| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 2, April 2023, pages 87-91
A Meningioma Mimic and Distinct Subtype of Primary Central Nervous System Lymphoma: Primary Dural Lymphoma
Sushanth Sreenivasana, c, Risha Solankib, Pragnan Kancharlab, Cyrus Khanb, Yazan Samhourib
aDepartment of Internal Medicine, West Penn Hospital, Pittsburgh, PA, USA
bDivision of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
cCorresponding Author: Sushanth Sreenivasan, Department of Internal Medicine, West Penn Hospital, Pittsburgh, PA, USA
Manuscript submitted March 11, 2023, accepted April 24, 2023, published online April 30, 2023
Short title: Primary Central Nervous System Lymphoma
doi: https://doi.org/10.14740/jh1113
| Abstract | ▴Top |
Primary central nervous system lymphoma (PCNSL) is an aggressive form of extranodal non-Hodgkin lymphoma that arises in the brain parenchyma, eyes, meninges, or spinal cord in the absence of systemic disease. Primary dural lymphoma (PDL), in contrast, arises from the dura mater of the brain. PDL is usually a low-grade B-cell marginal zone lymphoma (MZL), whereas other types of PCNSL are usually high-grade large B-cell lymphoma. This specific pathological subtype has important therapeutic and prognostic implications, making PDL a distinct subtype of PCNSL. Herein, we report a case of PDL in an African American patient, in her late thirties, who presented to our emergency room with chronic headaches. An emergent magnetic resonance imaging (MRI) of the brain showed a dural-based homogeneously enhancing extra-axial mass along the left hemisphere, which was contained within the anterior and parietal dural mater. A surgical specimen was collected after an emergency debulking procedure. The flow cytometry, done on the surgical specimen obtained, was positive for CD19+, CD20+, and CD22+, but negative for CD5- and CD10-. These findings were consistent with a clonal B-lymphoproliferative disorder. The surgical pathology specimen immunohistochemistry was positive for CD20+ and CD45+, but negative for Bcl-6Cyclin D1- and CD56-. The Ki67 was 10-20%. These findings were consistent with extranodal MZL. Given the location and pathology, the patient was diagnosed with PDL. Due to MZL’s indolent nature, location outside the blood-brain barrier, and known efficacy to bendamustine-rituximab (BR), we decided to treat our patient with BR. She completed six cycles without major complications, and her post-therapy brain MRI showed complete remission (CR). Our case adds to the sparse literature about PDL and highlights the efficacy of BR systemic chemotherapy on MZLs.
Keywords: Primary dural lymphoma; Primary central nervous system lymphoma; Marginal zone lymphoma; Diffuse large B-cell lymphoma
| Introduction | ▴Top |
Primary central nervous system lymphoma (PCNSL) is an aggressive form of extranodal non-Hodgkin lymphoma (NHL) that arises in the brain parenchyma, eyes, meninges, or spinal cord in the absence of systemic disease [1]. The most common location is brain parenchymal lesions. PCNSL of the meninges only is rare. Primary dural lymphoma (PDL) arises from the dura mater of the brain. It differs biologically from other CNS lymphomas [2, 3]. It is usually a low-grade B-cell marginal zone lymphoma (MZL), whereas other types of PCNSL are usually high-grade large B-cell lymphoma. This specific pathological subtype has important therapeutic and prognostic implications, making PDL a distinct subtype of PCNSL, with less known about its development other than its connection to other chronic stomach conditions.
The exact incidence of PDL is unknown; it was reported to be 2.4% in a series of 335 patients with PCNSL [4]. In contrast to brain parenchyma PCNSL which has slight male predominance, PDL occurs mainly in middle-aged women [4, 5]. A 5-year overall survival rate was reported at 96.7% and the progression-free survival rate was 81% [6]. Herein, we report a case of PDL in a woman, in her late thirties, and describe the diagnosis, disease course and treatment.
| Case Report | ▴Top |
Investigations
A woman, in her late thirties, with a past medical history of an ovarian teratoma presented to our hospital with chronic intermittent headaches that had progressively become worse over 6 months. She underwent a magnetic resonance imaging (MRI) of the brain that revealed a dural-based homogeneously enhancing extra-axial mass, measuring 11 cm, along the left hemisphere. It extended to the anterior interhemispheric falx and anterior right frontal. The mass effect caused 1 - 2 cm left-to-right midline shift on the underlying brain parenchyma along with significant vasogenic edema (Fig. 1a-c).
