| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 4, August 2023, pages 176-186
Reduced-Intensity Anthracycline-Free Chemoimmunotherapy in Elderly Patients With Newly Diagnosed or Relapsed Diffuse Large B-Cell Lymphoma
Binoy Yohannana, Adan Riosa, b
aDivision of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, USA
bCorresponding Author: Adan Rios, Division of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, USA
Manuscript submitted May 14, 2023, accepted July 14, 2023, published online August 31, 2023
Short title: Anthracycline-Free in Large B-Cell Lymphomas
doi: https://doi.org/10.14740/jh1144
| Abstract | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL); it has a cure rate of approximately 50% with standard anthracycline-based chemoimmunotherapy. However, the clinical outcomes of elderly unfit/frail DLBCL patients remain suboptimal due to poor tolerance of anthracycline-containing regimens. Herein, we report a series of seven elderly unfit patients with DLBCL who were treated with a reduced-intensity anthracycline-free chemoimmunotherapy (rituximab, cyclophosphamide, vincristine, and prednisone) regimen combined with lenalidomide (R2-COP). Five patients received R2-COP as first-line therapy, and two patients were treated for relapsed DLBCL. Four patients with newly diagnosed DLBCL and two with relapsed disease achieved complete remission. The R2-COP regimen was well tolerated. Interim positron emission tomography (PET) scans in four patients after two to three cycles showed a complete metabolic response. At a median follow-up of 24 months, six patients remain in complete remission. R2-COP is an effective anthracycline-free regimen with encouraging clinical activity in elderly DLBCL patients who are unfit for standard anthracycline-containing regimens.
Keywords: Diffuse large B-cell lymphoma; Elderly patients; Anthracycline-free; Chemoimmunotherapy; Complete metabolic response; Complete remission
| Introduction | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is an aggressive type of non-Hodgkin lymphoma (NHL) commonly seen in the sixth or seventh decade of life [1]. An anthracycline-based chemoimmunotherapy regimen (R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)) offers the best curative option and is the standard of care [2]. In elderly patients, this curative anthracycline-containing regimen carries a significant risk of cardiac toxicity and treatment-related mortality (TRM) [3]. Carson et al reported a large retrospective study of US veterans (age > 80) with newly diagnosed DLBCL, and TRM was noted to be high at 18% with infection being the leading cause of deaths. The baseline performance status of 2 or higher is an adverse prognostic marker with a higher risk of TRM when compared to performance status of 0 to 1 (27% vs. 8%) [4]. The attenuated chemoimmunotherapy regimen (R-mini-CHOP) is an acceptable standard of care in older DLBCL patients, but it has been studied only in fit patients and it carries a TRM of 6-8% [5, 6]. Treating relapsed or refractory DLBCL is even more challenging, as salvage therapies are poorly tolerated, and, unfortunately, most elderly patients die of their disease. Despite the promising results seen with R-mini-CHOP regimen in older fit DLBCL patients (age > 80 years), TRM remains high in unfit and frail patients, especially those with high-risk international prognostic index and hypoalbuminemia, which are independent adverse prognostic markers [5-7]. Hence, clinicians treating elderly, unfit/frail patients with DLBCL should carefully weigh the benefits of a curative anthracycline-containing regimen against the potential risk of TRM. With increasing life expectancy, the incidence of aggressive DLBCL is projected to increase over the next decade, and there is an unmet need for novel chemoimmunotherapy regimens that are effective and well tolerated. We report a series of seven elderly unfit/frail patients with DLBCL, who were treated with a reduced-intensity anthracycline-free chemoimmunotherapy regimen consisting of rituximab, cyclophosphamide, vincristine, and prednisone combined with lenalidomide. In our series, all seven patients had hypoalbuminemia, and six patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or higher highlighting the need for an anthracycline free reduced intensity therapy.
| Case Report | ▴Top |
Patients characteristics
The clinical characteristics of individual patients are provided in Table 1. All seven patients were ineligible for intensive cytotoxic chemotherapy due to age, frailty, and comorbidities. Hence, patients were treated with an anthracycline-free regimen, R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide (R2-COP). Patients 2, 3, 5, and 7 received R2-COP as first-line therapy for newly diagnosed DLBCL, whereas patients 1 and 6 were treated for relapsed disease. Patient 2 was undergoing hormonal therapy for stage 4 breast cancer when a staging positron emission tomography/computed tomography (PET/CT) showed a liver lesion and biopsy confirmed DLBCL.
