| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 12, Number 3, June 2023, pages 109-113
Amplification of Chromosome 1q Predicts Poor Overall Survival in Newly Diagnosed Multiple Myeloma Patients
Matevz Skergeta, b, d , Barbara Skopeca, b, Samo Zvera, b, Helena Podgornika, c
aDepartment of Hematology, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia
bFaculty of Medicine, University of Ljubljana, 1000 Ljubljana, Slovenia
cFaculty of Pharmacy, University of Ljubljana, 1000 Ljubljana, Slovenia
dCorresponding Author: Matevz Skerget, Department of Hematology, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia
Manuscript submitted May 7, 2023, accepted June 29, 2023, published online June 30, 2023
Short title: Amp(1q) Predicts Poor Survival in Myeloma
doi: https://doi.org/10.14740/jh1137
| Abstract | ▴Top |
Background: Chromosome 1q copy number alterations are common in newly diagnosed patients with multiple myeloma, and in most published studies, there is no distinction made between three copies or the addition of at least four copies. The impact of these copy number alterations on patient outcome and optimal treatment is not fully understood.
Methods: We retrospectively analyzed 136 transplant eligible patients with newly diagnosed multiple myeloma from our national registry, who were treated with first autologous stem cell transplantation (aHSCT) between January 1, 2018, and December 31, 2021. The primary endpoint was overall survival.
Results: Patients with at least four copies of chromosome 1q had the poorest prognosis, with an overall survival of only 28.3 months. In multivariate analysis, four copies of chromosome 1q were the only statistically significant factor for overall survival.
Conclusions: Despite the use of novel agents, transplantation, and maintenance therapy, patients with a gain of four copies of chromosome 1q have a very poor survival rate. Therefore, prospective studies using immunotherapy in this patient population are necessary.
Keywords: Myeloma; Amplification 1; Stem cell transplantation
| Introduction | ▴Top |
Multiple myeloma (MM) is an incurable plasma cell neoplasm with heterogeneous outcomes in progression-free survival (PFS) and overall survival (OS). Established cytogenetic risk factors predicting poor outcome include immunoglobulin heavy-chain (IgH) translocations (t)(4;14), t(14;16), t(14;20) and deletion (del) 17p and are incorporated to the definition of high-risk disease [1, 2]. Additional copies of any part of the long arm of chromosome 1 (1q+) are among the most common findings in patients with myeloma, occurring in 35-40% of patients with newly diagnosed multiple myeloma (NDMM) [3]. They occur as secondary events and are often associated with other high-risk cytogenetic changes and lead to increased expression of several oncogenes, including CKS1B, resulting in increased proliferation, dysregulated cell cycle, and antiapoptotic effect [4]. This results in high-risk disease, drug resistance and shorter OS [5, 6]. Additionally, 1q+ can be divided into two categories according to the number of copies gained. Chromosomal changes with three copies are defined as gain(1q) while patients with at least four copies are defined as amplification (amp)1q.
| Materials and Methods | ▴Top |
The objective of this retrospective observational study was to evaluate the impact of gain(1q) and amp(1q) on OS in a cohort of transplant eligible NDMM patients treated with proteosome inhibitors (PI) and/or immunomodulatory drugs (IMIDs) induction followed be single or tandem autologous stem cell transplantation (aHSCT). We included patients treated with first aHSCT between January 1, 2018, and December 31, 2021. Presence of t(11;14), t(4;14), t(14;16), t(14;20), del(17p), gain(1q), amp(1q) and hyperdiploidy was detected by fluorescence in situ hybridisation (FISH) on CD138-positive immunoselected plasma cells from bone marrow samples at time of diagnosis. Patients with insufficient material for cytogenetics were excluded. Statistical differences in baseline characteristics were evaluated using analysis of variance (ANOVA) for quantitative variables and Chi-squared for categorical variables. OS and overall survival from time of aHSCT (aHSCT-OS) were defined from the date of diagnosis, and date from aHSCT to death from any cause, respectively. Patients alive were censored at the last contact. OS and aHSCT-OS were estimated for defined cytogenetic risk groups using the Kaplan-Meier method and compared using the log-rank test. The impact of other variables on OS were assessed by univariate and multivariate Cox proportional hazards model. Statistical analysis was done using Jamovi on top of the R statistical language.
