| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 3, June 2023, pages 128-132
Hyperammonemic Encephalopathy: A Rare Presentation of Relapsed Multiple Myeloma
Sasmith R. Menakurua, c, Mona Attaa, Nischala Ammannagarib, Mohamad Younesb
aDepartment of Internal Medicine, Indiana University School of Medicine, Muncie, IN, USA
bHematology Oncology, New York Oncology Hematology, Albany, NY, USA
cCorresponding Author: Sasmith R. Menakuru, Department of Internal Medicine, Indiana University School of Medicine, Muncie, IN, USA
Manuscript submitted January 27, 2023, accepted April 20, 2023, published online April 30, 2023
Short title: Hyperammonemia Occurring in Relapsed MM
doi: https://doi.org/10.14740/jh1097
| Abstract | ▴Top |
Hyperammonemia is a rare cause of encephalopathy in multiple myeloma in the absence of hepatic involvement. This is the only reported case of a 74-year-old man who presented with multiple myeloma and achieved complete remission but developed hyperammonemia afterward. He was aggressively treated with a combination of chemotherapy and immunotherapy with a resolution of his encephalopathy; however, within 1 month relapsed with encephalopathy. He ultimately decided to pursue comfort care measures. The authors conclude that hyperammonemia in multiple myeloma is a rare but important differential in patients with encephalopathy of unknown causes. Aggressive treatment is of the utmost importance due to the high mortality associated with the condition.
Keywords: Hyperammonemia; Encephalopathy; Multiple myeloma; Treatment
| Introduction | ▴Top |
Multiple myeloma (MM) is the second most common hematologic malignancy characterized by the neoplastic proliferation of plasma cells, which in turn produces a monoclonal immunoglobulin [1]. MM can cause a wide spectrum of diseases with high mortality rates, ranging from skeletal destruction from plasma cells to renal dysfunction due to immunoglobulin deposition [2, 3]. Most patients will present subacutely; however, a small number of patients will present with acute findings such as spinal cord compression or hyperviscosity. MM defying events include calcium elevation, renal insufficiency, anemia, and bone disease. Diagnostic workup includes blood count with smear, serum protein electrophoresis (SPEP) and immunofluorescence, serum free light chains, renal and liver function testing, calcium level, lactate dehydrogenase (LDH), albumin, B2 microglobulin, urinalysis for proteinuria, urine electrophoresis and immunofluorescence, next generational testing, cytogenetics, and imaging including a whole body computed tomography (CT) and an whole body magnetic resonance imaging (MRI) if CT is negative to reveal bone lesions [4]. Definitive diagnosis involves bone marrow aspiration and biopsy for analysis of marrow cellularity, with possible additional study of cytogenetics and specific protein production. Upon diagnosis, patients can seek medical treatment with chemotherapy, targeted therapy such as enzyme inhibition and monoclonal antibodies, immunomodulatory medications, steroids, and bone-modifying drugs to significantly improve symptom development and overall mortality [5].
One of the rare complications associated with MM is encephalopathy which is attributed to hyperammonemia in the absence of liver involvement as seen in this case. Based on studies published thus far, it is proposed that ammonia is produced and released from myeloma cell lines themselves [6]. Another theory is that myeloma-related humoral factors influence ammonia acid metabolism leading to hyperammonemia [7]. A persistent finding in cases of hyperammonemia due to MM is that lactulose and hemodialysis do not lower ammonium levels, and therefore, the mainstay of treatment is chemotherapy with high-dose cyclophosphamide, bortezomib and dexamethasone [7-9].
In this report, we present a case of a patient who had achieved remission with medical management of MM before he eventually returned to the hospital with altered mentation. The evaluation revealed elevated levels of ammonia, as well as an increase in the free light chain ratio after prior improvement, and repeat bone marrow biopsy demonstrated recurrent marrow overgrowth with plasma cells. After chemotherapy was initiated, ammonia levels normalized, and the patient’s mentation returned to baseline. Delays in treatment can lead to a poor prognosis, and without treatment, there is a 100% mortality rate, according to a study done by Pham et al [9].
