| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 5, October 2022, pages 185-189
Dramatic Response After Switching MEK Inhibitors in a Patient With Refractory Mixed Histiocytosis
Anais Roesera, Fanelie Jouenneb, c, Laetitia Vercellinod, Julien Calvanib, e, Lauriane Goldwirtc, Gwenael Lorillona, Abdellatif Tazia, b, f
aNational Reference Center for Histiocytoses, Pulmonology Department, AP-HP, Hopital Saint-Louis, Paris, France
bInstitut de Recherche Saint Louis, Universite Paris Cite, INSERM U976, FR 75006, Paris, France
cDepartment of Pharmacology and Genomics, AP-HP, Hopital Saint-Louis, Paris, France
dDepartment of Nuclear Medicine, AP-HP, Hopital Saint Louis, Paris, France
eDepartment of Pathology, AP-HP, Hopital Saint Louis, Paris, France
fCorresponding Author: Abdellatif Tazi, Service de Pneumologie, Hopital Saint-Louis, 75475, Paris cedex 10, France
Manuscript submitted July 7, 2022, accepted August 22, 2022, published online October 31, 2022
Short title: Targeted Therapy in Refractory Histiocytosis
doi: https://doi.org/10.14740/jh1030
| Abstract | ▴Top |
We report the case of a patient with progressive multisystem mixed histiocytosis associating Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) involving the bone marrow, whose lesions harbored the MAP2K1 E102-I103del. After initial improvement under the MEK inhibitor trametinib, the treatment was only partially efficient and poorly tolerated. Eventually, although the trough blood level of trametinib at steady state was within expected ranges, the disease progressed to a life-threatening situation, with peritoneal involvement and anasarca. Switching to the MEK inhibitor cobimetinib as a salvage therapy resulted in a dramatic, rapid disease response, and the patient remains disease-free 3 years later with the treatment. The load of the MAP2K1 deletion in peripheral blood was correlated with the disease activity and strongly declined with cobimetinib, although it remained detectable at the last follow-up.
Keywords: Langerhans cell histiocytosis; Erdheim-Chester disease; Bone marrow; Peritoneum; Targeted therapy
| Introduction | ▴Top |
Since the identification of recurrent MAP kinase pathway gene alterations in L-group histiocytosis, targeted therapies such as BRAF and MEK inhibitors have dramatically changed the prognosis of severe forms of these rare diseases [1]. We herein report the benefit of switching MEK inhibitors as a salvage therapy in a case of severe multisystem refractory mixed histiocytosis associating Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD).
| Case Report | ▴Top |
Investigations
A 65-year-old nonsmoking woman was diagnosed in 2014 with isolated bone LCH confirmed on mastoid biopsy, with no BRAF mutation. She received methotrexate and corticosteroids that resulted in a complete metabolic response on 18 F-fluorodeoxyglucose positron emission tomography-computed tomography (18 F-FDG-PET-CT). Subsequently, she experienced multifocal bone and cutaneous involvement. Skin biopsy demonstrated superficial dermal infiltration by both CD163- CD1a+ histiocytes characteristic of LCH and CD163+ CD1a- suggesting concurrent non-LCH histiocytosis [1]. Despite treatment with vinblastine and subsequently cladribine, in December 2017, she presented with fever, abdominal pain, and multiple skin lesions, anemia (hemoglobin 6.9 g/dL) and an inflammatory syndrome (C-reactive protein (CRP) 92 mg/L). At this time, she was referred to our center.
Diagnosis
Bone marrow (BM) aspiration showed infiltration by histiocytic cells and some signs of hemophagocytosis. A BM core biopsy showed large sheets of CD163+/CD1a-/Langerin- histiocytes, with abundant foamy or eosinophilic cytoplasm. A few Touton cells were also observed (Fig. 1). PET-CT displayed hypermetabolism of the spleen (standardized uptake value (SUV)max 4.5) and diffuse sclerotic bone lesions (SUVmax 11.4) characteristic of ECD (Fig. 2a).