 Click to view | Table 1. Clinical Characteristics of Patients 1 - 7 |
This study was approved by the Institutional Review Board (IRB) (HSC-MS-23-0286).
Treatment and response
Response to treatment of each individual patient is reported in Table 2. A complete metabolic response (CMR) was achieved in all patients except for patient 6 within 1 - 6 months of starting therapy. An interim PET scan done after cycle 2 - 4 in patients 1, 2, 4, 6, 5 and 7, showed a CMR.
 Click to view | Table 2. Treatment and Response in Patients 1 - 7 |
Patient 1 initially presented in early 2018 with an oropharyngeal mass and bilateral cervical lymphadenopathy. The initial PET/CT is shown in Figure 1a. She was treated with six cycles of R-CHOP and achieved a CMR (Fig. 1b). Unfortunately, her disease relapsed 1 year later, and she presented with cervical lymphadenopathy (Fig. 1c). At the time of relapse, she was using a wheelchair and had significant difficulty performing her activities of daily living. Based on our clinical judgement, we felt that this patient would not tolerate a dose-intense regimen. As she was treated with vincristine at the time of her initial diagnosis and given her debility, we decided not to rechallenge with vincristine. She was treated with rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, oral prednisone 100 mg daily for 5 days, and lenalidomide 25 mg daily for 21 days followed by 1 week off. The patient tolerated this regimen well, and an interim PET/CT after two cycles showed a CMR (Fig. 1d). A PET/CT done 3 months after completing six cycles of therapy also showed a CMR. The patient remains in complete remission (CR) at the end of 24 months of follow-up.
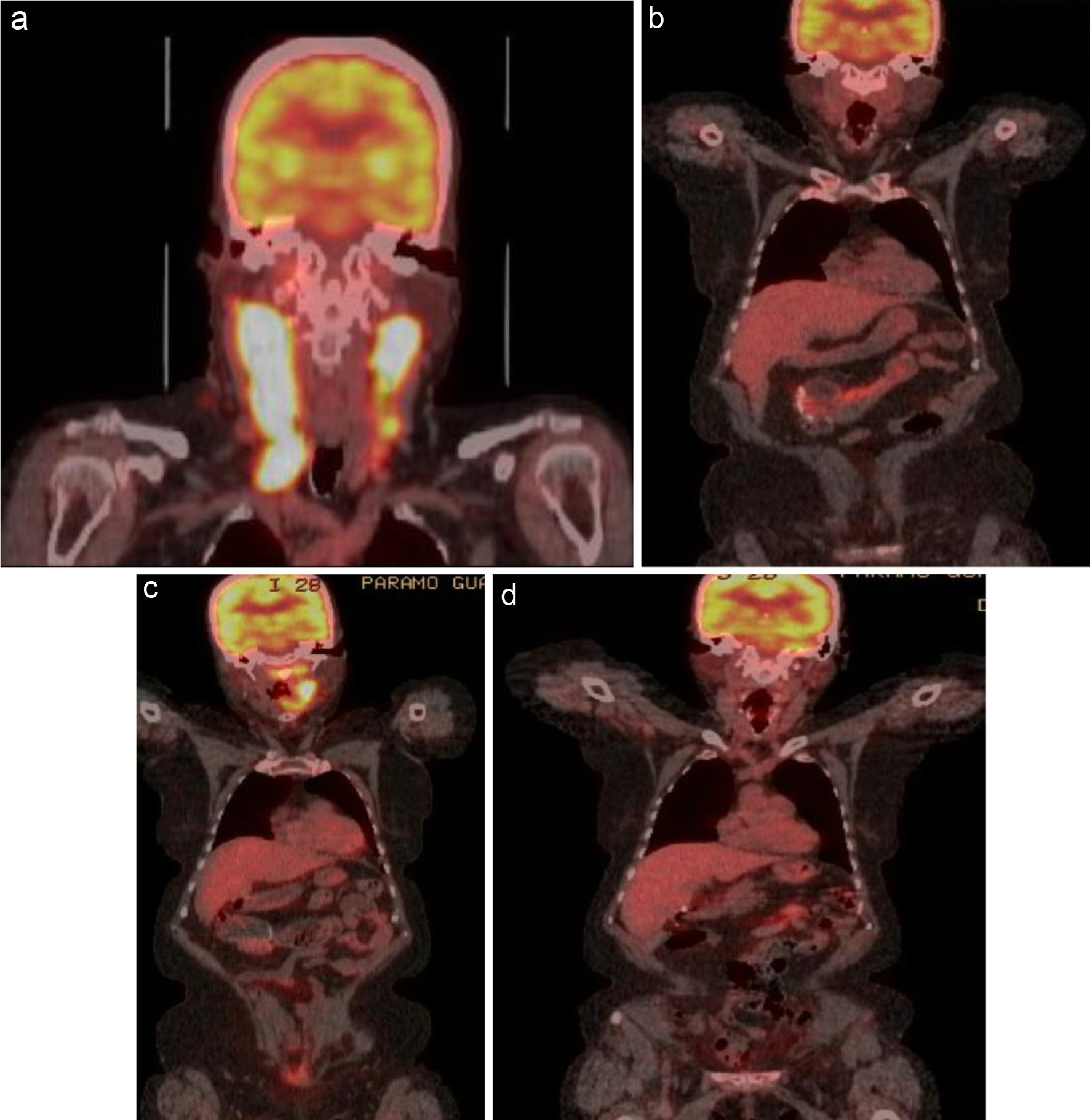 Click for large image | Figure 1. (a) FDG-avid uptake in the right oropharynx with an SUV of 27.4. Extensive lymph node involvement is present in the bilateral cervical chains (right greater than left), extending down to the level of the clavicle on the right. Areas of bilateral intensely FDG-avid lymph node conglomerates have an SUV of 27.4 on the right and 23 on the left. (b) PET/CT showing complete response after six cycles of R-CHOP. (c) PET/CT done 1 year later showing relapse. (d) Started on salvage therapy with R2-COP and achieved a CMR after two cycles. CMR: complete metabolic response; PET/CT: positron emission tomography/computed tomography; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; FDG: fluorodeoxyglucose; SUV: standardized uptake value; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide. |
