| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 1, February 2023, pages 7-15
Survival in Elderly Patients Diagnosed With Acute Myeloid Leukemia: A Hospital-Based Study
Diana Marcela Mendoza-Urbanoa, Maria Elena Tello-Cajiaoa, Joaquin Rosalesb, Fabian Emiliano Ahumadab, Luis Gabriel Parra-Laraa, c , Elizabeth Arrietab, d
aCentro de Investigaciones Clinicas (CIC), Fundacion Valle del Lili, Cali, Colombia
bServicio de Hemato-Oncologia, Departamento de Medicina Interna, Fundacion Valle del Lili, Cali, Colombia
cFacultad de Ciencias de la Salud, Universidad Icesi, Cali, Colombia
dCorresponding Author: Elizabeth Arrieta, Servicio de Hemato-Oncologia, Departamento de Medicina Interna, Fundacion Valle del Lili, Cali 760032, Colombia
Manuscript submitted September 16, 2022, accepted November 18, 2022, published online December 1, 2022
Short title: Survival in Elderly AML Patients
doi: https://doi.org/10.14740/jh1055
| Abstract | ▴Top |
Background: Acute myeloid leukemia (AML) is a hematological neoplasm that is more frequent in elderly patients. The objective of this study was to evaluate elderly patients’ survival with de novo AML and acute myeloid leukemia myelodysplasia-related (AML-MR), treated with intensive and less-intensive chemotherapy and supportive care.
Methods: A retrospective cohort study was conducted in Fundacion Valle del Lili (Cali, Colombia), between 2013 and 2019. We included patients ≥ 60 years old diagnosed with AML. The statistical analysis considered the leukemia type (de novo vs. myelodysplasia-related) and treatment (intensive chemotherapy regimen, less-intensive chemotherapy regimen, and without chemotherapy). Survival analysis was performed using Kaplan-Meier method and Cox regression models.
Results: A total of 53 patients were included (31 de novo and 22 AML-MR). Intensive chemotherapy regimens were more frequent in patients with de novo leukemia (54.8%), and 77.3% of patients with AML-MR received less-intensive regimens. Survival was higher in the chemotherapy group (P = 0.006), but with no difference between chemotherapy modalities. Additionally, patients without chemotherapy were 10 times more likely to die than those who received any regimen, independent of age, sex, Eastern Cooperative Oncology performance status, and Charlson comorbidity index (adjusted hazard ratio (HR) = 11.6, 95% confidence interval (CI) 3.47 - 38.8).
Conclusions: Elderly patients with AML had longer survival time when receiving chemotherapy, regardless of the type of regimen.
Keywords: Acute myeloid leukemia; Aged; Treatment patterns; Survival; Supportive care
| Introduction | ▴Top |
Acute myeloid leukemia (AML) is a hematological neoplasm characterized by the malignant clonal expansion of progenitor cells coupled with a differentiation arrest [1]. Although epidemiological behavior shows a higher frequency in people over 65 years old, this pathology can appear at any age. The Surveillance, Epidemiology, and End Results (SEER) program estimated that in 2020, approximately 19,940 new cases would be diagnosed with about 11,180 deaths in the United States alone [2, 3]. Historically, elder age groups have represented a challenge in cancer treatment [4]. A lower tolerance and treatment response is conditioned by a decreased functional-state and age-related morbidities, as well as history of hematologic disorders, such as myelodysplastic syndrome and tumor biology [5]. Moreover, as population continues to age, the number of new AML cases increases by approximately 2.2% each year, representing a challenge for decision-makers [6].
The population-based cancer registry in Cali estimated myeloid leukemia’s age-standardized incidence rate (ASIR) per 100,000 person-years was 3.1 for men and 2.1 for women between 2013 and 2017 [7]. In specified leukemias, the ASIR was 12.8 between 60 and 64 years of age and 48 between 75 and 79 years of age for men, while the ASIR was 8.2 and 27.5 for women, in the same age group between 2013 and 2017 [8]. The data suggest clinical differences by age that determine the AML’s behavior even between elderly patients. For that reason, elderly patients are a unique and heterogeneous group. The therapeutic approach and intention to treat depend on a comprehensive evaluation of the patients; however, this population’s best approach remains controversial [5, 9].
