| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 2, April 2023, pages 100-104
Acute Promyelocytic Leukemia Treatment Masking Hepatic Tuberculosis: A Management Dilemma
Kimberly Boldiga, e, Amy Kiamosa, Trevanne Matthews-Hewb, Reeba Ommanc, Walter Quand
aDepartment of Internal Medicine, University of Florida College of Medicine: Jacksonville, Jacksonville, FL 32209, USA
bDepartment of Hematology and Oncology, Mayo Clinic Jacksonville, Jacksonville, FL 32224, USA
cDepartment of Pathology, University of Florida College of Medicine: Jacksonville, Jacksonville, FL 32209, USA
dDepartment of Hematology and Oncology, University of Florida College of Medicine: Jacksonville, Jacksonville, FL 32209, USA
eCorresponding Author: Kimberly Boldig, Department of Internal Medicine, University of Florida College of Medicine: Jacksonville, Jacksonville, FL 32209, USA
Manuscript submitted March 3, 2023, accepted April 5, 2023, published online April 30, 2023
Short title: APL Treatment Masking Hepatic Tuberculosis
doi: https://doi.org/10.14740/jh1109
| Abstract | ▴Top |
Acute promyelocytic leukemia is a form of acute myeloid leukemia (AML) that is characterized by presence of a promyelocytic leukemia-retinoic acid receptor alpha fusion. In most patients, this fusion is detected on conventional karyotype as the t(15;17)(q24.1;q21.2) translocation, but some patients have cryptic translocations with a normal karyotype. Historically, AML is associated with a poor prognosis. Treatment with all-trans retinoic acid and arsenic trioxide assures long-term survival in the majority of patients. This treatment is generally well-tolerated but may cause hepatotoxicity. This is usually identified by transaminitis but resolves after temporary cessation of treatment. Our patient’s hepatotoxicity did not resolve following all-trans retinoic acid and arsenic trioxide cessation which posed a diagnostic dilemma. This prompted exploration of other possible causes of hepatotoxicity. An eventual liver biopsy identified acid-fast bacilli, confirming a diagnosis of hepatic tuberculosis. A broad differential diagnosis is imperative when investigating abnormalities in liver function, especially in chemotherapy patients when treatment cessation may cause cancer progression.
Keywords: Acute promyelocytic leukemia; Tuberculosis; Hepatotoxicity; ATRA; ATO
| Introduction | ▴Top |
Acute myeloid leukemia (AML) is classified as a group of hematopoietic neoplasms comprised of cells committed to the myeloid lineage. AML has an incidence of 3.7 per 100,000 persons and accounts for 25% of leukemias in the western world [1]. This malignancy is driven by the accumulation of genetic alterations which combine to halt cell maturation, increase clonal proliferation, and protect against programmed cell death [2]. There exists a large diversity and heterogeneity within AML because leukemic transformations often occur at multiple steps along the cellular differentiation pathway [3]. One of these transformations is responsible for the development of acute promyelocytic leukemia (APL), which is a biologically and clinically distinct variant of AML. Specifically, in APL, the t(15,17)(q24.1;q21.2) translocation produces a promyelocytic leukemia-retinoic acid receptor alpha (PML-RARA) fusion protein that binds with the retinoic acid receptor element in the promoter regions of several myeloid-specific genes and inhibits myeloid differentiation [4, 5]. This distinction of the APL subtype of AML is critically important because, without treatment, APL has a median survival of less than 1 month, often due to uncontrolled bleeding [6]. When recognized and treated appropriately, APL has the highest cure rate among all AML subtypes [5]. Treatment with all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) provides a cure for many patients [5]. It is generally a well-tolerated treatment; however, one notable side effect is hepatotoxicity. Generally, the hepatotoxic side effects improve when holding the medication [7]. We present a case that despite treatment cessation, liver enzymes remained elevated. A dilemma in management ensued with the discovery of coexisting hepatic tuberculosis (TB).
| Case Report | ▴Top |
Investigations
A 24-year-old man presented with headache and intermittent fevers of several months duration after immigrating from Africa 1 year prior. Initial laboratory testing revealed a white blood cell count of 26 × 109/L, blast cells, neutropenia, severe anemia, and thrombocytopenia. These laboratory findings were concerning for malignancy. A peripheral blood smear was conducted which demonstrated promyelocytes with Auer rods (Fig. 1).