Patient 2 was an 83-year-old woman with metastatic estrogen receptor-positive breast cancer, who was on monthly fulvestrant injections and was found to have a new fluorodeoxyglucose (FDG)-avid liver lesion on PET/CT (Fig. 2a). A biopsy of the liver lesion confirmed germinal center B-cell (GCB) subtype DLBCL. She received R-COP at standard doses and lenalidomide 25 mg daily for 21 days with 7 days off. An interim PET scan done after three cycles showed a CMR (Fig. 2b). After the third cycle, she became dehydrated and anorexic, and the patient stopped lenalidomide. Due to intolerance, lenalidomide was discontinued for the last three cycles. She completed the last three courses of treatment with R-CP (rituximab, cyclophosphamide, and prednisone). The patient remains in CR for her lymphoma at the end of 44 months of follow-up.
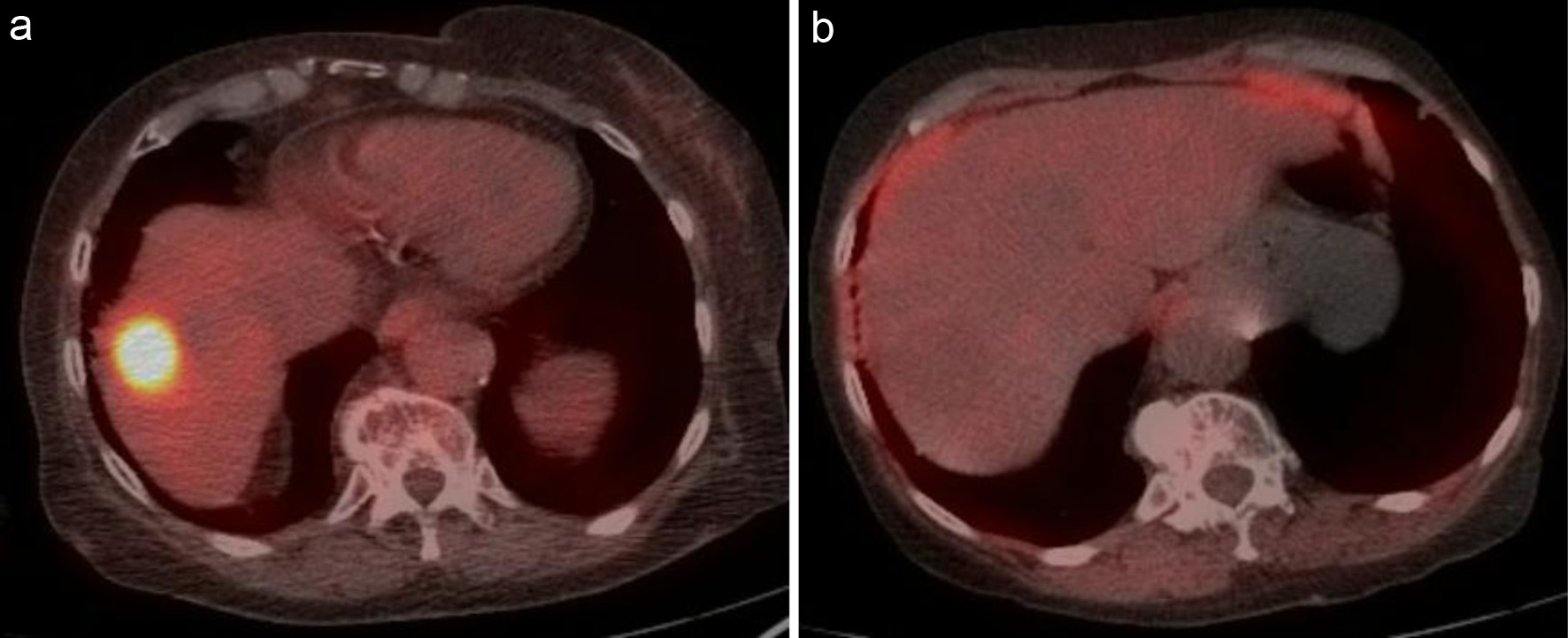 Click for large image | Figure 2. (a) Intense metabolically active lesion at the dome of the liver with an SUV of 21.7. (b) Interim PET/CT after two cycles showing complete resolution of abnormal metabolic activity within the liver lesion. PET/CT: positron emission tomography/computed tomography; SUV: standardized uptake value. |