Current literature supports the necessity of an individualized approach to make treatment decisions beyond chronological age alone [10, 11]. This observational study evaluated elderly patients’ survival with de novo and acute myeloid leukemia with myelodysplasia-related changes (AML-MRC), now called AML myelodysplasia-related (AML-MR), treated in routine clinical practice with intensive chemotherapy, less-intensive chemotherapy, and supportive care in a single-center located in Cali, Colombia.
| Materials and Methods | ▴Top |
Study design and patient selection
A single-center retrospective cohort study was conducted at Fundacion Valle del Lili (Cali, Colombia) between 2013 and 2019.
Patients ≥ 60 years old with diagnosed AML were included. The exclusion criteria were patients with acute promyelocytic leukemia, megakaryoblastic leukemia, and incomplete clinical records. To obtain cases, the Department of Data Management tallied the International Classification of Diseases, 10th Revision (ICD-10) codes related to AML in our databases and then, we obtained the clinical data through revision of the clinical records.
Baseline and clinical characteristics
Sociodemographic and clinical variables were described according to the type of AML diagnosed, whether de novo or AML-MR.
AML-MR was defined as a neoplasm with ≥ 20% blasts expressing a myeloid immunophenotype and harboring specific cytogenetic and molecular abnormalities associated with myelodysplastic neoplasms (MDS), arising de novo or following a known history of MDS or MDS/myeloproliferative neoplasms (MPN) [12].
Prophylaxis was defined according to the institutional protocol that includes management with acyclovir 400 mg every 12 h and posaconazole 300 mg every 12 h on day 1, followed by 300 mg daily. Patients do not receive antibiotic prophylaxis according to this protocol.
Patients were classified in two groups considering the chemotherapeutic scheme: 1) intensive chemotherapy regime: cytarabine and anthracyclines (7 × 3); 2) less-intensive chemotherapy regime: cytarabine and anthracyclines (5 × 2), fludarabine and cytarabine, azacitidine or methotrexate associated with 6-mercaptopurine. Palliative care treatment included pain and symptom control plus family support. The type of treatment was defined by a multidisciplinary group, where hematologists, geriatrics and palliative care physicians participated.
Eastern Cooperative Oncology Group (ECOG) performance status was obtained to determine patient’s aptness to tolerate therapies (0: asymptomatic; 1: symptomatic but completely ambulatory; 2: symptomatic, < 50% in bed during the day; 3: symptomatic, > 50% in bed, but not bedbound; 4: bedbound; 5: death) [13, 14]. Charlson comorbidity index (CCI) was estimated to predict 10-year survival in patients with multiple comorbidities [15, 16].
Measurable residual disease (MRD) was defined as the presence of leukemia cells down to levels of 1:104 to 1:106 white blood cells (WBCs), compared with 1:20 in morphology-based assessments [17]. It was determined using Navios EX™ flow cytometry (CE-IVD; 3 lasers, 10 colors; Beckman Coulter, Inc., USA) for immunophenotypic markers (CD15, CD117, CD33, CD13, human leukocyte antigen (HLA)-DR, CD34, CD16, CD11b, CD19, CD45, CD36, CD64, CD7, IREM, CD56, CD14, CD33) together with parameters of size and internal complexity (Forward Scatter/Side Scatter, FSC/SSC). The leukemia-associated immunophenotypes (LAIP) approach was implemented, which defines LAIPs at diagnosis and tracks these in subsequent samples.
Study outcomes
The primary outcome was the overall survival (OS). The time interval for survival analysis was from the date of diagnosis to the date of death or last follow-up (last day attended in the hospital), considering the chemotherapy regimen used and AML type.
Statistical analysis
Categorical variables were summarized in absolute and relative frequency tables, comparing them with χ2 or Fisher’s exact test. Quantitative variables were described with median (Me) and interquartile range (IQR), as they all had skewed distribution and were compared using Mann-Whitney’s U test. The bivariate analysis compared the chemotherapy regimen and some clinical characteristics using crude odds ratio (OR) to determine possible associations between variables. Survival was analyzed through confuser-adjusted Kaplan Meier functions, comparing them with the log-rank and Wilcoxon tests. A Cox regression was performed to model the hazard ratio (HR) considering variables of clinical or statistical importance. The Cox regression’s proportional risk assumption was verified by analyzing the Schoenfeld residuals and using goodness-of-fit graphical methods. A P value < 0.05 was considered significant for all statistical analyses. All analyzes used Stata® (Version 14.0, StataCorp LP, College Station, TX).
Ethics approval
The Institutional Review Board (Comite de Etica en Investigacion Biomedica of Fundacion Valle del Lili) approved this study (IRB/EC No. 1447); it followed the ethical principles for medical research outlined by the Declaration of Helsinki and considered the regulations of Resolution 8430/1993 of the Ministry of Health of Colombia.
| Results | ▴Top |
A total of 53 AML cases were included in the study; of these, 31 were de novo and 22 were AML-MR (Fig. 1).