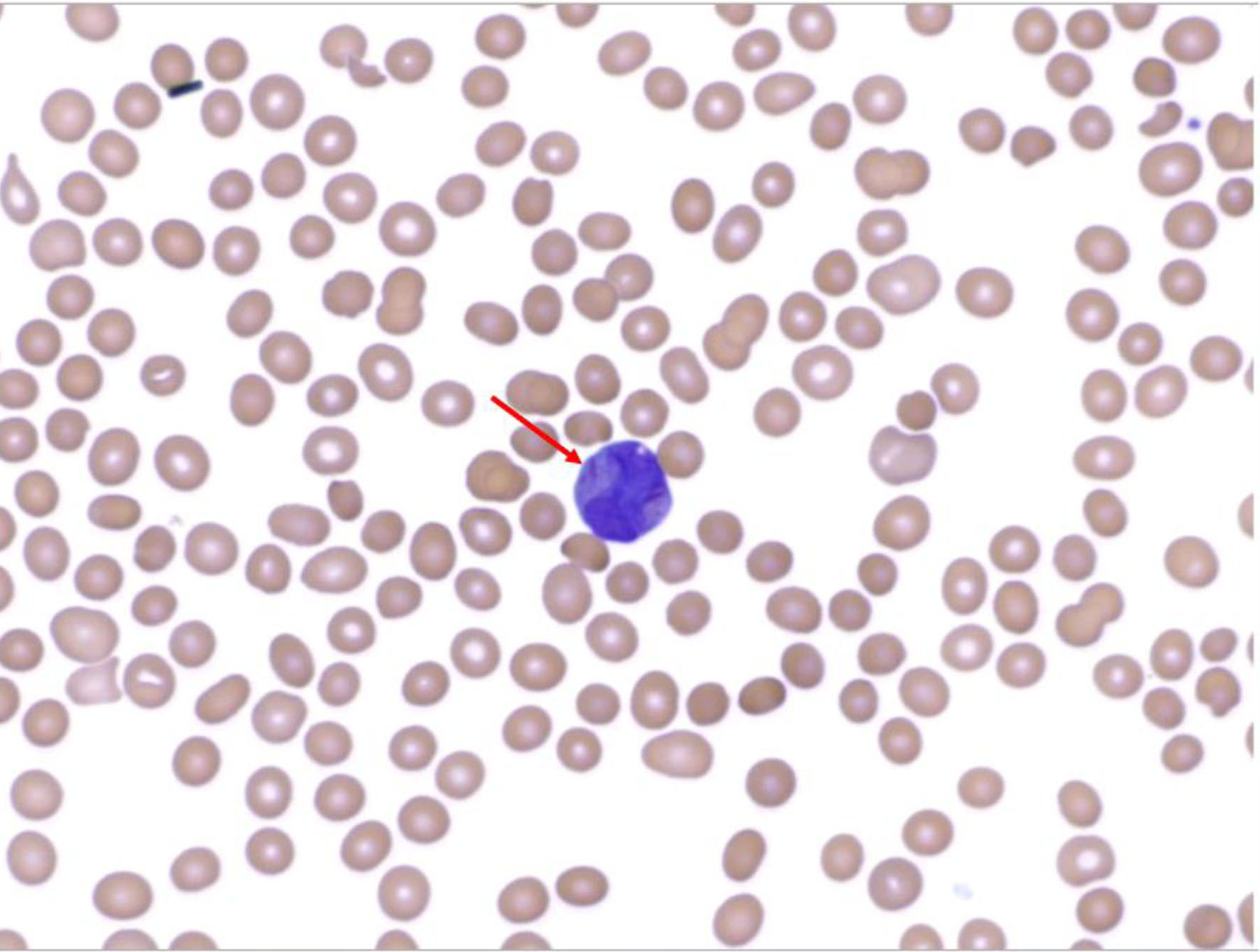 Click for large image | Figure 1. Peripheral blood smear demonstrating Auer rod within a promyelocyte. |
Diagnosis
A follow-up bone marrow biopsy demonstrated hypercellular marrow with increased promyelocytes and aspirate showed promyelocytes. PML-RARA fusion protein was detected by fluorescence in situ hybridization (FISH). Flow cytometry also showed CD34 negative, CD117 positive, human leukocyte antigen (HLA)-DR negative, and CD33 negative, suggestive of APL. Treatment with idarubicin, ATRA, and ATO was initiated. His clinical course was complicated by progressively worsening transaminitis (Table 1), prompting the decision to hold therapy with ATRA and ATO, given their known potential for hepatotoxicity. Despite holding these agents, his transaminitis persisted. Given the patient’s recent immigration from an endemic TB region, an interferon-gamma release assay was performed and returned positive. Chest X-ray and computed tomography (CT) demonstrated no acute cardiopulmonary findings or findings suggestive of TB. Serial sputum acid-fast bacillus (AFB) smears were negative. Given the clinical uncertainty between drug-induced liver injury (DILI) versus an infiltrative process, a liver biopsy was performed. It demonstrated caseating granulomas with AFB, confirming the diagnosis of hepatic TB (Fig. 2).