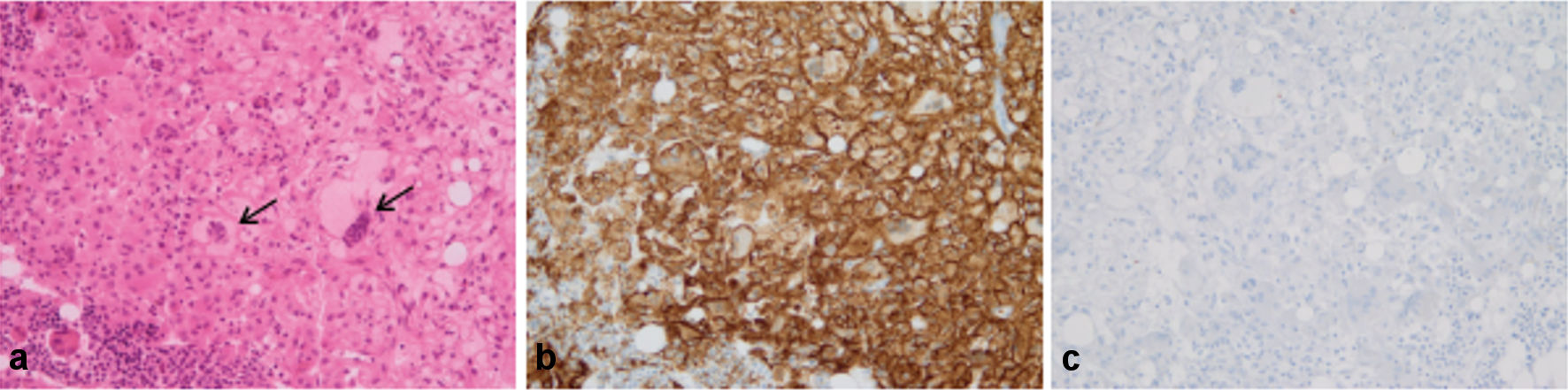 Click for large image | Figure 1. Bone marrow core biopsy. (a) Infiltration by large sheets of histiocytes, with abundant foamy or eosinophilic cytoplasm (H&E staining). A few Touton cells were also observed (arrows). (b) All histiocytes were intensely CD163+. (c) None of the cells were stained with anti-CD1a antibody (original magnification × 200). |
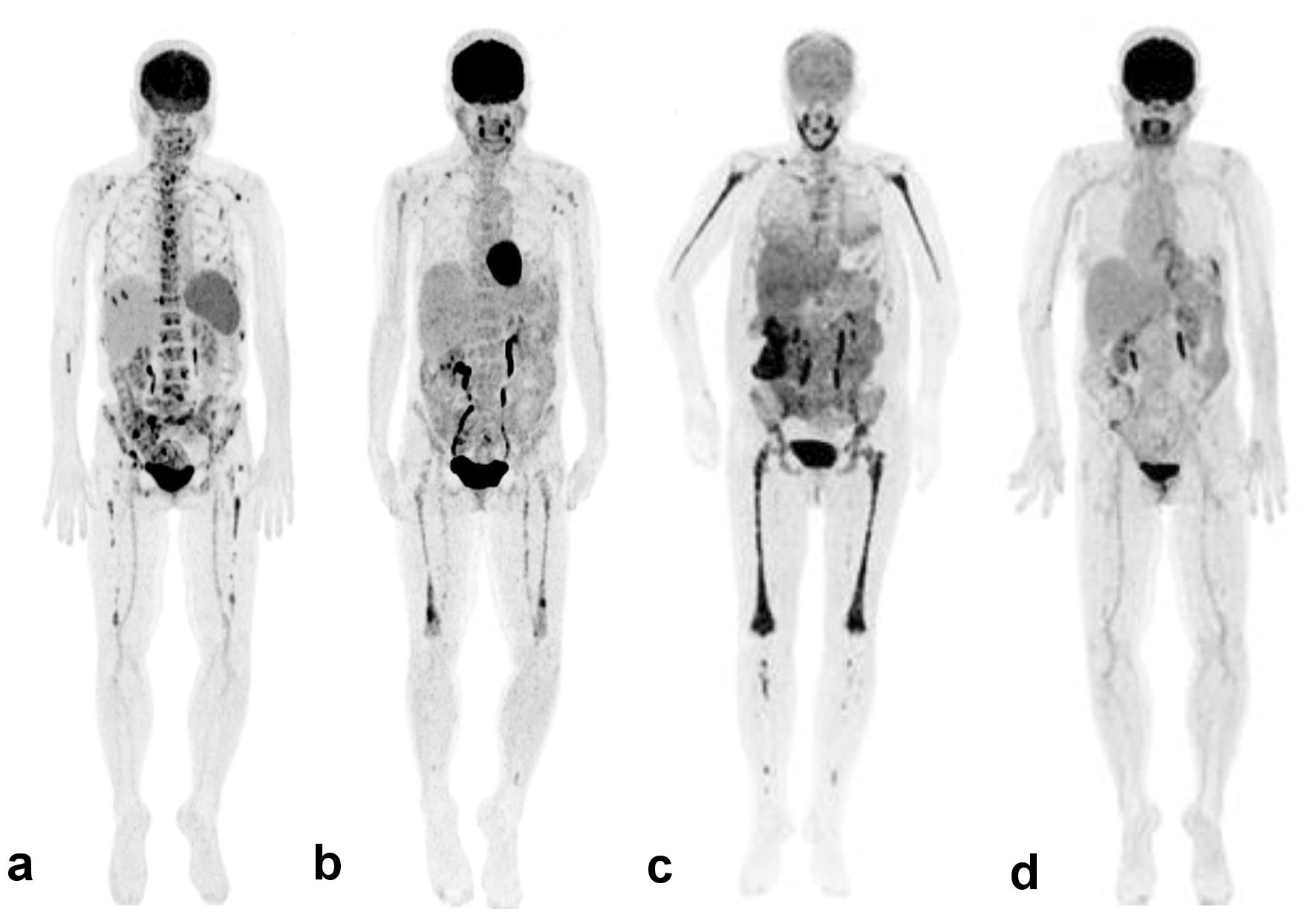 Click for large image | Figure 2. 18 F-FDG-PET-CT findings. Maximum intensity projection images of whole-body PET. (a) December 2017, at the time of initiation of trametinib. Diffuse bone marrow uptake, with focal hypermetabolic lesions in the axial and appendicular (especially in femurs) skeleton; diffuse homogeneous hypermetabolism in the spleen. (b) September 2018, under trametinib 1.5 mg/day. Decreased FDG uptake in the axial skeleton with extension of diffuse uptake in femurs, with a few focal lesions; decreased uptake in the spleen. (c) March 2019, at the time of initiation of cobimetinib. Diffuse intense hypermetabolism in the skeleton, with extension in peripheral bones; heterogeneous hypermetabolic uptake in the liver, intense hypermetabolism of the right colon and mild uptake of mesenteric infiltration; abundant non-hypermetabolic ascites. (d) November 2021, under cobimetinib 40 mg/day. Resolution of previous hypermetabolic lesions in the bone marrow, colon, and mesentery denoting a complete metabolic response. 18 F-FDG-PET-CT: 18 F-fluorodeoxyglucose positron emission tomography-computed tomography. |
Next-generation sequencing (NGS) of the previous mastoid and skin biopsies identified a deletion in MAP2K1 exon 3 (c.302-307del, p. E102-I103del) [2]. MAP2K1 deletion was detected in both BM and peripheral blood (variant allele frequencies (VAFs) of 0.84% and 1.20%, respectively). An NGS panel of 78 genes involved in hematological myeloid disorders [2] did not identify any additional mutations.