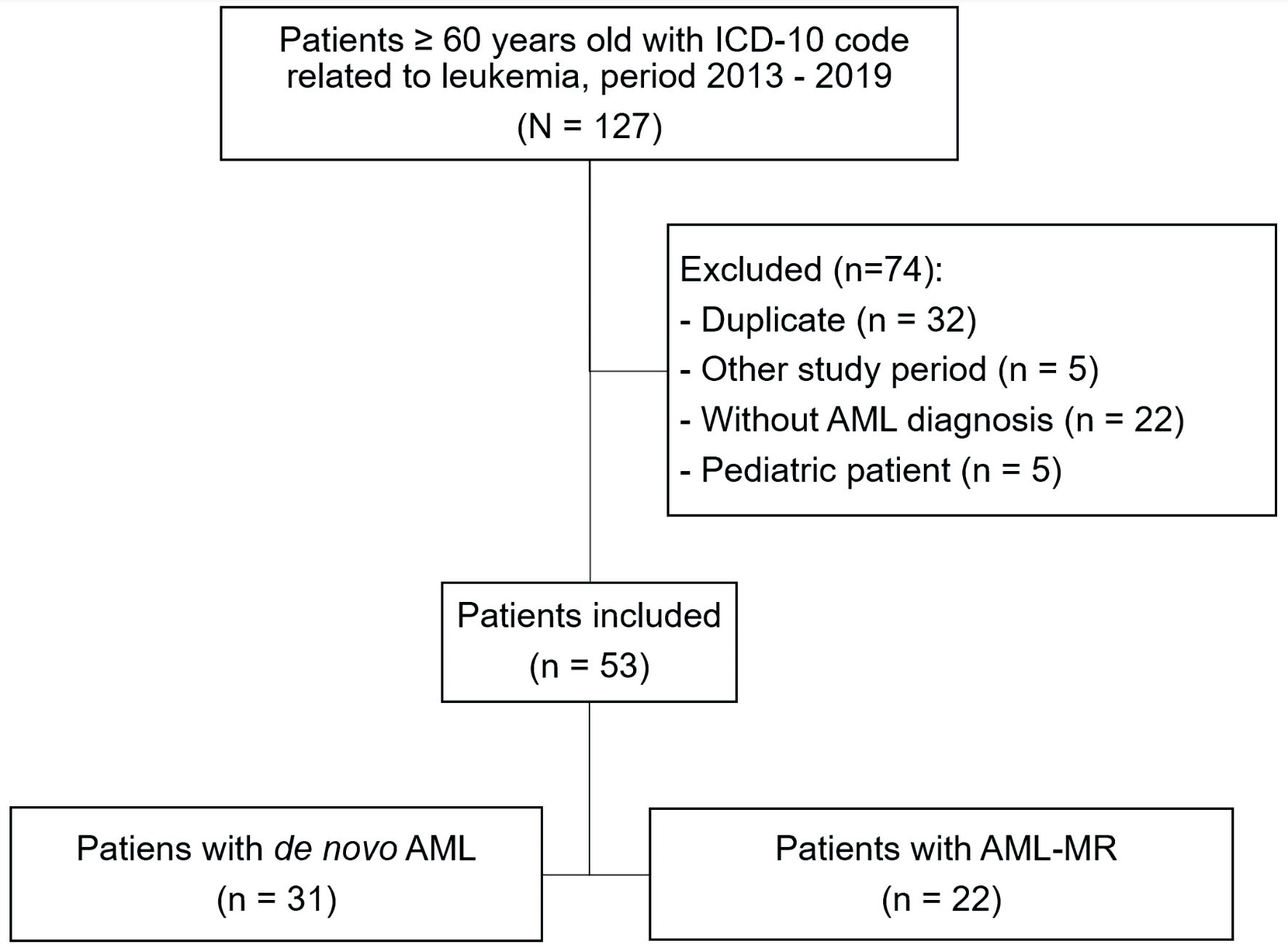 Click for large image | Figure 1. Flowchart of patient selection in the study. ICD-10: the International Classification of Diseases, 10th Revision; AML: acute myeloid leukemia; AML-MR: acute myeloid leukemia myelodysplasia-related. |
Sociodemographic and clinical characteristics, according to the AML type, are shown in Table 1. The median age was 71 years (IQR: 67 - 77 years), with similar sex distribution. Nearly 72% of patients were from urban areas. Patient’s performance status showed that 60.3% of them had an ECOG performance status of 1. Almost 80% had a CCI greater than 5. Chemotherapy with the intensive regimen was more common in patients with de novo AML (54.8%), while 77.3% of patients with AML-MR received less-intensive regimens. Thirteen percent of the patients did not receive any chemotherapy scheme. Most patients received blood transfusions as supportive management (64.2%, P = 0.004), but this procedure was more frequent in the AML-MR group (81.8%, P = 0.024), as well as the use of erythropoietin (36.4%, P = 0.002). Other clinical characteristics such as leukocyte count, extramedullary involvement, palliative care interventions, and intensive care unit (ICU) management did not have differences between AML types. The main complications during treatment according to the type of AML are shown here (Supplementary Material 1, www.thejh.org).
