prednisone 1 mg/kg daily and was tapered over a period of 3 months with gradual improvement in his hematological parameters: improvement of Hb to 10.2 g/dL, normalization of platelet count at 136/µL, and detectable haptoglobin at 176 mg/dL as reflected in Tables 1 - 3.
 Click to view | Table 1. Patient Demographics |
 Click to view | Table 2. Laboratory Features of ICI-A |
 Click to view | Table 3. Treatment and Outcomes |
Case 2
This is a 68-year-old Caucasian lady with de novo stage IV metastatic gastroesophageal adenocarcinoma with metastasis to the liver. She had received one line of therapy and had progressed after nine cycles of mFOLFOX. Hence, she was enrolled into a phase 1 clinical trial with dostarlimab (PD-1 inhibitor) in combination with niraparib and bevacizumab due to her germline BRCA2 mutation [9]. After 21 cycles, she developed acute grade 3 anemia (Hb 7.9 g/dL) with no other cytopenias prompting holding of therapy. There was no concern for acute blood loss. Anemia workup revealed normal iron stores with elevated ferritin levels (770 ng/mL), while reticulocyte percentage was 1.9% and serum vitamin B12 and folate levels were normal. However, patient had low haptoglobin level (16 mg/dL) with high LDH (259 U/L) (135 - 225 U/L). DAT was negative. Peripheral blood smear revealed anisopoikilocytosis and ovalocytes (including macro-ovalocytes), while no microspherocytes and very rare schistocytes and occasional teardrop forms were present. No blasts or immature forms were noted. In the absence of other cytopenias, leukoerythroblastosis or dysplastic features, a bone marrow biopsy was not considered necessary in this case. The anemia was approached as predominantly immune-mediated incited by the PD1 inhibitor as patient did not experience acute anemia for 21 cycles while on the same dose of the poly-(ADP ribose) polymerase (PARP) inhibitor. The anti-PD1 antibody schedule was extended to every 6-weekly dosing from every 3-weekly dosing and the dose of the PARP inhibitor was dropped to the next dose level. Her haptoglobin levels improved to 38 mg/dL and LDH normalized at 206 U/L, and she went on to complete 14 more cycles of dostarlimab in combination with niraparib and bevacizumab at this dose level. She achieved a best objective response of confirmed partial response at -46% but came off trial due to withdrawal of consent.
Case 3
A 65-year-old Caucasian lady with metastatic esophageal adenocarcinoma with metastases to the liver and lymph nodes started on a phase 1 clinical trial with inducible T-cell co-stimulator (ICOS) agonist monoclonal antibody and anti-PD-1 monoclonal antibody after failing two lines of prior cytotoxic chemotherapies. Patient presented with acute generalized jaundice and shortness of breath prior to cycle 3 and was noted to have Hb of 6.8 g/dL, indirect hyperbilirubinemia at 5.7 mg/dL, transaminitis (aspartate aminotransferase (AST) 206 U/L; alanine aminotransferase (ALT) 181 U/L; alkaline phosphatase (ALP) 336 U/L) and thrombocytopenia (platelets 109,000/µL). LDH was elevated at 7,175 U/L (135 - 225 U/L) with undetectable haptoglobin levels while DAT was negative and reticulocyte percentage was 7. Peripheral blood smear revealed spherocytes and nucleated RBCs and an enhanced DAT was negative. The case was approached as confirmed DAT negative AIHA secondary to ICI. She was started on IV methylprednisolone 1 mg/kg every 12 h (Q12H) along with supportive transfusions in the event of symptoms of bleeding or cardiac insufficiency. However, on day 4 of her hospital admission, she succumbed to acute hypoxic respiratory failure with persistent anemia secondary to hemolysis and new onset thrombocytopenia, as low at 32,000/µL. Peripheral blood smear on day 4 revealed anisopoikilocytosis, numerous spherocytes and schistocytes, teardrop forms, nucleated RBCs, burr cells. Left shifted hematopoiesis with bandemia and metamyelocytes were seen. No blasts or dysplastic neutrophils were noted. Platelets were reduced in number while large granular forms were seen. The prothrombin time was elevated at 21.6 s while the partial thromboplastin time was normal and international normalized ratio (INR) was elevated at 1.88. The D-dimer level was elevated at 6.11 µg/mL, FEU ADAMTS13 activity level was mildly low at 56% and no inhibitors were identified consistent with a drug-induced etiology. It was hence deemed that patient evolved to a clinical picture consistent with thrombotic microangiopathy (TMA) and leukoerythroblastic anemia that stemmed from ICI-AIHA. Unfortunately, patient and family elected for comfort care and eventually the patient expired. An autopsy revealed poorly differentiated adenocarcinoma, involving distal esophagus with metastatic adenocarcinoma in liver, hilar and paraaortic lymph nodes, myometrium, and involving multiple vessels in lung, heart, pancreas, adrenal, kidney, spleen and vaginal wall. Extramedullary hematopoiesis in spleen was present and a hypercellular bone marrow with decreased myeloid to erythroid ratio was noted with no evidence of metastatic disease to the bone marrow.