Treatment
Because we previously successfully treated a patient with refractory pulmonary LCH whose lesions harbored a MAP2K1 deletion with the MEK inhibitor trametinib [3], we started trametinib at 2 mg/day. The patient became afebrile within 24 h, and the cutaneous lesions strongly improved. She reported mild fluctuant diarrhea under treatment. Cardiac toxicity occurred in February 2018 with a decrease in left ventricle ejection fraction (LVEF) on cardiac echography. Trametinib was interrupted for a month, without progression of the disease, and was resumed at 1.5 mg/day in March 2018. The trough blood level of trametinib at steady state was 6.9 ng/mL, consistent with previously reported values [4]. In September 2018, new histiocytic skin lesions appeared, and PET-CT demonstrated the persistence of previous lesions, although with a lower FDG uptake (Fig. 2b). Cardiac echography evidenced the recurrence of decreased LVEF, again requiring the discontinuation of trametinib. A coronary angiography showed significant stenosis of the left anterior descending artery that was stented. Fever reappeared 24 h after trametinib discontinuation, as well as a flare of cutaneous lesions that persisted during the following month, after which LVEF was normalized. In November 2018, the patient presented with mild peripheral edema and fever, an inflammatory syndrome associated with hypoalbuminemia (17.7 g/L). Trametinib was resumed at 1.5 mg/day. She became afebrile, the skin lesions regressed, and PET-CT showed a decrease in the intensity of the bone lesions and a complete regression of spleen hypermetabolism. The trough blood level of trametinib at steady state was 7.6 ng/mL.