| Case Report | ▴Top |
In June 2019, a 74-year-old man with no significant past medical history was noted to be anemic with a hemoglobin of 9.2 g/dL (13.8 - 17.2), white cell count of 6.4 × 103/mm3 (4.5 - 11.0), platelets 96 × 109/L (150 - 450 ×109/L), mean corpuscular volume (MCV) 98 fL (80 - 100) and elevated total protein of 9.6 g/dL (6.0 - 8.3). The patient was not taking any medication at that time and lived a healthy lifestyle. The patient’s laboratory testing revealed calcium of 9.6 mg/dL (8.6 - 10.2) and a creatinine of 1 mg/dL (0.7 - 1.3). Further evaluation revealed beta-2 microglobulin of 6.68 µg/mL (0.70 - 1.80), albumin was 3.9 g/dL (3.4 - 5.4), and his LDH was 158 IU/L (105 - 333). His kappa/lambda ratio was 31.1 (0.26 - 1.65) with kappa light chains of 92.2 mg/L (3.3 - 19.4) and lambda light chain of 2.7 mg/L (5.7 - 26.3). Immunoglobulin testing revealed immunoglobulin G (IgG) of 5,250 g/L (6.0 - 16.0), SPEP revealed a paraprotein of 4.1 g/dL (6.4 - 8.3), and immunofixation revealed IgG kappa. A skeletal survey did not reveal lytic lesions and bone marrow aspirate and biopsy confirmed the diagnosis of MM and 90% involvement of the bone marrow. Cytogenetics showed loss of chromosome Y patient, deletion of 13q and gain of 1 q. Given these findings the patient was classified as having an international myeloma working group (IMWG) uniform response criteria stage 3.
The patient was treated with lenalidomide, bortezomib, and dexamethasone (RVD) and attained complete remission after a total of 12 cycles. Patient was referred for autologous stem cell transplantation, but he declined. He continued lenalidomide maintenance with follow-up every month. By June 2022, his laboratory results for SPEP, kappa light chains, kappa/lambda ratio, calcium, hemoglobin, and creatinine were within normal limits and the patient was doing remarkably well. According to the IMWG uniform response criteria the patient was defined as having a complete response.
However, in September 2022 he was admitted to the hospital for altered mental status with rapid deterioration. On physical examination the patient had an increased respiratory rate, slurred speech, tremor of the hands, and marked confusion. His SPEP revealed an increased paraprotein of 1.1 g/dL, his kappa light chains increased to 47.7, his kappa/lambda ratio was 5.1, and he was found to have hyperammonemia to 62 µmol/L (< 30 µmol/L). His calcium had increased to 11.9 mg/dL, hemoglobin had dropped to 9.1 g/dL and his creatinine was 1.3 mg/dL. A bone marrow biopsy revealed 100% involvement of the bone marrow with sheets of aberrant plasma cells. According to the IMWG uniform response criteria the patient relapsed from a complete response.
His ammonia levels continued to increase, and his encephalopathy continued to worsen despite treatment with lactulose and rifaximin. His aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, gamma-glutamyl transferase (GGT) were unremarkable and MRI of the liver showed no concerning findings. A lumbar puncture and MRI of the brain were unrevealing. Given that all testing was unrevealing, his confusion was attributed to hyperammonemia secondary to MM. He was started on bortezomib and dexamethasone without significant improvement in his symptoms. He was transferred to a higher center and was then started on daratumumab, carfilzomib and dexamethasone. Patient developed an allergic reaction to carfilzomib necessitating its discontinuation. He was continued on daratumumab combined with dexamethasone with the addition of bortezomib to his regimen, which led to the resolution of his encephalopathy, decline in serum ammonia levels and significant improvement in his kappa light chains and monoclonal protein after 5 weeks of treatment.
By November 2022, his SPEP was 0.25 g/dL, kappa light chains were 14.6, and the kappa/lambda ratio was 6.08 and the patient was doing well. The patient’s ammonia levels were recorded after discharge during his follow-up appointments (Fig. 1). Unfortunately, he was admitted to the hospital 1 month later with altered mental status again with marked elevation of ammonia levels (114 µmol/L) and an increase in the free light chain ratio (26.1). His ammonia level peaked at 212 µmol/L 5 days later, and given his rapid deterioration and poor performance status, he was transitioned to comfort care.