 Click to view | Table 1. Laboratory Values |
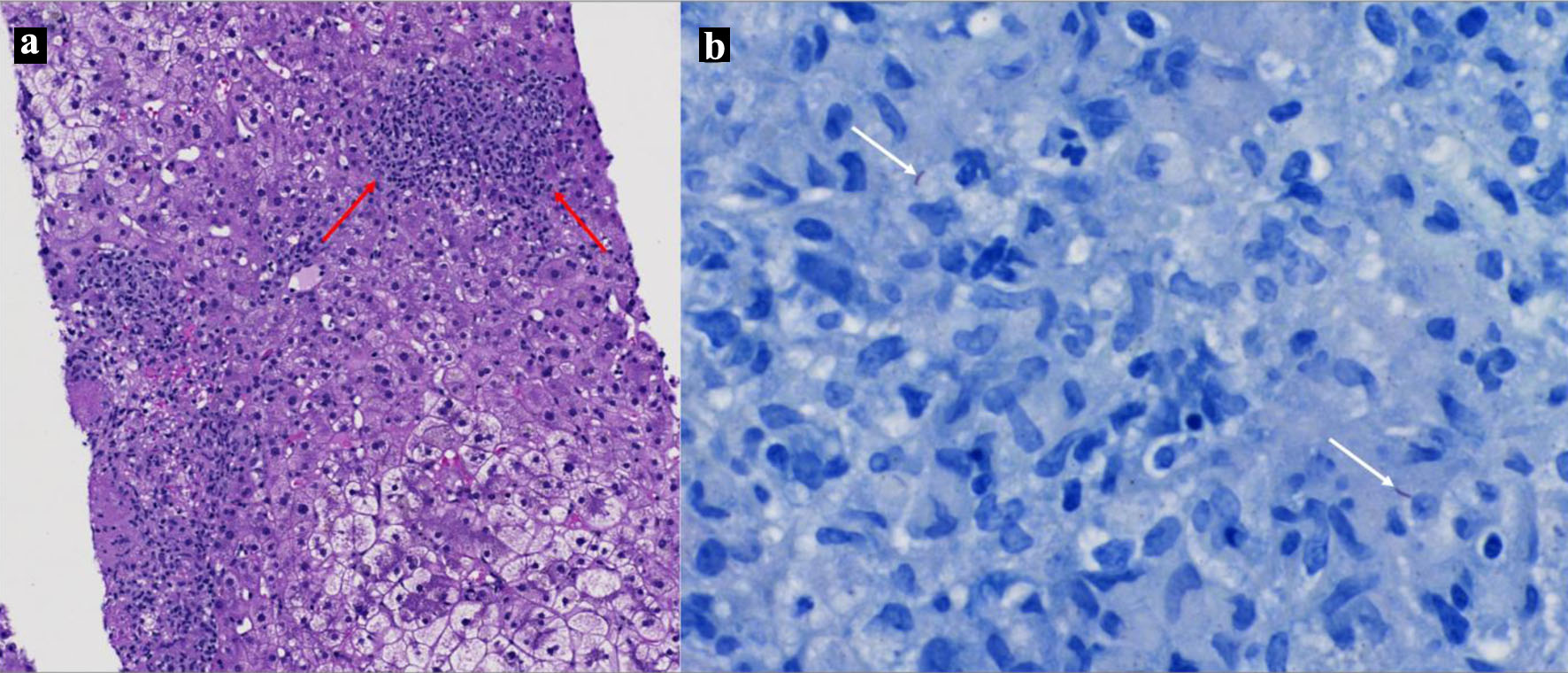 Click for large image | Figure 2. (a) H&E of liver biopsy specimen demonstrating necrotizing granulomas (red arrows). (b) Liver biopsy demonstrating two acid bacilli (white arrows). |
Treatment
TB treatment was initiated with rifampin, isoniazid, pyridoxine, and ethambutol, as he did not have multidrug-resistant disease. ATRA and ATO were temporarily held for 1 and 2 weeks, respectively until liver enzymes improved. The dose of ATO was adjusted to half of the original dose once reinitiated, then increased to his therapeutic dosage when liver function normalized.