 Click to view | Table 1. Demographic and Clinical Characteristics of Patients Included in the Study (N = 53) |
The median follow-up was 208 days (IQR: 36 - 454 days). The median survival between the two diagnostic groups was similar. About 74% of the entire cohort had died by the time follow-ups concluded.
Chemotherapy regimens
Table 2 describes chemotherapy regimens and their relation to some clinical features. Of 46 patients who received chemotherapy, 43.5% had an intensive regimen with cytarabine and anthracycline in a 7 × 3 scheme. While less-intensive regimens included cytarabine and anthracyclines in a 5 × 2 scheme (21.7%), fludarabine/cytarabine (13%), azacitidine (13%), or methotrexate and 6-mercaptopurine (8.7%).
 Click to view | Table 2. Factors Related to the Chemotherapy Scheme |
Clinical factors associated with lower use of intensive chemotherapy regimen were a diagnosis of AML-MR (OR = 0.9, 95% CI: 0.014 - 0.47), an ECOG performance status over 2 (OR = 0.05, 95% CI: 0.001 - 0.39), and a CCI of 5 or higher (OR = 0.04, 95% CI: 0.0008 - 0.36). Other factors such as the use of salvage treatment and the presence of MRD were not related to the type of chemotherapy used.
Survival analysis
The median days of OS among patients treated with chemotherapy was higher than those who did not receive any regimen (250 days vs. 28 days, P = 0.001). However, there was no difference in survival time based on chemotherapy regimen administered (Table 3). Also, Kaplan Meier’s function showed that at any time during follow-up, the adjusted survival probability was higher among the chemotherapy group vs. without chemotherapy (P = 0.006). There were no statistical differences in survival time, according to the chemotherapy regimen used (P = 0.938). However, the probability of surviving longer tends to be remarkable in the less-intensive chemotherapy group (Fig. 2). No differences in survival functions were found according to the type of AML (Supplementary Material 2, www.thejh.org).
 Click to view | Table 3. Overall Survival by Chemotherapy Scheme |
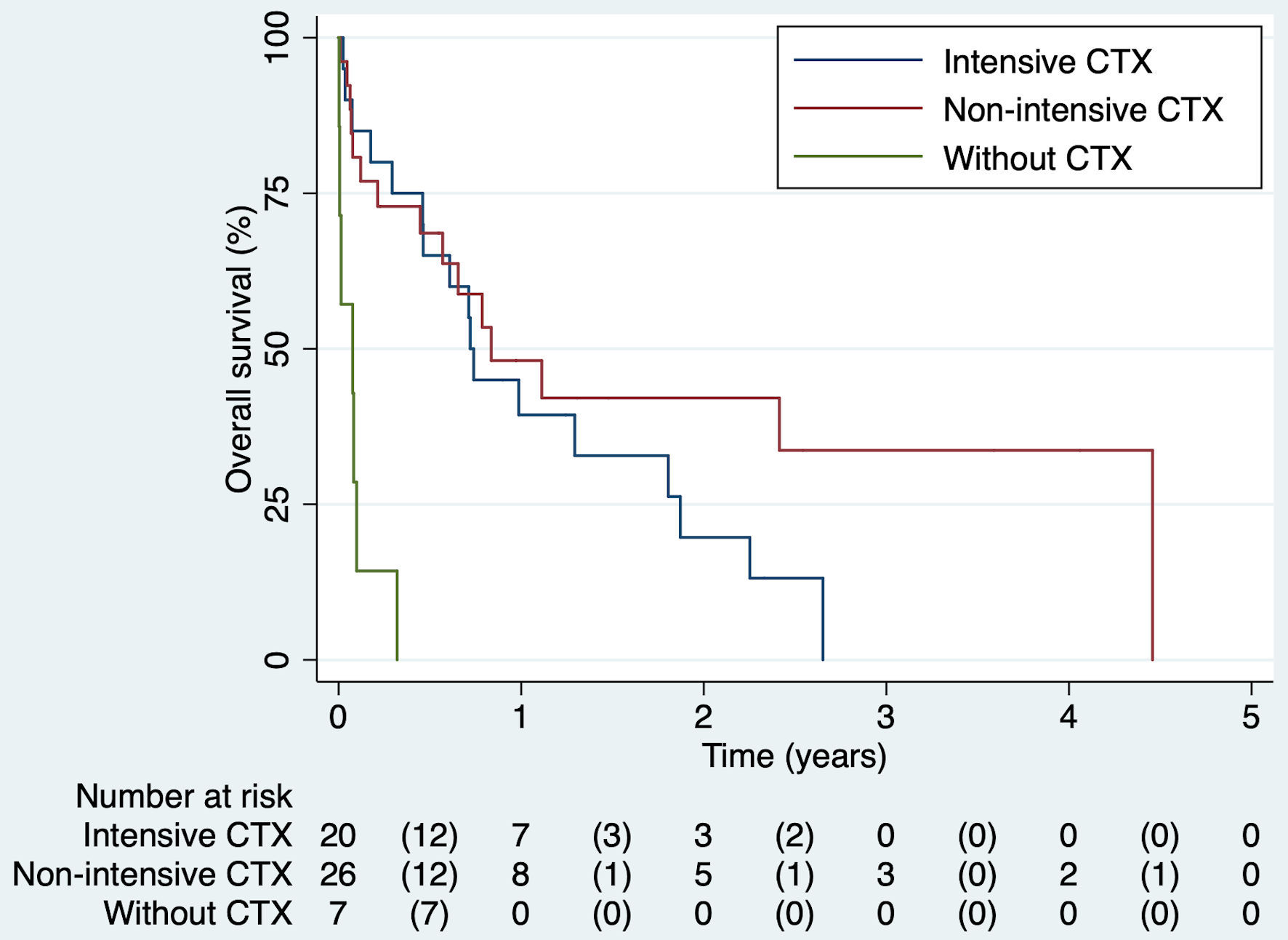 Click for large image | Figure 2. Kaplan’s survival function by chemotherapy scheme. CTX: chemotherapy. |