Case 4
Our last patient whose diagnosis of stage III primary right renal cell carcinoma, clear cell type extended back to 2002, was addressed by definitive right radical nephrectomy and adjuvant sunitinib. She had recurrence-free interval of 14 years, following which oligometastatic spread was managed with metastasectomies. In 2016, she developed stage IV disease with metastases to the pancreas, peritoneum and left adrenal gland and hence started on a phase 1 clinical trial of pegilodecakin (pegylated interleukin (IL)-10) combined with nivolumab (PD-1 inhibitor) [10]. Prior to clearance for cycle 5 therapy, she experienced acute onset fatigue, abdominal discomfort with early satiety, fevers and generalized malaise. Workup revealed grade 2 thrombocytopenia (platelets 68,000/µL), grade 2 anemia (8.2 g/dL) with massive acute splenomegaly (13 × 14 cm, previously 4 × 12 cm). On admission, she was febrile (38.7 °C) and tachycardic (up to 114 beats/min (bpm)), otherwise hemodynamically stable. Further laboratory workup revealed DAT immunoglobulin G (IgG) positivity and negative eluate suggesting drug-induced hemolytic anemia with elevated LDH at 4,896 U/L (135 - 225 U/L), mild transaminitis, undetectable haptoglobin levels, reticulocyte percentage (3.9%) and normal bilirubin levels. Her peripheral blood smear review revealed signs of functional asplenia, microspherocytes and artifactual changes in the RBCs with some blister cells. Heinz body stain was negative. Her ferritin levels were notable at 25,472 ng/mL. Based on the following symptoms and signs: fever of 38.7 °C, massive splenomegaly on computed tomography (CT), eventual pancytopenia (platelets 37,000/µL; Hb 7.7 g/dL; absolute neutrophil count 1,320), ferritin rising up to 32,110 ng/dL and IL-2 receptor CD25 levels elevated at 9,020 pg/mL, the working diagnosis of hemophagocytic syndrome was made. Bone marrow biopsy revealed hypercellular (80%) bone marrow with trilineage hematopoiesis and rare hemophagocytic macrophages with no evidence of morphologic and immunohistochemical support for metastatic tumor infiltration. She was started on a regimen of etoposide 100 mg/m2 every 3 days (Q3D) × 3 doses, daily dexamethasone 10 mg twice a day (BID) tapered over 1 month, and one dose of tocilizumab 4 mg/kg. At her first month follow-up, ferritin dropped to 1,952 ng/mL, and at 3 months follow-up it fell to 750 ng/mL. Her cytopenias and IL2-CD25 levels (420 pg/mL) normalized as well. She had responded well to therapy with reduction of LDH, improvement in splenomegaly, and normalization of her liver enzymes. A bone marrow aspiration performed post treatment showed absence of hemophagocytic macrophages with trilineage hematopoiesis and regenerative features. She developed progression of disease 1 year later and was started on axitinib in 2017. She has been deemed to have stable disease for past 5 years with no recurrence of signs of hemophagocytosis.
| Discussion | ▴Top |
To our knowledge this case series of four patients with hematological immune anemias induced by investigational anti-PD-1 or anti-PD-L1, is the first study to shed light on the incidence and provide descriptive evidence of its occurrence in patients enrolled in early phase clinical trials. The spectrum of ICI-A that has been reported in literature include hemolytic anemia, thrombocytopenia, neutropenia, thrombotic microangiopathy (TMA) and hemophagocytosis. Largest case series of ICI-A examined so far come from the VigiBase and the Food and Drug Administration database [11, 12]. Although the frequency of hematological toxicities is low, those that did occur were often clinically serious and life-threatening [13]. Despite the serious nature of the hematologic irAEs, to this date, clear mechanistic studies have not elucidated the triggers and pathogenesis of these irAEs while some studies attribute it to the removal of the checkpoint brakes and indiscriminate T-cell activation [14, 15].