Follow-up and outcomes
The patient had an excellent hematological response to ATRA and ATO induction with repeat bone marrow showing molecular remission. Treatment was subsequently followed by a consolidation phase of therapy. He remained on his TB medication to complete a 6-month duration of therapy.
| Discussion | ▴Top |
ATRA/ATO has improved survival in APL by reducing the early treatment related mortality and risk of relapse. This treatment has been shown to improve survival when compared to ATRA with chemotherapy [7]. Arsenic trioxide binds the promyelocytic leukemia protein component of PML-RARA oncoprotein leading to apoptosis of leukemic promyelocytes [7]. ATRA then binds the RARA component of the PML-RARA oncoprotein, which is believed to cause degradation [8]. This subsequently causes the maturation of the APL cells into granulocytes [9-11].
Drug toxicities due to ATRA/ATO are generally minor, but potential adverse effects include hepatotoxicity, leukopenia, QT time prolongation, and differentiation syndrome [6]. When compared to ATRA-chemotherapy, it is associated with fewer cases of neutropenia, thrombocytopenia, mucositis, and infections [6, 7]. The hepatotoxicity associated with ATRA/ATO has been estimated to affect anywhere from 24% to 75% of patients, in comparison to 6% of patients treated with ATRA-chemotherapy [6, 7, 12, 13]. Lo-Coco et al described grade 3 or 4 hepatotoxicity occurring in 63% of patients receiving ATRA/ATO. Grade 3-4 hepatotoxicity was defined as an increase in serum bilirubin and/or serum glutamic-oxaloacetic transaminase (SGOT) and/or alkaline phosphatase > 5 times the upper limit of normal [7]. Hepatic toxicity usually resolves after temporary discontinuation and dose adjustments [7, 12-15]. Some cases of hepatic toxicity have resolved spontaneously even in the setting of continuing treatment [6]. Lo-Coco et al describe the re-initiation of ATRA/ATO treatment at 50% of the previous dose for 7 days once the serum bilirubin and/or SGOT and/or alkaline phosphatase had decreased to below four times upper limit of normal [7]. Subsequently, ATRA/ATO was resumed at full dosage, in the absence of worsening of previous toxicity. If hepatotoxicity recurred, ATRA/ATO was discontinued [7]. The first 3 weeks following induction of therapy have been described as important periods of monitoring for hepatic toxicity [12]. Zacholski et al identified a correlation between increased ATO dosages and the development of neurotoxicity; however, there was no increased incidence of hepatotoxicity identified following dose fluctuations [16]. It is critical to differentiate DILI from secondary causes of liver toxicity as delay in treatment can increase the risk for severe bleeding events or lead to cancer progression [17].