Follow-up and outcomes
In March 2019, she developed profuse lower limb edema, ascites and bilateral pleural effusions associated with intermittent diarrhea and profound hypoalbuminemia (15 g/L) with inflammatory anemia (hemoglobin 8.4 g/dL, CRP 40 mg/L). There was no cardiac dysfunction. Serum creatinine was normal without proteinuria. Mild anicteric cholestasis and vitamin K deficiency (factor VII 34%) were present with normal liver function (factor V 130%). We suspected a protein-losing enteropathy, but alpha-1 antitrypsin clearance was normal. Video capsule endoscopy showed scarce ulcerations in the small intestine. Ascites liquid was exudative (proteins 32 g/L, leucocytes 970/mm3, 36% lymphocytes and few histiocytes), as were the pleural effusions. PET-CT demonstrated intense hypermetabolic sclerotic bone lesions (SUVmax distal femur 8.2), as well as colonic (SUVmax 11.8), epiploic and mesenteric increased FDG uptake, and heterogeneous hepatic hypermetabolism associated with bilateral pleural effusions and abundant ascites (Fig. 2c). Trametinib was stopped, and the patient again became febrile. She suddenly experienced an ischemic stroke secondary to paroxysmal atrial fibrillation. Her general condition rapidly deteriorated, and she became bedridden.
On April 1, 2019, she was empirically switched to another MEK inhibitor cobimetinib, at a dose of 40 mg/day. Concurrently, her volume depletion was treated, and refeeding was initiated. Her condition dramatically improved, and she completely recovered from her stroke within 6 weeks. She tolerated cobimetinib well. The trough blood concentration of cobimetinib at steady state was 222 ng/mL, consistent with expected values [5]. Hemoglobin was 10.8 g/dL, CRP was 5 mg/L, and albumin was 37 g/L. In November 2021, she was still doing well under cobimetinib. PET-CT did not demonstrate any sign of disease activity (Fig. 2d).
Interestingly, whereas the load (VAF) of MAP2K1 deletion in the peripheral blood was high under trametinib treatment, it significantly decreased under cobimetinib treatment (Fig. 3).
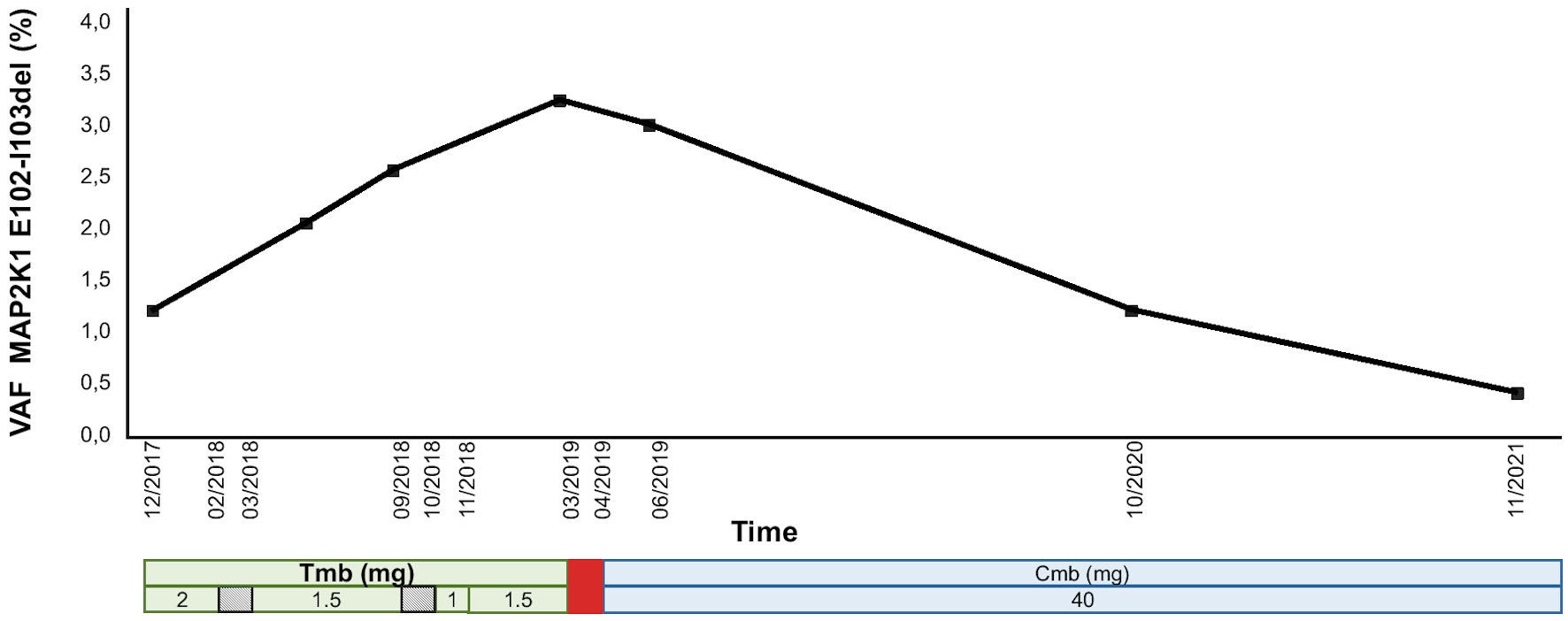 Click for large image | Figure 3. MAP2K1 E102-I103 deletion at the DNA level in the peripheral blood under MEK inhibitor treatment. The treatment regimen is shown at the bottom. Trametinib was transiently interrupted (diagonal stripes rectangle), each time because of LVEF decrease, and ultimately stopped (red rectangle) in the face of severe progression of the disease. The patient was then switched to cobimetinib. The data are expressed in terms of variant allelic frequency (VAF %). MEK inhibitor doses are in mg. VAF: variant allele frequency; Tmb: trametinib; Cmb: cobimetinib; LVEF: left ventricular ejection fraction. |
| Discussion | ▴Top |