This study complies with the Declaration of Helsinki and was approved by the local ethical committee at the University Medical Center in Ljubljana (KSVE 140223). All patients gave written consent for data collection and analysis in our national registry and international registries for bone marrow transplantation.
| Results | ▴Top |
We included 136 NDMM patients. Most patients received induction therapy with bortezomib, dexamethasone and IMIDs (65%), conditioning with melphalan 200 mg/m2 (85 %), and 23% of patients received tandem aHSCT. At our institution tandem aHSCT is the preferred choice for patients with high-risk cytogenetics. Maintenance therapy with lenalidomide or bortezomib was given to almost half of all patients. Second-line therapy options available at our institution at the time were daratumumab combinations, second-generation PI combinations, and pomalidomide. Treatment across the subgroups was similar. Baseline characteristics showed variations between groups based on the international staging system (ISS) stage and the number of tandem transplants. Baseline characteristics and treatment are presented in Table 1.
 Click to view | Table 1. Baseline Patient Characteristics and Treatment |
OS and aHSCT-OS were analyzed for four subgroups; patients with established high-risk cytogenetics including del(17p), t(4;14), t(14;16), t(14;20), patients harboring isolated gain(1q), patients harboring isolated amp(1q), and patients with other cytogenetic changes (Fig. 1). Patients with isolated gain(1q) had similar OS as patients with high-risk cytogenetics. Patients with isolated amp(1q) had the worst prognosis with a median survival of only 28.3 months despite availability of novel agents at relapse.
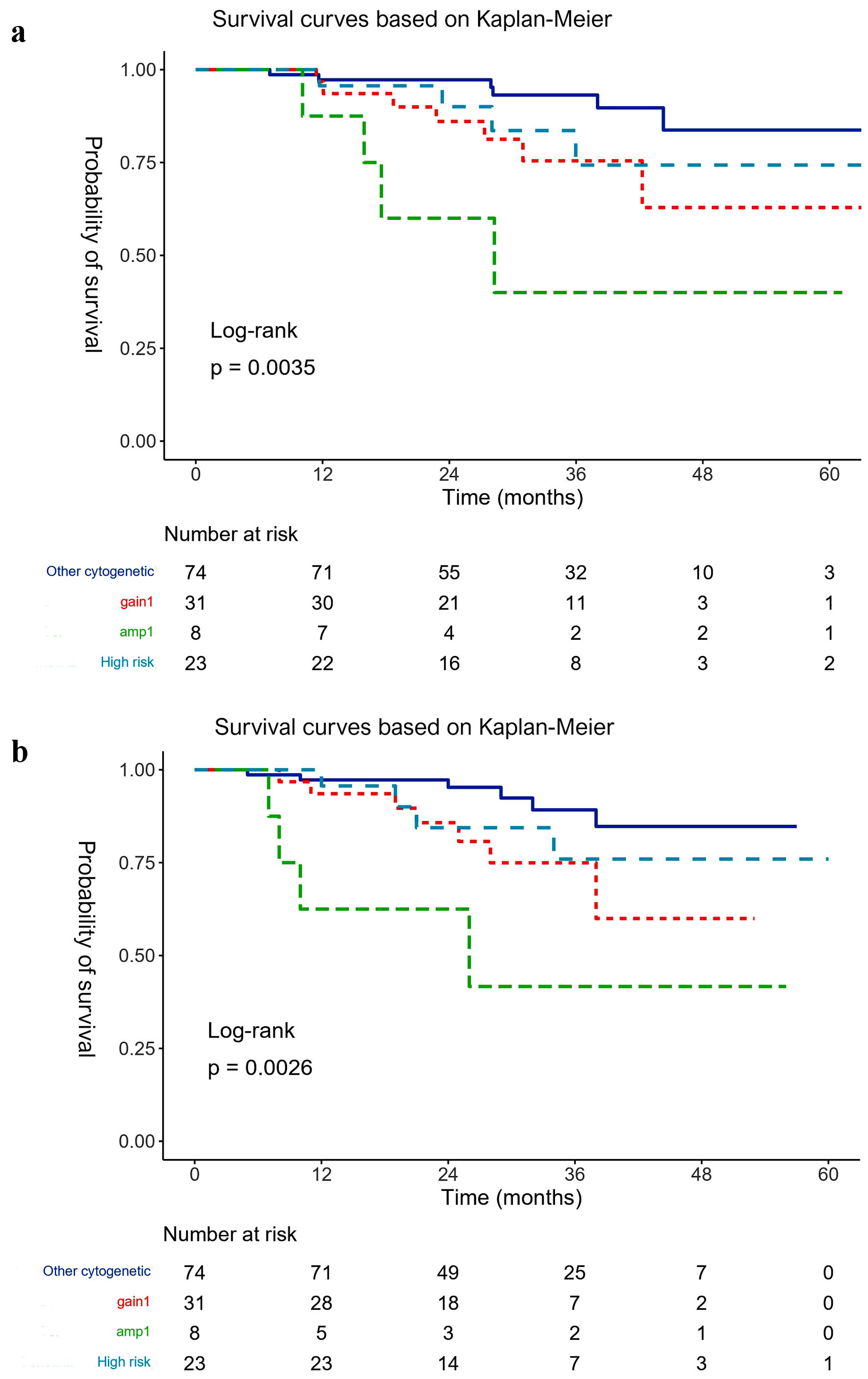 Click for large image | Figure 1. (a) Overall survival (OS) and (b) overall survival from time of stem cell transplantation (aHSCT-OS) (based on Kaplan-Meier estimates). Gain(1q): isolated gain(1q) three copies of 1q; Amp(1q): isolated amp(1q) > 3 copies of 1q; High risk: del(17p), t(4;14), t(14;16), t(14;20); Other cytogenetics: all cytogenetic changes without gain(1q) or amp(1q) or high risk. |
In multivariate analysis amp(1q) was associated with a significantly higher risk of death (hazard ratio (HR): 7.06, P = 0.011). Conversely, a hyperdiploid karyotype was found to reduce the risk of death (HR: 0.32, P = 0.045). However, no statistically significant differences were observed in OS among the groups receiving different induction treatment, tandem transplantation, or lenalidomide maintenance (Fig. 2)
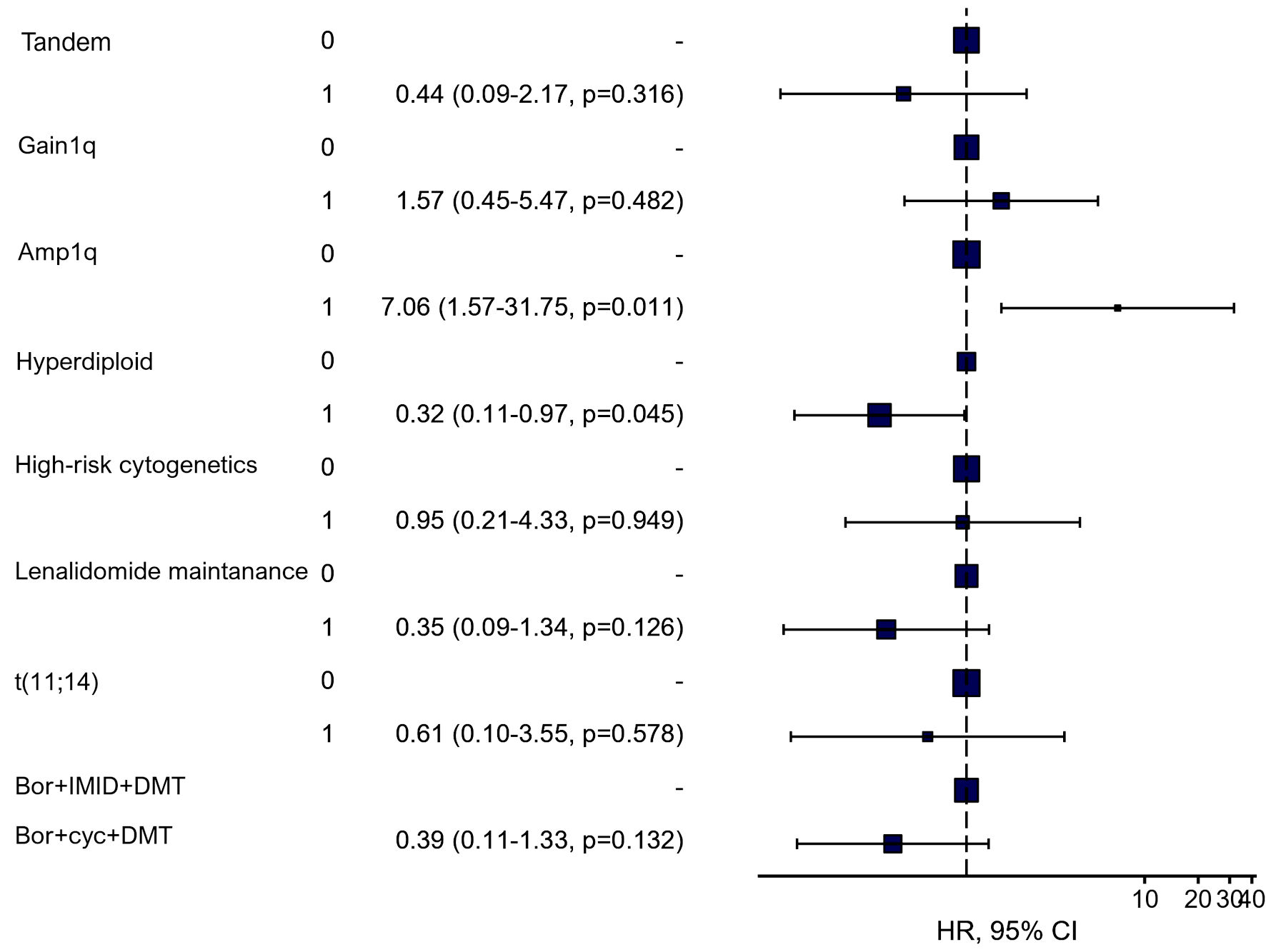 Click for large image | Figure 2. Multivariable subgroup analysis using Cox proportional-hazards model for overall survival (HR, 95% CI, and P value). Gain(1q): three copies of 1q; Amp(1q): > 3 copies