Hepatic TB is a rare form of infectious hepatic disease. Hepatic involvement of disseminated TB is known as secondary hepatic TB [18]. This type of intra-abdominal TB occurs in 3.5% of cases [17]. Primary hepatobiliary TB is even more rare, occurring in 1% of cases [17]. The primary complaint by patients is often abdominal pain, although other common symptoms include abdominal distention, weight loss, ascites, diarrhea, nausea, and vomiting [19]. Hepatic TB can cause elevated transaminitis or alkaline phosphatase depending on the involvement of liver parenchyma or porta and ducts [17]. Diagnosis of hepatic TB can be challenging as symptomatology may be broad and nonspecific. Imaging studies, such as computerized tomography or ultrasound, may be helpful to establish the diagnosis; however, they have been found to be sensitive but not specific [17]. The diagnosis usually requires a liver biopsy or positive culture of a liver specimen, as in the case of our patient [18].
Few cases have been described of patients with APL and TB. Palta et al described a patient with dyspnea who was diagnosed with TB and subsequently found to have APL after abnormalities were seen on complete blood count differential [20]. Abdullah et al described a case of a patient with APL and persistent fevers. After extensive testing, without explanation for fever, a lung biopsy was performed which was consistent with TB [21]. Our patient initially presented without any symptoms suggestive of a TB diagnosis and had leukocytosis on initial laboratory testing which was thought to be explained by APL. No other laboratory or imaging findings suggested a diagnosis of TB until the transaminitis occurred and persisted following APL treatment. Hematological malignancies may predispose to a risk for activation of latent TB or opportunistic infections. Coburn et al found that 4.6% of patients with hematological disease had active TB compared to 0.2% of patients without hematological disease [22].
Medications used to treat TB are often involved in drug-drug interactions due to their metabolism by cytochrome p450 enzymes [23]. Minimal information has been reported in literature to describe drug interactions between TB treatment and ATRA/ATO. However, both drug regimens increase risk for hepatotoxicity [6, 7, 23]. Conversely, ATRA has shown to be efficacious in the treatment of TB both in vitro and in vivo [24]. Treatment mechanisms of ATRA relate to the activation of host innate and adaptive immune responses while stimulating the release of proinflammatory cytokines [24]. The pro-inflammatory environment created within the hepatic parenchyma secondary to the targeted attack of the infection may have caused persistent transaminitis despite the cessation of APL therapy. This conclusion was made whilst considering that the patient had normal liver function prior to its initiation while still harboring the infection.
Learning points
Chemotherapy-related side effects can be difficult to differentiate from clinical or laboratory indicators of an underlying infectious etiology. In our case, treatment of APL revealed a transaminitis causing concern for a DILI. However, because the transaminitis remained despite the cessation of ATRA-ATO, it prompted further investigation into the liver dysfunction. Identifying the underlying pathology of TB allowed our patient to be treated for both concurrent disease states. Making the distinction between drug-induced and other secondary causes of liver injury is imperative when the goal of treatment is curative as in the case of APL. The presence of unexplained abnormalities in liver function should prompt a broader differential diagnosis, which may include extrapulmonary TB in certain at-risk patient populations.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consent has been obtained.
Author Contributions
Kimberly Boldig and Walter Quan conceptualized the project. Kimberly Boldig and Amy Kiamos wrote the initial manuscript draft. Trevanne Matthews-Hew edited the manuscript. Reeba Omman provided pathologic images. Walter Quan was senior editor of the manuscript.
Data Availability
All data underlying the results are available in the article, and no additional source data are required. Further inquiries can be directed to the corresponding author.
Abbreviations
AFB: acid-fast bacillus; AML: acute myeloid leukemia; APL: acute promyelocytic leukemia; ATO: arsenic trioxide; ATRA: all-trans retinoic acid; CT: computed tomography; DILI: drug-induced liver injury; FISH: fluorescence in situ hybridization; PML-RARA: promyelocytic leukemia-retinoic acid receptor alpha; SGOT: serum glutamic-oxaloacetic transaminase
| References | ▴Top |
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099-2107.
doi pubmed - Kishtagari A, Levine RL, Viny AD. Driver mutations in acute myeloid leukemia. Curr Opin Hematol. 2020;27(2):49-57.
doi pubmed - Liquori A, Ibanez M, Sargas C, Sanz MA, Barragan E, Cervera J. Acute Promyelocytic Leukemia: A Constellation of Molecular Events around a Single PML-RARA Fusion Gene. Cancers (Basel). 2020;12(3):624.