The efficacy of targeted therapies in severe forms of ECD and mixed histiocytosis was first reported in BRAFV600E-mutated patients treated with the BRAF inhibitor vemurafenib [6]. The MEK inhibitor cobimetinib was also shown to be efficient, regardless of the mutational status of the histiocytic lesion [7, 8]. Similarly, trametinib was used with success in several patients whose lesions harbored a MAP2K1 mutation [9]. However, although both BRAF and MEK inhibitors are highly efficient, some patients experience only a partial response under these treatments [6, 8, 10].
ECD rarely infiltrates the BM [11]. Cohen-Aubart et al recently reported a series of 22 ECD patients with peritoneal and mesenteric involvement, among whom one patient presented with peripheral edema [12]. An ECD patient with ascites has also been previously reported [13]. In our patient, PET-CT demonstrated a highly hypermetabolic mesenteric thickening at the time of worsening of her disease with anasarca, including ascites. In contrast, liver involvement in the course of ECD has been exceptionally described [14, 15]. The patient’s hepatic manifestations were most likely related to LCH, in which the liver is a well-known localization [16].
Substantial progress has been made in the knowledge of mixed histiocytosis [17, 18]. In their review of the literature, Bonometti et al have better characterized the clinical spectrum and outcome of mixed histiocytosis. Our patient presented the most frequent type observed in adults (type-1 mixed histiocytosis), i.e., multisystem histiocytic disorder associating LCH and ECD involving the bones and skin [18]. Molecular alterations of the MAPK pathway are more frequently observed in mixed histiocytosis lesions, paving the way for targeted treatments in case of progressive disease. Pathogenic studies strongly suggest that mixed histiocytosis originate from a unique BM-derived neoplastic clone, bearing the driver MAPK molecular abnormality [19]. In addition, about 10% of these patients also develop hematologic malignancies [18, 20].
MEK inhibitors may induce side effects, mainly acne and digestive disturbances that may require a reduction in the dose of treatment [9]. Although less frequent, cardiac toxicity is well described under these drugs, requiring transient or definitive cessation of treatment [9]. Although peripheral edema secondary to cardiac toxicity may occur under MEK inhibitors [8], in the present case, both pleural effusions and ascites were exudative (and not transudate as in cardiac failure) and occurred concurrently with the reappearance of fever as well as other manifestations of progression of her histiocytic disorder. Prolonged inflammatory syndrome secondary to uncontrolled disease under trametinib, as well as mesenteric involvement, probably accounted for the development of severe hypoalbuminemia.