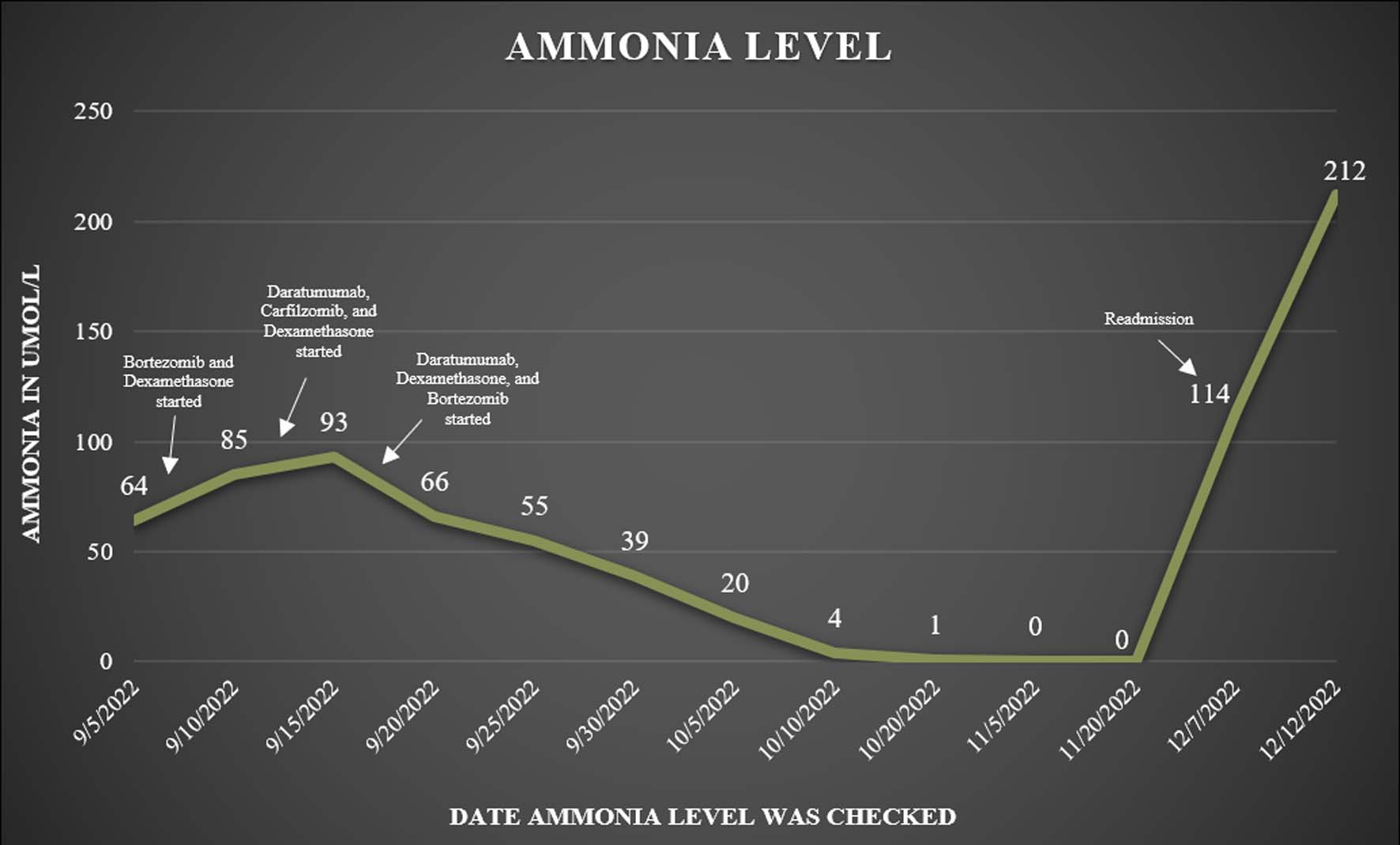 Click for large image | Figure 1. Patient’s ammonia levels recorded after discharge during his follow-up appointments. |
| Discussion | ▴Top |
MM can rarely present as encephalopathy due to hyperammonemia in the absence of liver involvement; however, it has never been reported as the presenting sign of a relapse occurring after a patient had been in remission for several years [10]. In most cases, neurological manifestations of MM are due to hypercalcemia, hyperviscosity, infection, or medication side effects. Hyperammonemia due to MM is often associated with advanced stages of disease (International Staging System (ISS)-3), plasma cells in the peripheral circulation, cytological abnormalities, and extramedullary locations [11, 12]. Patients with an advanced stage of the disease and poor prognostic factors such as 1q gain are more susceptible to the development of hyperammonemia. Hyperammonemia encephalopathy due to MM has been associated with high mortality, and is characterized by lethargy, confusion, and asterixis, with reported cases progressing rapidly to coma and subsequent death [13].