of 1q; High risk: del(17p), t(4;14), t(14;16), t(14;20); Bor + IMID + DMT: bortezomib, immunomodulatory drug, dexamethasone; Bor + Cyc + DMT: bortezomib, cyclophosphamide, dexamethasone; HR: hazard ratio; CI: confidence interval. |
| Discussion | ▴Top |
The use of PI and IMIDs in induction treatment and maintenance, and aHSCT is known to improve PFS and OS in patients with high-risk disease. The impact of novel agents on outcomes of patients with 1q+ is more conflicting. Recent data show worse PFS and OS [6-8]. The largest retrospective study from the Mayo Clinic confirmed a decreased PFS and OS regardless of 1q copies number [8]. On the contrary, in studies by Schmidt [6] and D’Agostino [7] using either bortezomib, lenalidomide and dexamethasone (VRd) or carfilzomib, lenalidomide and dexamethasone (KRd) induction, there was a clear tendency for worse PFS in patients with amp(1q) compared to gain(1q), and in the study by D’Agostino only amp(1q) was statistically significant for OS [7]. In subgroup analysis, treatment with KRd and aHSCT completely abrogated the negative impact of gain(1q) but not amp(1q). Whether the use of induction treatments with daratumumab containing quadruplets or second-generation PI, or the use of novel treatments including bispecific antibodies, and chimeric antigen receptor (CAR) T-cells in relapse, improves OS in these poor risk patient population, remains elusive.