The median time to onset of heme-irAE in this retrospective series was 3.5 weeks from last dose of ICI, which was similar in other studies (3 - 10 weeks) examining similar hematological factors and for other types of irAEs [4, 16]. The time to occurrence of heme-irAEs was varied in our study (2 - 6 weeks) although our search parameters were limited to 10 weeks post investigational agent use to avoid overlap with consecutive lines of therapies that may confound results, although in literature heme-irAEs can potentially occur at any time [11]. However, the number of cycles varied between two and 21 cycles, with a median of eight cycles consistent with similar studies as reported above where reported occurrences have happened as late as 39th cycle [6]. Notably, warm (IgG-mediated) and cold (C3-mediated) immune hemolytic anemias were observed with anti-PD-1 or anti-PD-L1 immunotherapies. The incidence of heme-irAEs with anti-PD-1 versus anti-PD-Ll ICI were similar concordant with published literature [12].
Timely detection of ICI-A is pertinent when dealing with investigational ICI therapies. Two patients (50%) in our series developed ICI-AIHA that was reversible allowing them to pursue further systemic therapies. Concordant to findings by Leaf et al, we found severity of hemolysis observed was proportional in DAT-positive and DAT-negative patients [6]. Hence, it is necessary to maintain increased vigilance for ICI-A in DAT-negative patients as well. Early detection and prompt institution of therapy reverses this spectrum of ICI-A as reported here and in literature. There is no clear consensus on rechallenge but about 50% of patients in retrospective databases who underwent rechallenged had no relapse in ICI-A. In our cohort, three patients experienced durable response close to 1 year and hence this highlights importance of carefully gauging ICI-based treatments to improve patient outcomes while monitoring these adverse events which can be set to remission.
In our study, one of the four patients had a concomitant medical history of untreated B-cell CLL which has a higher predisposition for AIHA and Evan’s syndrome, as seen in our case secondary to clonal propagation of immune dysregulation through immunosuppressive cytokines or by downregulation of surface molecules. Exposure to ICI has also been seen in this subset of patients as supported by published literature [17, 18]. Nine percent of patients in the REISAMIC registry were also noted to experience similar effects which increases the concern for detrimental responses and hence careful selection of these patients into standard of care or clinical trials with exposure to ICI should be weighed with caution.
Hemophagocytosis is an uncommon heme-irAE where Davis et al [11] reported an incidence of 26 patients (15%) from the VigiBase implicated mainly by complicated CTLA-4-based regimens rather than anti-PD-1/PD-L1 therapy with high mortality. In our case series, one patient (25%) developed hemophagocytosis secondary to exposure to pegylated IL-10 agent and anti-PD1 monoclonal antibody but due to early identification and therapy, she went into remission from that complication. Interestingly, our other patient who initially presented with characteristics consistent with ICI-AIHA eventually was complicated with microangiopathic phenomenon which is the first ever reported case to demonstrate such a unique presentation after exposure to ICI, although it carries a high mortality of 33% as translated in our patient [19].
Several limitations exist in this case series by virtue of its retrospective nature. It is probable that under-detection and diagnosis of ICI-A plays into the low capture of these cases. Therefore, the question continues to remain as to what the true incidence of ICI-A are and their spectrum in clinical practice. This scenario is further challenged by confounding effects of chronic disease and prior treatment. All patients in this case series received a combinatory investigational regimen where side effects may not be clearly delineated yet while in early phase drug development. The impact of the synergistic effects of combinatorial therapies in triggering ICI-A remains to be elucidated. The small sample size also precluded us from performing statistical tests to identify risk factors predisposing to ICI-A. Nevertheless, despite the retrospective nature of the study and the small patient numbers, longitudinal data on clinical and laboratory parameters from prospective trials helped us to capture any degree of toxicity, establish their temporal association to ICI therapy, monitor course of the adverse event, and durable response to therapy.