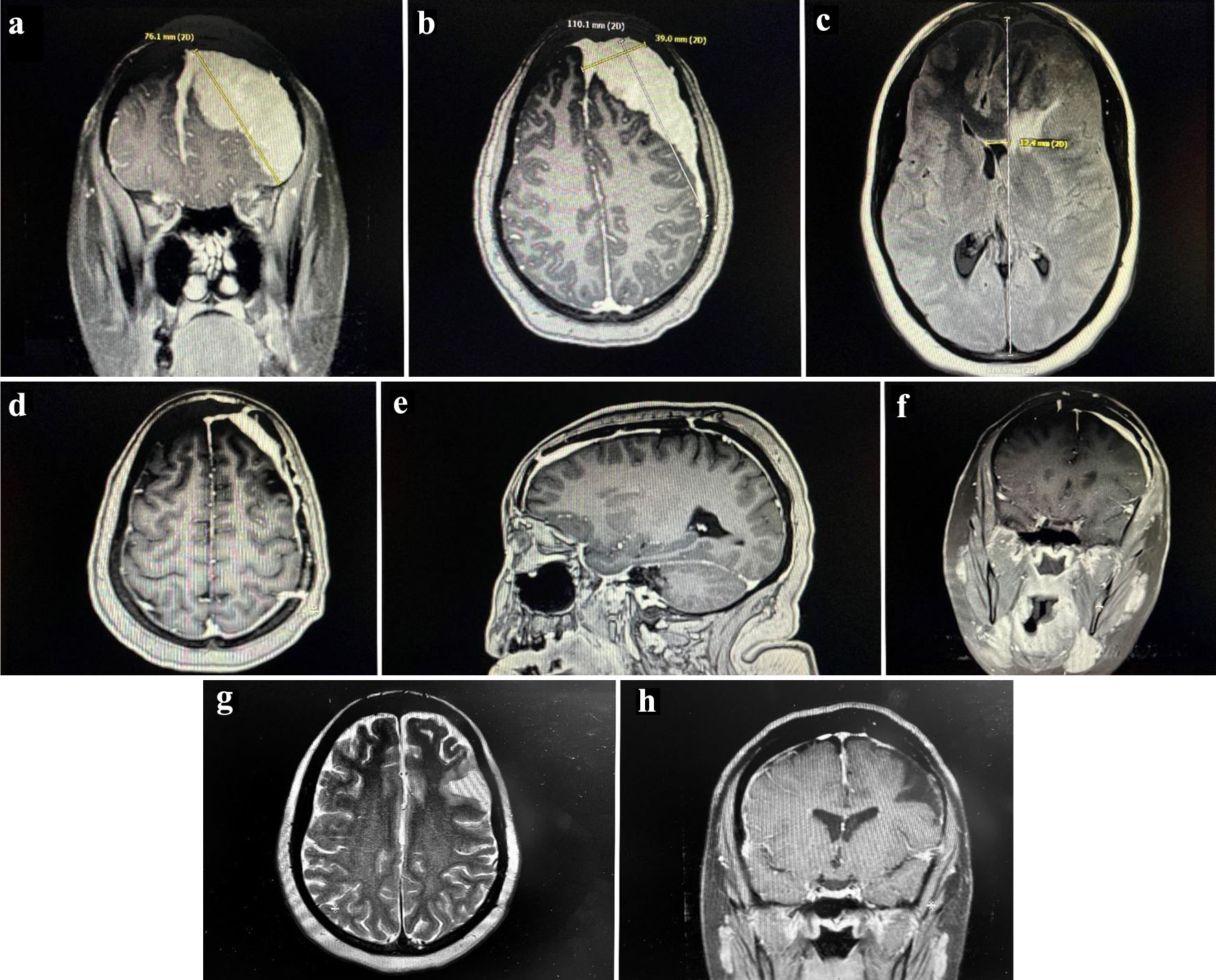 Click for large image | Figure 1. Pre-operative MRI scan. Dural-based homogeneously enhancing extra-axial mass along the left hemisphere with extension to the anterior interhemispheric falx and anterior right frontal lobe measuring 11 cm. There is significant mass effect and vasogenic edema. (a) Coronal section of the mass. (b) Sagittal section of the mass. (c) Transverse section of the mass. Post-operative MRI scan. Diffuse dural thickening through the left hemisphere, most notably along the left frontal lobe, reflecting postoperative changes of residual tumor. (d) Transverse section. (e) Sagittal section. (f) Coronal section. Post-chemotherapy MRI scan. Unchanged dural thickening and enhancement along the left hemisphere and anterior interhemispheric falx post-operatively. Some encephalomalacia in the left frontal lobe, but no new pathological enhancement seen. (g) T2 transverse section. (h) T1 axial section. MRI: magnetic resonance imaging. |
Diagnosis
Her significant laboratory findings were a white blood cell (WBC) 8.37 with a normal differential, uric acid 2.5, lactate dehydrogenase (LDH) 229 and negative disseminated intravascular coagulation (DIC) panel. Her cerebral spinal fluid collected was colorless, predominantly lymphocytic 84%, protein of 55 (reference: 15 - 45) and void of any malignant cells.