We identified MAP2K1 E102-I103del as a driver molecular alteration of the MAPK pathway in LCH lesions as well as in the BM infiltrated by ECD cells and in the peripheral blood [21]. In addition, as previously performed for the circulating BRAFV600E mutation [22, 23], we were able to monitor the load of the MAP2K1 deletion during treatment, initially with trametinib and subsequently with cobimetinib. Blood MAP2K1 deletion levels correlated with the activity of the disease and strongly decreased under cobimetinib, albeit it remained detectable at the end of follow-up, while the patient was in complete remission both clinically and on PET-CT.
Given that the trough blood level of trametinib at steady state was within expected ranges at the time of severe progression of the disease, non-pharmacological mechanisms are most likely involved in the better response to cobimetinib than to trametinib. A successful therapeutic response to trametinib in an LCH patient whose lesions harbored the same MAP2K1 E102-I103del has been previously reported [24]. However, recent in vitro experiments suggest that among MAP2K1 mutations identified in histiocytic disorders, the MAP2K1 E102-I103del is less sensitive to trametinib [25, 26]. This probably accounts for the partial and transient response to trametinib in this severe form of multisystem mixed histiocytosis.
Learning points
This observation illustrates mixed histiocytosis associating LCH and an unusual severe form of ECD and provides clues for management, in the case of disease progression under initial MEK inhibitor treatment.
Acknowledgments
The authors would like to thank E. Savariau for her technical assistance.
Financial Disclosure
This study was supported by the French Ministry of Health.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient included in the study.
Author Contributions
AR acquired the data and drafted the manuscript. FJ performed and analyzed the molecular genotyping. LV analyzed and interpreted the 18F-FDG PET/CT findings. JC analyzed and interpreted the histological findings. LG performed and interpreted drug monitoring during the patient’s follow-up. GL managed the patient and provided the data. AT designed the study and revised the manuscript. All authors have approved the version of the manuscript submitted for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
LCH: Langerhans cell histiocytosis; ECD: Erdheim-Chester disease; MAP kinase: mitogen activating kinase; PET: positron emission tomography; CT: computed tomography; CRP: C-reactive protein; BM: bone marrow; NGS: next-generation sequencing; LVEF: left ventricular ejection fraction; VAF: variant allele frequency
| References | ▴Top |
- Emile JF, Cohen-Aubart F, Collin M, Fraitag S, Idbaih A, Abdel-Wahab O, Rollins BJ, et al. Histiocytosis. Lancet. 2021;398(10295):157-170.
doi - Jouenne F, Chevret S, Bugnet E, Clappier E, Lorillon G, Meignin V, Sadoux A, et al. Genetic landscape of adult Langerhans cell histiocytosis with lung involvement. Eur Respir J. 2020;55(2):1901190.
doi pubmed - Lorillon G, Jouenne F, Baroudjian B, de Margerie-Mellon C, Vercellino L, Meignin V, Lebbe C, et al. Response to trametinib of a pulmonary langerhans cell histiocytosis harboring a MAP2K1 deletion. Am J Respir Crit Care Med. 2018;198(5):675-678.
doi pubmed - Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773-781.
doi - FDA clinical pharmacology and biopharmaceutics reviews - Cobimetinib NDA 206192.2015.
- Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Toledano D, Barete S, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411-418.
doi pubmed - Cohen Aubart F, Emile JF, Maksud P, Galanaud D, Cluzel P, Benameur N, Aumaitre O, et al. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180(1):150-153.
doi pubmed - Diamond EL, Durham BH, Ulaner GA, Drill E, Buthorn J, Ki M, Bitner L, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567(7749):521-524.
doi pubmed - Jouenne F, Benattia A, Tazi A. Mitogen-activating protein kinase pathway alterations in Langerhans cell histiocytosis. Curr Opin Oncol. 2021;33(2):101-109.
doi pubmed - Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, Raje NS, et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4(3):384-388.
doi pubmed - Tzankov A, Kremer M, Leguit R, Orazi A, van der Walt J, Gianelli U, Hebeda KM. Histiocytic cell neoplasms involving the bone marrow: summary of the workshop cases submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) organized by the European Bone Marrow Working Group, Basel 2016. Ann Hematol. 2018;97(11):2117-2128.