Patient 3 had coronary artery disease and underwent coronary artery bypass graft in 2018. He presented with a mass in the right nasal cavity. PET/CT revealed a hypermetabolic mass involving the right nasal ala with extension into the right nostril and nasal cavity (Fig. 3a, b). He was treated with R2-COP for six cycles, and post-treatment PET/CT showed a CMR (Fig. 3c, d).
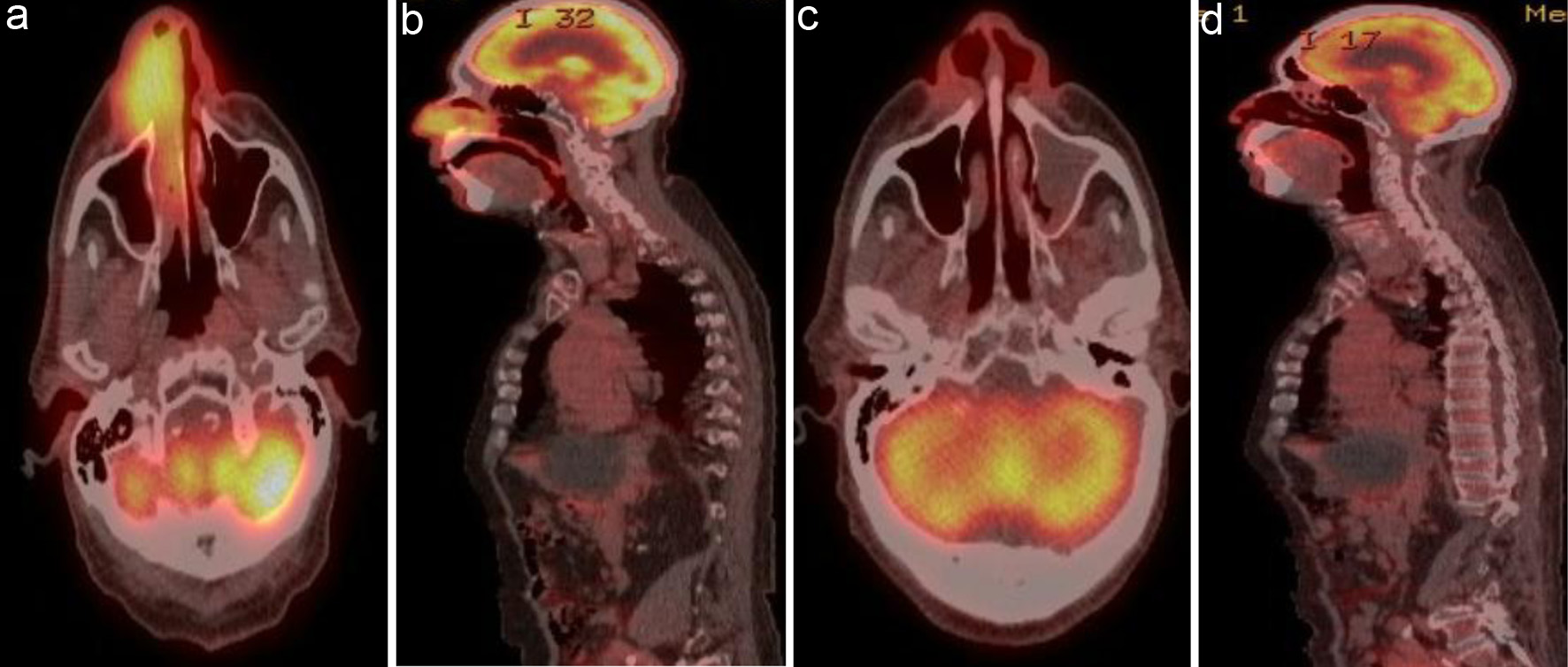 Click for large image | Figure 3. (a) Coronal view and (b) sagittal view of 5.1 × 3.6 cm hypermetabolic mass involving the right nasal ala with extension into the right nostril and nasal cavity. (c) Coronal view and (d) sagittal view of post-treatment PET/CT showing complete resolution of previously noted increased metabolic activity in the nasal cavity. PET/CT: positron emission tomography/computed tomography. |
Patient 4 was initially diagnosed with DLBCL based on biopsy of a right neck mass in January 2018 at an outside hospital. She received one cycle of CHOP at that time, but she did not receive rituximab due to insurance problems. She developed recurrent multidrug-resistant extended spectrum beta-lactamase (ESBL) Escherichia coli (E. coli) urinary tract infection and was unable to complete subsequent cycles of chemoimmunotherapy. However, she had an excellent response on PET/CT after her one cycle of chemotherapy, and she was placed on surveillance. She presented to our hospital in December 2020 with a large oropharyngeal