The patient underwent an emergent craniotomy and debulking of the mass, during which a surgical specimen was obtained. The tumor was entirely contained within the anterior and parietal dural matter (Fig. 1d-f). The flow cytometry, done on the surgical specimen obtained, was positive for CD19+, CD20+, and CD22+, but negative for CD5- and CD10-. These findings were consistent with a clonal B-lymphoproliferative disorder. The surgical pathology specimen immunohistochemistry was positive for CD20+ and CD45+, but negative for Bcl-6Cyclin D1- and CD56-. The Ki67 was 10-20%. These findings were consistent with extranodal MZL. Her Ebstein-Barr virus titers were negative (Fig. 2a-d).
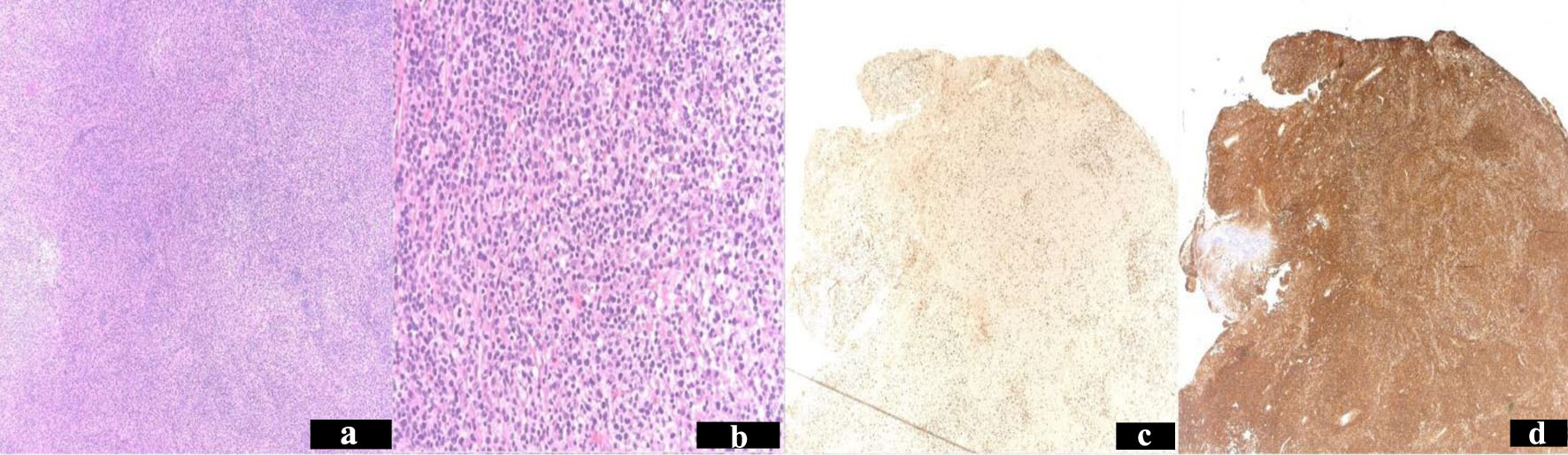 Click for large image | Figure 2. Resected tumor pathology stains. Hematoxylin and eosin stain of left frontal craniotomy tumor resection specimen shows focal residual germinal centers, partial plasmacytic differentiation and scattered large transformed cells. Low power × 40 (a). high power × 200 (b). Immunohistochemistry stain Ki67 proliferation index 10-20% (c). Stained CD45 diffusely positive (d). |
An MRI of her complete spine and computed tomography (CT) of chest abdomen and pelvis did not reveal any other sites of lymphomatous spread. A final diagnosis of PDL was made from tumor geography and histology.