The adjusted Cox regression model showed that at any time during follow-up, patients who did not receive chemotherapy were 10 times more likely to die compared to those who received chemotherapy, regardless of age, sex, ECOG performance status, and CCI (HR = 11.6, 95% CI: 3.47 - 38.8). The Cox model’s Schoenfeld scale plot showed a horizontal line, and the rho test was not significant (P = 0.969). The Martingale residue plot and the survival curve represented with Nelson Aalen’s method drew a 45-degree line. The proportionality criterion was satisfied.
| Discussion | ▴Top |
AML disproportionately affects elderly population. It represents a treatment challenge due to patients’ decreased functional status and the neoplasm’s more aggressive behavior in this age group. Developing a standardized treatment approach is complex, and the best therapeutic choice for this population remains controversial. It has been shown that the efficacy and tolerability of treatment deteriorates markedly with age [5, 18].
This study evaluated the treatment and survival of elderly patients with de novo and AML-MR. We found that patients who received chemotherapy had a significantly higher OS compared with patients with non-pharmacological intervention. However, survival analyzes of chemotherapy schemes did not evidence statistically significant differences between intensive or less-intensive therapy.
These results have been supported in previous studies that involved elderly patients. A phase 3 clinical trial showed that AML elderly patients who received intensive induction chemotherapy had improved survival at 2.5 years and higher remission rates than supportive care [19]. A retrospective study conducted in the USA analyzed, among Medicare beneficiaries, the treatment patterns and outcomes of elderly AML patients. It found a significant survival benefit between those who received antileukemic therapy (with intensive and less-intensive schemes) compared to support treatment [20]. These findings are consistent with the recommendation published by the National Cancer Comprehensive Network guidelines in their third version, which established that age alone should not be the sole factor in determining the treatment scheme chosen in AML patients [21]. Our results reported an adjusted chance of dying 10 times greater for patients who did not receive chemotherapy.