mass causing airway obstruction (Fig. 4a, b) and needed an emergent tracheostomy. She was treated with R2-COP at standard doses, and interim PET after two cycles showed a CMR (Fig. 4c). She completed a total of five cycles. Post-treatment PET/CT after five cycles showed a CMR. She remains in CR after 24 months of follow-up.
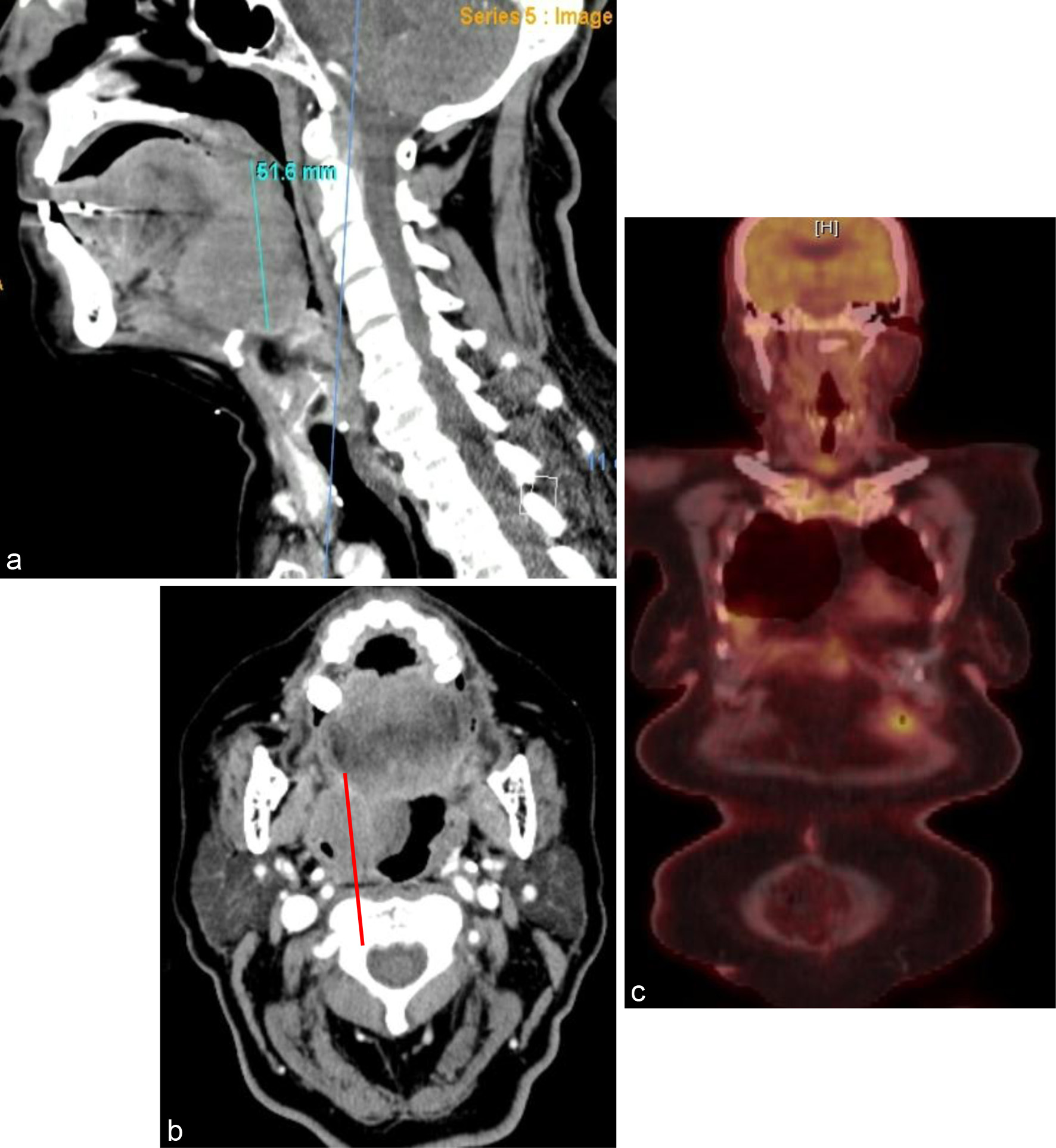 Click for large image | Figure 4. (a) Sagittal view and (b) coronal view of a 4.4 × 5.2 × 5.2 cm solid mass along the right base of the tongue with narrowing of the oropharynx. (c) Interim PET/CT after two cycles showing complete metabolic response. PET/CT: positron emission tomography/computed tomography. |
Patient 5 was a 72-year-old woman with systemic lupus erythematosus and Sjogren syndrome who presented with cervical lymphadenopathy. A core biopsy revealed a GCB-subtype DLBCL with a Ki-67 of 80%. Bone marrow biopsy did not reveal any morphologic or immunophenotypic evidence of lymphoma. She also had a PET/CT done that showed FDG-avid lymphadenopathy above and below the diaphragm with possible FDG-avid nodules in the pleura (Fig. 5a). The patient completed three cycles of R2-COP and achieved a CMR (Fig. 5b). A PET/CT done after completing six cycles of treatment showed a CMR. She remains in CR after 20 months of follow-up.