Treatment
After surgery, treatment with bendamustine and rituximab (BR) (q 28-day cycle, standard dosing) was initiated and the patient completed six cycles without any complications. We chose to treat our patient with a partial resection and chemotherapy rather than radiotherapy (RT) due to the proven efficacy of BR in extranodal MZL and to treat possible microscopic systemic disease.
Follow-up and outcomes
Post-therapy MRI of the brain revealed stable post-operative changes in the brain but did not identify any signs of malignancy. She achieved CR after 6 months of treatment and continues to do well after 3 years (Fig. 1g, h).
| Discussion | ▴Top |
The dura mater is devoid of lymphoid tissue; however, it is hypothesized that chronic inflammation of the dura, due to chronic infection and/or autoimmune disorder, may attract polyclonal lymphocytes from which monoclonal lymphoma could arise. Histologically, MZL is the most frequent pathology seen in PDL [7, 8]. Chronic infections like Helicobacter pylori [9] and Chlamydia psittaci [10] have been associated with extranodal MZL (extranodal mucosa-associated lymphoid tissue (EMALT) lymphoma) of the stomach and ocular adnexa, respectively. Sjogren disease has been associated with MZL of the parotid gland. However, PDL has not been definitively associated with any infectious or autoimmune disease, but reported amongst patients with hepatitis C, scleroderma, Graves’ disease, and Sjogren disease [11-14].
PDLs are diagnosed with both advanced imaging and tissue biopsy. The imaging test of choice is MRI with gadolinium contrast which usually shows a single or multiple homogeneously enhanced extra-axial mass [4]. Other MRI findings include meningeal thickening, dural tail sign, underlying parenchymal vasogenic edema, early invasion of the underlying brain calvarial hyperostosis, and bone erosion [15]. However, these features are not specific to PDL but are also shared by meningiomas, fibrous tumors or chronic subdural hematomas. In one report, 14 out of 15 PDL cases were misdiagnosed as meningiomas on imaging alone [5]. Our patients’ MRI findings were classical for PDL, and led our radiologists’ differential to include meningioma, neurosarcoidosis, fibrous tumor and lymphoma. It has been postulated that due to its rarity, no standard treatment exists for PDL and variation of conditions. RT, surgery and multiple chemotherapy regimens have been used in a single modality or in combination [15]. Due to its indolent pathology and being a localized disease, surgical resection and focal RT have been used successfully [8, 16, 17]. However, complete resection can be difficult due to multiple tumors, infiltrative behavior, or en plaque presentation. Karschnia et al found that surgery, partial or complete tumor resection, improved their patient cohort’s overall survival (P = 0.044), but not progression-free survival [18]. If the tumor is in several bodily areas, most cases require RT/chemotherapy as a follow-up. Thus, making either chemotherapy or another multimodal approach more viable in select cases.
RT is a viable treatment option in nodal and other extranodal MZL, and PDL is not an exception. RT as an option should be exhausted before beginning chemotherapy or methotrexate [4]. De la Fuente et al postulate that CR can be achieved with local therapy, surgery followed by focal RT, in MZLs limited to a single site [19]. In their study, 12/13 patients that received focal RT to a single tumor achieved CR in their follow-up period [19].
High-dose methotrexate (HDMTX)-based regimens are the standard of care for brain parenchyma PCNSL which has an aggressive histopathology. HDMTX is favored for these lymphomas because of the drug’s ability to penetrate the blood-brain barrier. However, an HDMTX chemotherapy regimen may be excessive for PDL, which usually lies within the dura. Developmentally, dura mater originates from the mesoderm while the CNS parenchyma originates from the ectoderm. Therefore, the dura develops outside the blood-brain barrier, and hence easily exposed to systemic therapies. The MALT2008-01 trial established BR efficacy against MALT lymphoma by purporting a 100% overall response rate (ORR) after three cycles and 98% CR rate in untreated CD20-positive MALT lymphoma [20]. Due to the proven efficacy of BR against extranodal MZL and BR’s overall tolerability, we decided to treat our patient with chemotherapy after a partial debulking procedure. Tsutsumi et al reported two cases of dural MZL that were treated successfully with BR, even though they had evidence of systemic disease, not only dural disease.