doi pubmed pmc - Jimenez JJ, Chale RS, Abad AC, Schally AV. Acute promyelocytic leukemia (APL): a review of the literature. Oncotarget. 2020;11(11):992-1003.
doi pubmed pmc - Stahl M, Tallman MS. Acute promyelocytic leukemia (APL): remaining challenges towards a cure for all. Leuk Lymphoma. 2019;60(13):3107-3115.
doi pubmed pmc - Pei R, Cao J, Ma J, Zhang P, Liu X, Du X, Chen D, et al. Long term curative effects of sequential therapy with all-trans retinoic acid, arsenious oxide and chemotherapy on patients with acute promyelocytic leukemia. Hematology. 2012;17(6):311-316.
doi pubmed - Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121.
doi pubmed - Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90(2):124-133.
doi pubmed - Zhu J, Koken MH, Quignon F, Chelbi-Alix MK, Degos L, Wang ZY, Chen Z, et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94(8):3978-3983.
doi pubmed pmc - Lallemand-Breitenbach V, Guillemin MC, Janin A, Daniel MT, Degos L, Kogan SC, Bishop JM, et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189(7):1043-1052.
doi pubmed pmc - de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10(11):775-783.
doi pubmed - Hao L, Zhao J, Wang X, Wang H, Wang H, Xu G. Hepatotoxicity from arsenic trioxide for pediatric acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2013;35(2):e67-70.
doi pubmed - Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342-3347.
doi pubmed pmc - Leu L, Mohassel L. Arsenic trioxide as first-line treatment for acute promyelocytic leukemia. Am J Health Syst Pharm. 2009;66(21):1913-1918.
doi pubmed - Chen L, Zhu HM, Li Y, Liu QF, Hu Y, Zhou JF, Jin J, et al. Arsenic trioxide replacing or reducing chemotherapy in consolidation therapy for acute promyelocytic leukemia (APL2012 trial). Proc Natl Acad Sci U S A. 2021;118(6):e2020382118.
doi pubmed pmc - Zacholski K, Hambley B, Hickey E, Kashanian S, Li A, Baer MR, Duong VH, et al. Arsenic trioxide dose capping to decrease toxicity in the treatment of acute promyelocytic leukemia. J Oncol Pharm Pract. 2022;28(6):1340-1349.
doi pubmed pmc - Evans RP, Mourad MM, Dvorkin L, Bramhall SR. Hepatic and intra-abdominal tuberculosis: 2016 update. Curr Infect Dis Rep. 2016;18(12):45.
doi pubmed - Subramanyam SG, Kilpadi AB, Correa M, Pai M. Hepatic TB: four cases and a review of the literature. Trop Doct. 2006;36(2):121-122.
doi pubmed - Rasheed S, Zinicola R, Watson D, Bajwa A, McDonald PJ. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Dis. 2007;9(9):773-783.
doi pubmed - Palta A, Dhiman P, Cruz SD. ZBTB16-RARalpha variant of acute promyelocytic leukemia with tuberculosis: a case report and review of literature. Korean J Hematol. 2012;47(3):229-232.
doi pubmed pmc - Abdullah AS, Adel AM, Hussein RM, Abdullah MA, Yousaf A, Mudawi D, Mohamed SF, et al. Hypercalcemia and acute pancreatitis in a male patient with acute promyelocytic leukemia and pulmonary tuberculosis. Acta Biomed. 2018;89(3-S):23-27.
doi pubmed pmc - Coburn RJ, England JM, Samson DM, Walford DM, Blowers R, Chanarin I, Levi AJ, et al. Tuberculosis and blood disorders. Br J Haematol. 1973;25(6):793-799.
doi pubmed - Yew WW. Clinically significant interactions with drugs used in the treatment of tuberculosis. Drug Saf. 2002;25(2):111-133.
doi pubmed - Bahlool AZ, Grant C, Cryan SA, Keane J, O'Sullivan MP. All trans retinoic acid as a host-directed immunotherapy for tuberculosis. Curr Res Immunol. 2022;3:54-72.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