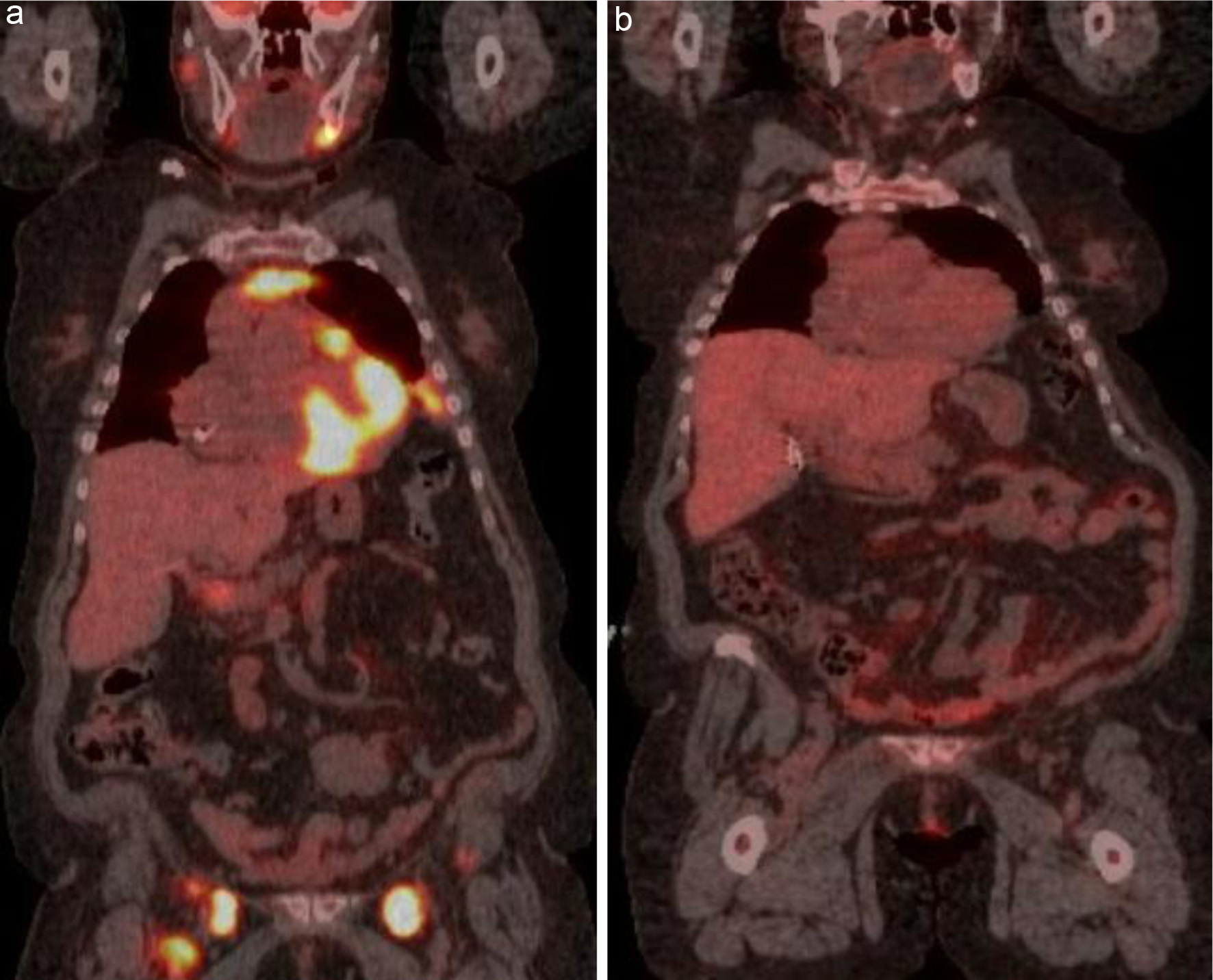 Click for large image | Figure 5. (a) FDG-avid lymphadenopathy in multiple stations above and below the diaphragm. (b) Interim PET/CT after three cycles showing a complete metabolic response. PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose. |
Patient 6 presented with a mediastinal mass, recurrent pleural effusions, and a left renal mass (Fig. 6a). Biopsy of the left renal mass confirmed DLBCL. He was treated with R2-COP for the first three cycles; after an interim scan showed a CMR, vincristine was dropped for the last three cycles. He remains in CR after 48 months of follow-up (Fig. 6b).