Conclusions
Risk assessment for predisposition, early recognition, and prompt institution of appropriate therapy are vital in mitigating ICI-related anemia. This is of utmost importance when studying investigational agents. Guidelines for treatment, monitoring and re-challenge in patients with heme-irAEs are evolving and gaining grounds in understanding this rare entity to clinical relevance.
Acknowledgments
We thank the patients who participated in this study, and their families for supporting them.
Financial Disclosure
None to declare.
Conflict of Interest
BEN, CLE, MN and MBM have no interests declared. TAY received research funding (paid to his institution) from Artios, AstraZeneca, Bayer, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, ImmuneSensor, Ipsen, Jounce, Karyopharm, Kyowa, Merck, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, and Vertex Pharmaceuticals. In addition, he has received fees for consulting with Almac, Aduro, AstraZeneca, Atrin, Axiom, Bayer, Bristol Myers Squibb, Calithera, Clovis, Cybrexa, EMD Serono, F-Star, Guidepoint, Ignyta, I-Mab, Jansen, Merck, Pfizer, Repare, Roche, Rubius, Schrodinger, Seattle Genetics, Varian, and Zai Labs. CMRH received research funding from Daichii Sankyo pharmaceuticals and Aspen pharmaceuticals. AN reports research funding from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Eli Lilly, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Kymab, PsiOxus, Immune Deficiency Foundation (Spouse), Advisory board: CytomX Therapeutics, Novartis, Kymab, Genome. Travel and accommodation expenses: ARMO BioSciences. SPP reports research funding through the institution: AbbVie, Inc., ABM therapeutics, Inc., Acepodia, Inc., Alkermes, Inc., Aminex Therapeutics, Amphivena Therapeutics, Inc., BioMarin Pharmaceutical, Inc., Boehringer Ingelheim, Bristol Myers Squib, Chugai Pharmaceutical Co., Ltd., Daichi Sankyo, Inc., Eli Lilly, Five Prime Therapeutics, Genmab A/S, GlaxoSmithKline, Helix BioPharma Corp., Incyte Corp., Jacobio Pharmaceuticals Co., Ltd., Medimmune, LLC., Medivation, Inc., Merck Sharp and Dohme Corp., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Principia Biopharma, Inc., Puma Biotechnology, Inc., Rapt Therapeutics, Inc., Seattle Genetics, Taiho Oncology, Tesaro, Inc., TransThera Bio. DSH reports research funding from AbbVie, Adaptimmune, Aldi-Norte, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, GSK, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, miRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda, Turning Point Therapeutics. Travel, Accommodations, Expenses: Bayer, LOXO, miRNA, Genmab, AACR, ASCO, SITC. Consulting or Advisory Role: Alpha Insights, Amgen, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Prime Oncology, Seattle Genetics, Takeda, Trieza Therapeutics, WebMD. Other ownership interests: Molecular Match (Advisor), OncoResponse (Founder), Presagia Inc (Advisor). SF reports research funding from AstraZeneca, Abbisko, Anaeropharma Science, Arrien Pharmaceuticals, BeiGene, BioAtla, LLC, Boehringer Ingelheim, Eli Lilly & Co., Hookipa Biotech, Huya Bioscience International, IMV, Inc., Innovent Biologics, Co., Ltd., Lyvgen Biopharm, Co., Ltd., MacroGenics, Medivir AB, Millennium Pharmaceuticals, Inc., Nerviano Medical Sciences, NeuPharma, inc., Novartis, OncoMed Pharmaceuticals, Parexel International, LLC, Sellas Life Sciences Group, Soricimed Biopharma, Inc., Tolero Pharmaceuticals, NovoCure, Turnstone Biologics, Taiho Oncology, Abbisko (other). NGD receives honoraria from BMS, Jazz Pharmaceuticals, Novartis, Incyte, Otsuka, Immunogen, Pfizer, Astellas Pharma, AbbVie. His consulting or advisory role includes Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Otsuka, Incyte, Karyopharm Therapeutics, Sunesis Pharmaceuticals, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics. His research funding includes Bristol Myers Squibb, Pfizer, Immunogen, Genentech, Nohla Therapeutics, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Novartis, Karyopharm Therapeutics, Incyte, Sunesis Pharmaceuticals, Sobi.