CNS prophylaxis is generally not part of the treatment paradigm of PDL, due to extranodal MZL indolent nature and location outside the blood-brain barrier. In the case series, De la Fuente et al do not report any cases of CNS prophylaxis being used in PDL with MZL histology.
Learning points
Our case adds to the sparse literature about PDL and emphasizes the importance of recognizing it as a separate entity from parenchymal PCNSL which is an aggressive disease and carries a poor prognosis. Surgery, RT, and/or chemoimmunotherapy have been used in the treatment of extranodal MZL and are all appropriate treatments for PDL.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Consent for publication of individual healthcare data and images was obtained from the patient described in the case report.
Author Contributions
SS and YZ were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BR: bendamustine-rituximab; CR: complete remission; HDMTX: high-dose methotrexate; MALT: mucosa-associated lymphoid tissue; MZL: marginal zone lymphoma; NHL: non-Hodgkin lymphoma; PCNSL: primary central nervous system lymphoma; PDL: primary dural lymphoma; RT: radiotherapy
| References | ▴Top |
- Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18(17):3144-3150.
doi pubmed - Lv ZW, Cheng KL, Tian HJ, Han XM. Primary diffuse large B-cell lymphoma of the dura with skull and scalp involvement: A case report and brief review of the literature. Oncol Lett. 2016;11(6):3583-3588.
doi pubmed pmc - Kumar S, Kumar D, Kaldjian EP, Bauserman S, Raffeld M, Jaffe ES. Primary low-grade B-cell lymphoma of the dura: a mucosa associated lymphoid tissue-type lymphoma. Am J Surg Pathol. 1997;21(1):81-87.
doi pubmed - Iwamoto FM, DeAngelis LM, Abrey LE. Primary dural lymphomas: a clinicopathologic study of treatment and outcome in eight patients. Neurology. 2006;66(11):1763-1765.
doi pubmed - Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23(24):5718-5727.
doi pubmed - Bustoros M, Liechty B, Zagzag D, Liu C, Shepherd T, Gruber D, Raphael B, et al. A rare case of composite dural extranodal marginal zone lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma. Front Neurol. 2018;9:267.
doi pubmed pmc - Zinzani PL, Magagnoli M, Galieni P, Martelli M, Poletti V, Zaja F, Molica S, et al. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999;17(4):1254.
doi pubmed - Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, Patterson BJ, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003;101(7):2489-2495.
doi pubmed - Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4(8):644-653.
doi pubmed - Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, Caggiari L, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96(8):586-594.
doi pubmed - Estevez M, Chu C, Pless M. Small B-cell lymphoma presenting as diffuse dural thickening with cranial neuropathies. J Neurooncol. 2002;59(3):243-247.
doi pubmed - Kambham N, Chang Y, Matsushima AY. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) arising in dura. Clin Neuropathol. 1998;17(6):311-317.
pubmed - Miller M, Loffe V, Ruffin WK, Giri PG. Primary MALT lymphoma of the dura in a patient with active scleroderma. Clin Adv Hematol Oncol. 2004;2(12):815-819.
pubmed - Sanjeevi A, Krishnan J, Bailey PR, Catlett J. Extranodal marginal zone B-cell lymphoma of malt type involving the cavernous sinus. Leuk Lymphoma. 2001;42(5):1133-1137.
doi pubmed - Iwamoto FM, Abrey LE. Primary dural lymphomas: a review. Neurosurg Focus. 2006;21(5):E5.
doi pubmed - Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, et al. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15(4):1624-1630.
doi pubmed - Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21(22):4157-4164.
doi pubmed - Karschnia P, Batchelor TT, Jordan JT, Shaw B, Winter SF, Barbiero FJ, Kaulen LD, et al. Primary dural lymphomas: Clinical presentation, management, and outcome. Cancer. 2020;126(12):2811-2820.
doi pubmed - de la Fuente MI, Haggiagi A, Moul A, Young RJ, Sidani C, Markoe A, Vega F, et al. Marginal zone dural lymphoma: the Memorial Sloan Kettering Cancer Center and University of Miami experiences. Leuk Lymphoma. 2017;58(4):882-888.
doi pubmed pmc - Salar A, Domingo-Domenech E, Panizo C, Nicolas C, Bargay J, Muntanola A, Canales M, et al. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2014;1(3):e104-111.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