Our study has limitations due to its retrospective nature and small patient numbers in subgroups limiting statistical power. Potential treatment and follow-up bias in patients with proven high-risk cytogenetics could potentially lead to better outcomes in these patients compared to patients with isolated gain(1q) and amp(1q). On the contrary, OS is not prone to bias compared to PFS, and the large OS difference cannot be solely explained by an imbalance in induction therapy, as OS benefit is difficult to achieve in clinical trials in patients with NDMM and only after prolonged follow-up. Due to the retrospective approach and failure to stick to certain timepoints for response assessment in real world patients, we were unable to satisfactorily analyze differences in response between subgroups. Of note, the main objective of our study was to analyze survival, which is the most important parameter in patients, and cannot be distorted by clinician bias. Due to small patient numbers, we were not able to show a difference in OS for patients with amp(1q) regarding tandem transplantation vs. single transplant or maintenance therapy.
Despite the limitations mentioned, our study clearly demonstrated that amp(1q) is a clinically and statistically significant marker of poor prognosis. The emerging negative prognostic significance of amp(1q) requires a clear distinction from gain(1q). Furthermore, randomized trials that include novel agents often do not report 1q+ and do not distinguish between gain(1q) and amp(1q), so data for best treatment options are lacking. Available data show an unsatisfactory response to combinations based on PI and IMIDs even with aHSCT, and studies incorporating novel treatment approaches in this population might change the poor outcome of patients with amp(1q). Future randomized clinical trials selectively including patients with 1q+ and especially amp(1q) to establish the best combination treatment are needed.
Acknowledgments
None to declare.
Financial Disclosure
Authors have no source of funding regarding this study.
Conflict of Interest
Authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
MS and HP designed the study and analyzed the data. All authors participated in the study and shared responsibility for its results.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210-2221.
doi pubmed pmc - Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863-2869.
doi pubmed pmc - Walker BA, Leone PE, Chiecchio L, Dickens NJ, Jenner MW, Boyd KD, Johnson DC, et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood. 2010;116(15):e56-65.
doi pubmed - Luo S, Su T, Zhou X, Hu WX, Hu J. Chromosome 1 instability in multiple myeloma: Aberrant gene expression, pathogenesis, and potential therapeutic target. FASEB J. 2022;36(6):e22341.
doi pubmed - Zhan F, Colla S, Wu X, Chen B, Stewart JP, Kuehl WM, Barlogie B, et al. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood. 2007;109(11):4995-5001.
doi pubmed pmc - Schmidt TM, Barwick BG, Joseph N, Heffner LT, Hofmeister CC, Bernal L, Dhodapkar MV, et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019;9(12):94.
doi pubmed pmc - D'Agostino M, Ruggeri M, Aquino S, Giuliani N, Arigoni M, Gentile M, Olivero M, et al. Impact of gain and amplification of 1q in newly diagnosed multiple myeloma patients receiving carfilzomib-based treatment. Forte Trial Blood. 2020;(Suppl 1):38-40.
doi - Abdallah N, Greipp P, Kapoor P, Gertz MA, Dispenzieri A, Baughn LB, Lacy MQ, et al. Clinical characteristics and treatment outcomes of newly diagnosed multiple myeloma with chromosome 1q abnormalities. Blood Adv. 2020;4(15):3509-3519.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