Informed Consent
Obtained during trial enrollment.
Author Contributions
Conception and design: BEN, BAS, AN, and CMRH. Acquisition of data: BEN, CLE, MN, EC, and JG. Analysis and interpretation of data: BEN. Critical revision for intellectual content: DSH, TAY, NGD, SF, MBM, CMRH, and AN.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
ICI: immune checkpoint inhibitor; ICI-A: immune checkpoint inhibitor anemia; SD: stable disease; PR: partial response; RECIST: Response Evaluation Criteria in Solid Tumors; DAT: direct antiglobulin test; AIHA: autoimmune hemolytic anemia; LDH: lactate dehydrogenase
| References | ▴Top |
- Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grunwald V, Kahler KC, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7-18.
doi pubmed - Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391.
doi pubmed - De Velasco G, Je Y, Bosse D, Awad MM, Ott PA, Moreira RB, Schutz F, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312-318.
doi pubmed - Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, Champiat S, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol. 2019;6(1):e48-e57.
doi - Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. 2021;9(1):e001719.
doi pubmed - Leaf RK, Ferreri C, Rangachari D, Mier J, Witteles W, Ansstas G, Anagnostou T, et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am J Hematol. 2019;94(5):563-574.
doi pubmed - CTCAE Version 5.0. Mar 29, 2022. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Yap TA, Bessudo A, Hamilton E, Sachdev J, Patel MR, Rodon J, Evilevitch L, et al. IOLite: phase 1b trial of doublet/triplet combinations of dostarlimab with niraparib, carboplatin-paclitaxel, with or without bevacizumab in patients with advanced cancer. J Immunother Cancer. 2022;10(3):e003924.
doi pubmed - Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, Autio KA, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019;20(11):1544-1555.
doi - Davis EJ, Salem JE, Young A, Green JR, Ferrell PB, Ancell KK, Lebrun-Vignes B, et al. Hematologic complications of immune checkpoint inhibitors. Oncologist. 2019;24(5):584-588.
doi pubmed - Tanios G, Doley PB, Munker R. Autoimmune hemolytic anemia and checkpoint inhibitors: 68 cases from the FDA database and critical review. Blood. 2018;132(Supplement 1):2324-2324.
doi - Wilson NR, Lockhart JR, Garcia-Perdomo HA, Oo TH, Rojas-Hernandez CM. Management and Outcomes of Hematological Immune-related Adverse Events: Systematic Review and Meta-analysis. J Immunother. 2022;45(1):13-24.
doi pubmed - Ammann S, Lehmberg K, Zur Stadt U, Janka G, Rensing-Ehl A, Klemann C, Heeg M, et al. Primary and secondary hemophagocytic lymphohistiocytosis have different patterns of T-cell activation, differentiation and repertoire. Eur J Immunol. 2017;47(2):364-373.
doi pubmed - Hollinger MK, Giudice V, Cummings NA, Rivell G, Zhang H, Kajigaya S, Keyvanfar K, et al. PD-1 deficiency augments bone marrow failure in a minor-histocompatibility antigen mismatch lymphocyte infusion model. Exp Hematol. 2018;62:17-23.
doi pubmed - Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
doi pubmed - Ni D, AlZahrani F, Smylie M. AIHA and pancytopenia as complications of pembrolizumab therapy for metastatic melanoma: a case report. Case Rep Oncol. 2019;12(2):456-465.
doi pubmed - Autore F, Pasquale R, Innocenti I, Fresa A, Sora F, Laurenti L. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: a comprehensive review. Cancers (Basel). 2021;13(22):5804.
doi pubmed - Ghanem P, Marrone K, Shanbhag S, Brahmer JR, Naik RP. Current challenges of hematologic complications due to immune checkpoint blockade: a comprehensive review. Ann Hematol. 2022;101(1):1-10.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