A phase 3, multicenter, randomized, double-blind, placebo-controlled trial evaluated azacitidine plus venetoclax vs. azacitidine plus placebo in untreated patients aged ≥ 75 years old who were ineligible for intensive chemotherapy. Their findings showed that patients who received azacitidine plus venetoclax OS was longer (median OS 14.7 months vs. 9.6 months; HR = 0.66, 95% CI: 0.52 - 0.85, P < 0.001) and the incidence of remission was higher (36.7% vs. 17.9%, P < 0.001), but with a higher incidence of febrile neutropenia (42% vs. 19%) [22]. However, the use of venetoclax has only recently been implemented in Latin America (since 2019 in some countries), that is why future studies are required to evaluate its efficacy in older patients with AML in the region.
Although there were no statistically significant differences between intensive and less-intensive chemotherapy regimens in this study, data suggest a higher survival trend among patients who received less-intensive modalities. Nonetheless, a longer follow-up and sample size would be required to verify this finding. Quintas-Cardama et al showed that survival in patients treated with less-intensive therapy was non-inferior to that achieved with intensive chemotherapy. The study states that despite hypomethylating therapy resulting in lower complete remission rates, it may result in leukemia control and mortality reduction, particularly in AML elderly patients with adverse cytogenetic presentations [23]. Meanwhile, Dombret et al found improvement in OS in the less-intensive group compared with conventional care. It also reported low complete response rate but a survival benefit between the participants [24]. These findings could explain that less-intensive schemes are better tolerated by elderly patients [25].
Whereas there is some evidence to suggest that less-intensive chemotherapy is the best option in AML elderly patients, other studies favor intensive schemes. Bell et al compared intensive induction chemotherapy vs. treatment with hypomethylating agents in AML elderly patients [26]. They demonstrated that the median OS was significantly higher in patients treated with intensive regimens even when it is well known that hypomethylating agents are usually selected for elderly patients that tend to have more comorbidities [20].
Despite these results, many elderly patients had considerable morbidity and mortality from intensive chemotherapy, increasing significantly with age, and decreased performance status [27]. A retrospective analysis of elderly patients receiving intensive chemotherapy at MD Anderson Cancer Center demonstrated a 28-day mortality risk higher between comorbid patients than healthy ones. Patients with a score ≥ 3 on the Hematopoietic Stem Cell Transplant comorbidity index had a 29% chance of 28-day mortality risk compared with 11% for patients with lower scores [28]. Likewise, Appelbaum et al reported a 30-day mortality risk for patients with ECOG performance status of 2, as 31%, in 66 to 75 years old patients [29]. The previous results suggest that the cornerstone to decide the treatment option in an elderly leukemia patient is to determine their functional state because it determines which treatment goal to pursue [30, 31]. Therefore, looking beyond OS as the primary endpoint may be appropriate in elderly patients with AML. Also, considering the disease-related quality of life as a suitable endpoint, mostly because they rarely achieve a cure [32].
A comprehensive geriatric assessment as a standard of evaluation between elderly patients should be mandatory [9, 33]. This approach classifies patients as fit, vulnerable, or frail predicting the therapy response and adverse effects likelihood [11, 34]. Although these tools have been validated for AML patients, their use is not widespread in clinical practice. This study found that our patients’ functional status was evaluated through the ECOG performance status and CCI. In our context, the hematologists did not use a comprehensive geriatric assessment as literature recommends, but we had the support of a multidisciplinary group.
Another finding of our study was that AML-MR patients had a lower probability of receiving an intensive chemotherapy regime compared with de novo AML patients (13.6% and 54.8%, respectively, P = 0,003). Considering our results, the subtype of AML determined the curative intention. Other studies also reported the preference for less-intensive therapies in this group [35, 36], which could be explained by a decrease at complete remission rates, OS, and worse prognosis observed among these patients [37]. Other factors, such as age, comorbidities, lower functional status, and cytotoxic treatment history, also condition the therapeutic election [38]. In fact, a study reported in 2014 that prior treatment with hypomethylating agents could induce tumoral cells and immunological changes that will result in resistance to the same pharmacological class in the future [39].
When comparing the de novo and AML-MR patients, the literature reports among the second group a trend of older age, comorbidities, low-risk cytogenetics, and worse ECOG performance status [36, 40]. Identified factors that have an impact in AML-MR are the presence of mutations in specific cellular populations that may define secondary nature and adverse outcomes of AML (ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1 and ZRSR2), presence of MDS-defining cytogenetic abnormalities, or disease secondary to a previously treated myeloid malignancy (treated-secondary AML, ts-AML), cytogenetic abnormalities that include complex karyotype-3 or more abnormalities balanced translocations and unbalanced translocations, degree of differentiation, myeloid lineage involved, and dysplastic changes [38, 41, 42].