The pathophysiology of hyperammonemia in patients with MM is not well understood, but according to a study done by Otsuki et al, human myeloma cell lines produce an elevated amount of ammonia in vitro than non-MM cells [5]. Toxicity arises from the conversion of excess ammonia into glutamine by astrocytes, which leads to an osmotic transmembrane gradient and the ensuing cerebral edema. The overproduction is possibly due to mutations in the enzymes involved in the urea cycle. Another theory is thought to be due to excess protein synthesis in myeloma cells, including the synthesis of immunoglobulins and cytokines that induce excess ammonia biosynthesis [6]. Ikewaki et al postulated that the mechanism that is a possible of hyperammonemia is the hepatic infiltration by MM cells leading to liver failure due to the presence of myeloma cells in the peripheral blood [13]. Howman et al theorized that liver infiltration of the plasma cells could result in portal hypertension and a portosystemic shunt that results in hyperammonemia [14]. Gaiani et al suggested an alteration in urea metabolism that would increase protein synthesis and subsequent catabolism of plasma cells leading to hyperammonemia [15]. In our case, we suspected hyperammonemia was due to the MM cells themselves without hepatic involvement, as the patient’s liver function tests were within normal limits, his MRI of the liver was normal, and he did not respond to lactulose or rifaximin.
A review by Pham et al [9] presented 40 patients with MM-induced hyperammonemia-induced encephalopathy and showed that the median age was 67 years with a male-to-female ratio of 1.8:1. The median ammonia level was found to be 114 µmol/L, and IgG was the most common type followed by IgA. Inpatient mortality was 48%, and a 31% mortality in patients with hyperammonemia who received MM-directed treatment, versus 100% mortality in those who did not [16]. In cases of hyperammonemia, aggressive treatment with chemotherapy and immunotherapy is the mainstay. It has also been found that ammonia levels and a patient’s state of consciousness return to baseline when the underlying MM responds to treatment. A similar review by Lora-Tamayo et al showed that 22 of the 25 patients with hyperammonemia due to MM had lower ammonia levels after treatment, and 15 of the 25 survived [17].
A review performed by Talamo et al [18] of 279 patients with encephalopathy due to MM found that 70 patients had hyperammonemia due to liver dysfunction. Of the 279 patients, only eight patients had a serum ammonia greater than 47 µmol/L, which is the upper limit of normal, and only two patients were found to have encephalopathy due to MM as they did not have concomitant cause factors such as hypercalcemia, central nervous system disease, or plasma hyperviscosity. These two patients had a rapid improvement in their neurological function after therapy with normalization of their ammonia levels [18].
In current literature, most patients with hyperammonemia due to MM are of an initial presentation or a refractory case (Table 1) [12, 19, 20]. The case reported here is rare, as our patient was in remission, but thereafter relapsed and the initial presentation was of hyperammonemia and not typical MM symptoms. The patient responded well to daratumumab, dexamethasone and bortezomib therapy and eventually had his mental status to baseline. However, he relapsed again with higher levels of ammonia than before, and due to his rapid deterioration, he was transitioned to comfort care. The authors urge physicians to consider MM in cases of hyperammonemia of unknown cause, as timely management with aggressive treatment is of the utmost importance. It also underscores the gain of 1q as an aggressive and poor prognostic sign in MM.
 Click to view | Table 1. Hyperammonemia Due to MM in Current Literature |
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
No conflict of interest to declare.
Informed Consent
Informed consent has been taken from the patient.