doi pubmed - Cohen-Aubart F, Ungureanu I, Razanamahery J, Charlotte F, Valmary-Degano S, Helias-Rodzewicz Z, Cazals-Hatem D, et al. Peritoneal or mesenteric tumours revealing histiocytosis. BMJ Open Gastroenterol. 2021;8(1):e000622.
doi pubmed - Ota M, Sakamoto M, Sato K, Yoshida Y, Funakubo Asanuma Y, Akiyama Y, Yamakawa M, et al. Immunopathological analysis of Erdheim-Chester disease with massive ascites. Intern Med. 2012;51(19):2825-2830.
doi pubmed - Gupta A, Aman K, Al-Babtain M, Al-Wazzan H, Morouf R. Multisystem Erdheim-Chester disease; a unique presentation with liver and axial skeletal involvement. Br J Haematol. 2007;138(3):280.
doi pubmed - Sung YE, Lee YS, Lee J, Lee KY. Erdheim-chester disease involving lymph nodes and liver clinically mimicking lymphoma: A Case Report. J Pathol Transl Med. 2018;52(3):183-190.
doi pubmed - Abdallah M, Genereau T, Donadieu J, Emile JF, Chazouilleres O, Gaujoux-Viala C, Cabane J. Langerhans' cell histiocytosis of the liver in adults. Clin Res Hepatol Gastroenterol. 2011;35(6-7):475-481.
doi pubmed - Hervier B, Haroche J, Arnaud L, Charlotte F, Donadieu J, Neel A, Lifermann F, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124(7):1119-1126.
doi pubmed - Bonometti A, for Associazione Italiana Ricerca Istiocitosi AO. The triptych of mixed histiocytosis: a systematic review of 105 cases and proposed clinical classification. Leuk Lymphoma. 2021;62(1):32-44.
doi pubmed - Durham BH, Roos-Weil D, Baillou C, Cohen-Aubart F, Yoshimi A, Miyara M, Papo M, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130(2):176-180.
doi pubmed - Bonometti A, Ferrario G, Parafioriti A, Giardino D, Simonetti F, Ginori A, Passoni E, et al. MAP2K1-driven mixed Langerhans cell histiocytosis, Rosai-Dorfman-Destombes disease and Erdheim-Chester disease, clonally related to acute myeloid leukemia. J Cutan Pathol. 2021;48(5):637-643.
doi pubmed - Janku F, Diamond EL, Goodman AM, Raghavan VK, Barnes TG, Kato S, Abdel-Wahab O, et al. Molecular profiling of tumor tissue and plasma cell-free DNA from patients with non-Langerhans cell histiocytosis. Mol Cancer Ther. 2019;18(6):1149-1157.
doi pubmed - Evseev D, Kalinina I, Raykina E, Osipova D, Abashidze Z, Ignatova A, Mitrofanova A, et al. Vemurafenib provides a rapid and robust clinical response in pediatric Langerhans cell histiocytosis with the BRAF V600E mutation but does not eliminate low-level minimal residual disease per ddPCR using cell-free circulating DNA. Int J Hematol. 2021;114(6):725-734.
doi pubmed - Heritier S, Helias-Rodzewicz Z, Lapillonne H, Terrones N, Garrigou S, Normand C, Barkaoui MA, et al. Circulating cell-free BRAF(V600E) as a biomarker in children with Langerhans cell histiocytosis. Br J Haematol. 2017;178(3):457-467.
doi pubmed - Papapanagiotou M, Griewank KG, Hillen U, Schimming TT, Moeller LC, Fuhrer D, Zimmer L, et al. Trametinib-induced remission of an MEK1-mutated langerhans cell histiocytosis. JCO Precis Oncol. 2017;1:1-5.
doi pubmed - Gao Y, Chang MT, McKay D, Na N, Zhou B, Yaeger R, Torres NM, et al. Allele-specific mechanisms of activation of MEK1 mutants determine their properties. Cancer Discov. 2018;8(5):648-661.
doi pubmed - Lian T, Li C, Wang H. Trametinib in the treatment of multiple malignancies harboring MEK1 mutations. Cancer Treat Rev. 2019;81:101907.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