Our results do not show differences between these populations. This could be explained because both groups were similar in age, gender, ECOG performance status, and CCI; as well as the exposure of hypomethylating agents in both groups and the frequency of complications during treatment. Finally, the low number of patients could be explained by the fact that the study was conducted in only one center. Therefore, we recommend exploring the de novo AML and AML-MR Colombian population in future prospective studies with a larger sample size and exploring associated biological and genetic factors.
Limitations
The results of this study should be interpreted in the context of a retrospective cohort. First, it was conducted retrospectively at a single center, limiting the sample size and implying selection and information biases. Secondly, it was not possible to describe chemotherapy response because post-chemotherapy myelogram data were not available in most clinical records. It is an important parameter to consider in the treatment of AML patients, so it should be included in future studies. Third, the heterogeneity of less-intensive schemes administered to the patients’ limits developing a pharmacological specific analysis because low number of patients on each treatment. Although less-intensive chemotherapy schemes often include cytarabine plus anthracyclines in a 5 + 2 regimen, this therapy was used in vulnerable patients (those dependent on instrumental activities of daily living (IADL) and with some comorbidity), while the 7 + 3 scheme was used in fit patients (independent patients in basic activities of daily living (BADL) and IADL, and without associated comorbidity) [43]. Regarding the use of venetoclax, the National Institute for Food and Drug Surveillance (INVIMA) approved its use in Colombia in 2020, but for AML treatment it was authorized in 2021, so the patients included in the study could not receive it. In addition, the approval by the regulatory body does not imply that the patient would have had access to the drug since that also depends on aspects related to the healthcare system (i.e., access to the health system, drug supply management, financing, coverage, among others). Fourth, the lack of a geriatric assessment model used at patients’ functional status evaluation revealed the necessity of hematologists and palliativist having familiarity with these tools. Specially because these instruments are validated for AML patients, and evidence favors them over others predicting mortality and chemotherapy toxicity. Finally, due to the dynamic cohort, patients admitted in the last year of the study (2019) had shorter follow-up times. To mitigate bias, we adjusted the analyzes for the cohort effect.
Despite the limitations, these findings contribute to the better knowledge and characterization of AML in the elderly population because we described the treatment patterns and survival in our context. Besides, we included de novo and AML-MR, which allowed us to independently analyze the effect of treatment in both types of AML, thus being one of the few studies that include patients with both diagnoses. This is useful for the medical community that handles patients with similar characteristics.
Conclusions
Elderly AML patients (over 60 years old) had a significant survival benefit with chemotherapy intervention compared with supportive care or non-pharmacological treatment. However, it was nonrandomized and retrospective and based on experience with a small patient population at one center.
| Supplementary Material | ▴Top |
Suppl 1. Main complications during treatment according to the type of AML.
Suppl 2. Kaplan’s survival function by type of AML.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
As this is a retrospective study, patient informed consent was not required.
Author Contributions
DM Mendoza-Urbano and E. Arrieta participated at conceptualization; J. Rosales and FE Ahumada planned the methodology; DM Mendoza-Urbano and ME Tello-Cajiao did the validation; ME Tello-Cajiao and LG Parra- Lara performed the formal analysis; DM Mendoza-Urbano and ME Tello-Cajiao prepared the original draft; LG Parra- Lara and FE Ahumada reviewed and edited the first draft; J. Rosales performed the project supervision. All authors made significant contributions to writing the manuscript, read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Kantarjian H. Acute myeloid leukemia—major progress over four decades and glimpses into the future. Am J Hematol. 2016;91(1):131-145.
doi pubmed - Surveillance Epidemiology and ERP. Cancer stat facts: leukemia - Acute Myeloid Leukemia (AML) [Internet]. Cancer Stat Facts. 2020 [cited Apr 1, 2020]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html.
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70-87.
doi pubmed - Mamdani H, Santos CD, Konig H. Treatment of acute myeloid leukemia in elderly patients-a therapeutic dilemma. J Am Med Dir Assoc. 2016;17(7):581-587.
doi pubmed - Keiffer G, Palmisiano N. Acute myeloid leukemia: update on upfront therapy in elderly patients. Curr Oncol Rep. 2019;21(8):71.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Registro Poblacional de Cancer de Cali (RPCC). Tasas de inicidencia estandarizadas por edad por 100.000 per-ano. Todas las localizaciones, en hombres y mujeres. Cali. 2022.