Author Contributions
SRM and MY saw the case. SRM did research on the topic and wrote the paper. MA and NA edited the paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860-1873.
doi pubmed - Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med. 1997;336(23):1657-1664.
doi pubmed - Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, Delforge M, et al. Corrigendum to 'Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up': [Ann Oncol 2021; 32(3): 309-322]. Ann Oncol. 2022;33(1):117.
doi pubmed - Otsuki T, Yamada O, Sakaguchi H, Ichiki T, Kouguchi K, Wada H, Hata H, et al. In vitro excess ammonia production in human myeloma cell lines. Leukemia. 1998;12(7):1149-1158.
doi pubmed - Leon-Ruiz M, Oliva-Navarro J, Alonso-Singer P, Castaneda-Cabrero C, Benito-Leon J. Multiple myeloma-induced hyperammonemic encephalopathy: another important potential cause of triphasic waves. J Clin Neurophysiol. 2022;39(7):647.
doi pubmed - Vahora I, Panneerselvam E, Deoker A. Hyperammonemic encephalopathy in multiple myeloma: a case report. Cureus. 2023;15(1):e33626.
doi pubmed pmc - Jaruvongvanich V, Spanuchart I, P OC, Kitamura C, Sumida L, Roytman M. An unusual cause of altered mental status in multiple myeloma: an extraosseous manifestation. Hawaii J Med Public Health. 2016;75(4):109-112.
pubmed pmc - Pham A, Reagan JL, Castillo JJ. Multiple myeloma-induced hyperammonemic encephalopathy: an entity associated with high in-patient mortality. Leuk Res. 2013;37(10):1229-1232.
doi pubmed - Costa LJ, Usmani SZ. Defining and managing high-risk multiple myeloma: current concepts. J Natl Compr Canc Netw. 2020;18(12):1730-1737.
doi pubmed - Passucci M, Fazio F, Petrucci MT. A case of fatal hyperammonaemic encephalopathy in a patient with end-stage multiple myeloma. Mediterr J Hematol Infect Dis. 2023;15(1):e2023012.
doi pubmed pmc - Murtaza G, Lu H, Faqah A, Konowitz N, Kuruvilla A, Adhikari S. Multiple myeloma-induced hyperammonemic encephalopathy. J Hematol. 2017;6(1):29-31.
doi pubmed pmc - Ikewaki J, Ogata M, Imamura T, Kohno K, Nakayama T, Kadota J. Development of hyperammonemic encephalopathy in patients with multiple myeloma may be associated with the appearance of peripheral blood myeloma cells. Leuk Lymphoma. 2009;50(4):667-669.
doi pubmed - Howman R, Thakerer A, Pitman M, Ding N, Thompson PA, Khot A, Harrison SJ. Bortezomib, cyclophosphamide, and dexamethasone: highly effective for rapid reversal of myeloma-associated hyperammonemic encephalopathy. Leuk Lymphoma. 2010;51(12):2299-2302.
doi pubmed - Gaiani A, Pompanin S, Zambello R, Briani C, Cagnin A. Steroid-responsive hyperammonemic encephalopathy as first manifestation of multiple myeloma. Neurol Sci. 2017;38(3):503-505.
doi pubmed - Issa N, Blondeau B, Dimicoli-Salazar S, Marit G, Morlat P, Camou F. [Hyperammonemic encephalopathy as the presenting feature of a relapsing multiple myeloma]. Rev Med Interne. 2016;37(8):567-569.
doi pubmed - Lora-Tamayo J, Palom X, Sarra J, Gasch O, Isern V, Fernandez de Sevilla A, Pujol R. Multiple myeloma and hyperammonemic encephalopathy: review of 27 cases. Clin Lymphoma Myeloma. 2008;8(6):363-369.
doi pubmed - Talamo G, Cavallo F, Zangari M, Barlogie B, Lee CK, Pineda-Roman M, Kiwan E, et al. Hyperammonemia and encephalopathy in patients with multiple myeloma. Am J Hematol. 2007;82(5):414-415.
doi pubmed - Douedi S, Kapadia S, AlAzzawi M, Sen S. Hyperammonemic encephalopathy: a unique presentation of multiple myeloma. Cureus. 2021;13(1):e12781.
doi pubmed pmc - Nakamura H, Takada K, Murase K, Ikeda H, Iyama S, Manabe T, Kobune M. Multiple myeloma with hyperammonemia treated with novel agents: a case series of three patients. Intern Med. 2023;62(5):775-778.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