- Registro Poblacional de Cancer de Cali (RPCC). Cali, Colombia. Tasas de incidencia y mortalidad de leucemias especificas por edad, crudas y ajustadas por edad durante el periodo 1962-2017. Cali. 2022.
- Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy—a systematic review. Leuk Res. 2014;38(3):275-283.
doi pubmed - Lazarevic VL, Bredberg A, Lorenz F, Ohlander E, Antunovic P, Cammenga J, Wennstrom L, et al. Acute myeloid leukemia in very old patients. Haematologica. 2018;103(12):e578-e580.
doi pubmed - Soto-Perez-de-Celis E, Aapro M, Muss H. ASCO 2020: The Geriatric Assessment Comes of Age. Oncologist. 2020;25(11):909-912.
doi pubmed - Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703-1719.
doi pubmed - Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
doi pubmed - Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135-1141.
doi pubmed - Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
doi pubmed - Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682.
doi pubmed - Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, Grimwade D, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275-1291.
doi pubmed - Ferrara F. Conventional chemotherapy or hypomethylating agents for older patients with acute myeloid leukaemia? Hematol Oncol. 2014;32(1):1-9.
doi pubmed - Lowenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, Cauchie C, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7(9):1268-1274.
doi pubmed - Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127-1138.
doi pubmed - Tallman MS, Wang ES, Altman JK, Appelbaum FR, Bhatt VR, Bixby D, Coutre SE, et al. Acute myeloid leukemia, Version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Quintas-Cardama A, Ravandi F, Liu-Dumlao T, Brandt M, Faderl S, Pierce S, Borthakur G, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120(24):4840-4845.
doi pubmed - Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299.
doi pubmed - Podoltsev NA, Stahl M, Zeidan AM, Gore SD. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev. 2017;31(2):43-62.
doi pubmed - Bell JA, Galaznik A, Farrelly E, Blazer M, Murty S, Ogbonnaya A, Eaddy M, et al. A retrospective study evaluating treatment patterns and survival outcomes in elderly patients with acute myeloid leukemia treated in the United States with either 7+3 or a hypomethylating agent. Leuk Res. 2019;78:45-51.
doi pubmed - Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
doi pubmed - Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624-627.
doi pubmed - Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-3485.
doi pubmed - Rao AV. Fitness in the elderly: how to make decisions regarding acute myeloid leukemia induction. Hematology Am Soc Hematol Educ Program. 2016;2016(1):339-347.
doi pubmed - Luger SM. Acute myeloid leukemia: How to treat the fit patient over age 75? Best Pract Res Clin Haematol. 2019;32(4):101105.
doi pubmed - Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma. 2018;59(2):274-287.
doi pubmed - Hamaker ME, van Huis-Tanja LH, Rostoft S. Optimizing the geriatrician's contribution to cancer care for older patients. J Geriatr Oncol. 2020;11(3):389-394.
doi pubmed - Balducci L, Stanta G. Cancer in the frail patient. A coming epidemic. Hematol Oncol Clin North Am. 2000;14(1):235-250.
doi pubmed - Zeichner SB, Arellano ML. Secondary adult acute myeloid leukemia: a review of our evolving understanding of a complex disease process. Curr Treat Options Oncol. 2015;16(8):37.
doi pubmed - Ossenkoppele G, Montesinos P. Challenges in the diagnosis and treatment of secondary acute myeloid leukemia. Crit Rev Oncol Hematol. 2019;138:6-13.
doi pubmed - Ostgard LS, Norgaard JM, Sengelov H, Severinsen M, Friis LS, Marcher CW, Dufva IH, et al. Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29(3):548-555.
doi pubmed - Koenig KL, Sahasrabudhe KD, Sigmund AM, Bhatnagar B. AML with myelodysplasia-related changes: development, challenges, and treatment advances. Genes (Basel). 2020;11(8).
doi pubmed - Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280-1288.
doi pubmed - Weinberg OK, Seetharam M, Ren L, Seo K, Ma L, Merker JD, Gotlib J, et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood. 2009;113(9):1906-1908.
doi pubmed - Montalban-Bravo G, Kanagal-Shamanna R, Class CA, Sasaki K, Ravandi F, Cortes JE, Daver N, et al. Outcomes of acute myeloid leukemia with myelodysplasia related changes depend on diagnostic criteria and therapy. Am J Hematol. 2020;95(6):612-622.
doi pubmed - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Balducci L, Extermann M. Management of the frail person with advanced cancer. Crit Rev Oncol Hematol. 2000;33(2):143-148.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