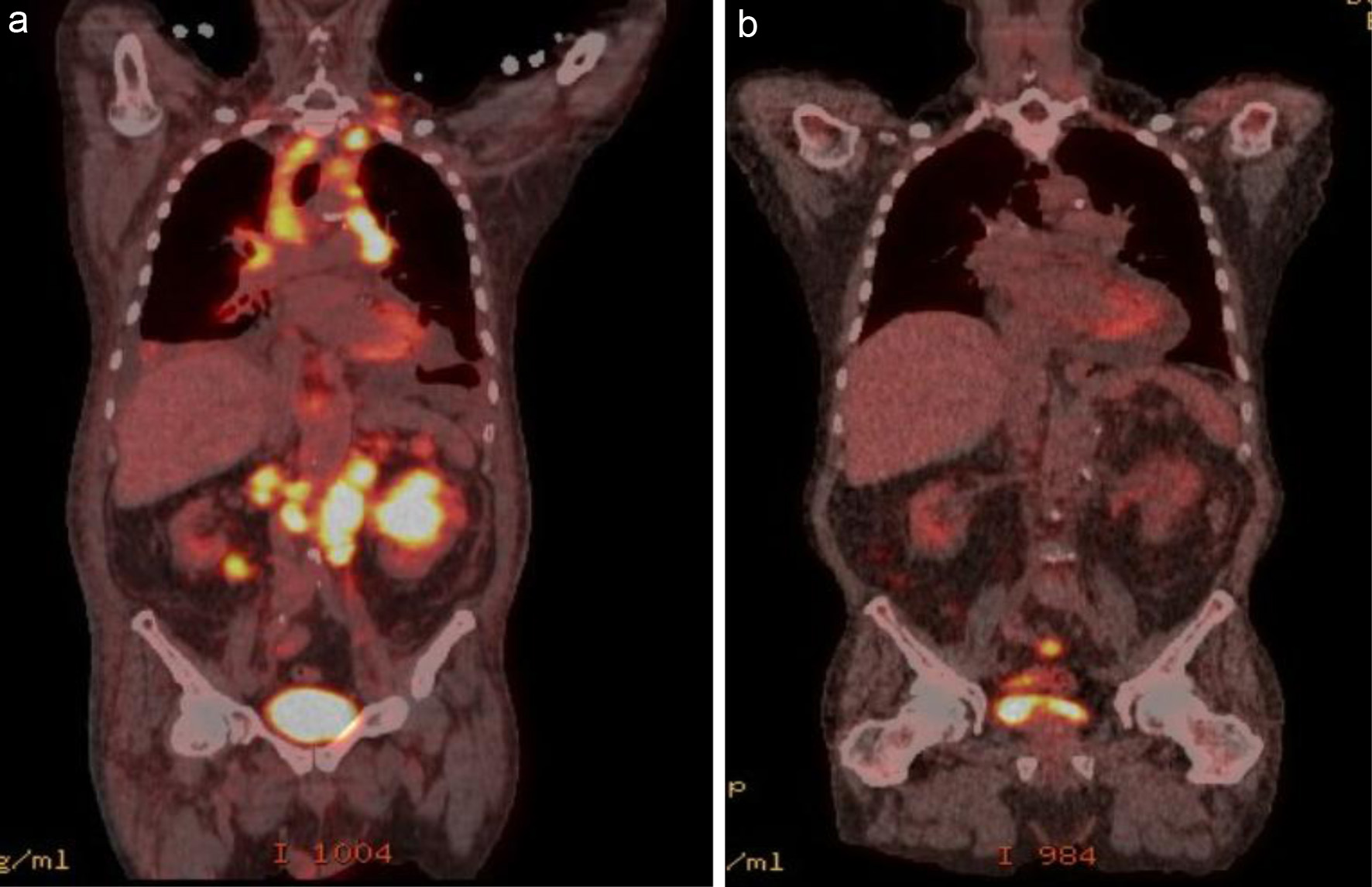 Click for large image | Figure 6. Pretreatment (a): extensive FDG-avid lymphomatous involvement above and below the diaphragm, including cervical, mediastinal, retrocrural, and retroperitoneal lymphadenopathy, as well as foci in the lungs, spleen, and bilateral kidneys. (b) PET/CT after four cycles of R2-COP showing a complete metabolic response. PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide. |
Patient 7 presented with an enlarging neck mass, hoarseness of voice, and difficulty swallowing. PET/CT scan showed a left tongue-base mass with intense FDG uptake, as well as lymphadenopathy in multiple levels of the left and right neck, right axilla, and inguinal region (Fig. 7a). He was treated with three cycles of R2-COP, and an interim PET scan showed a CMR (Fig. 7b). The patient completed two additional cycles of R2-COP, and the end-of-treatment PET scan showed a CMR. Unfortunately, patient died from hepatitis B reactivation 3 months after the completion of chemoimmunotherapy.
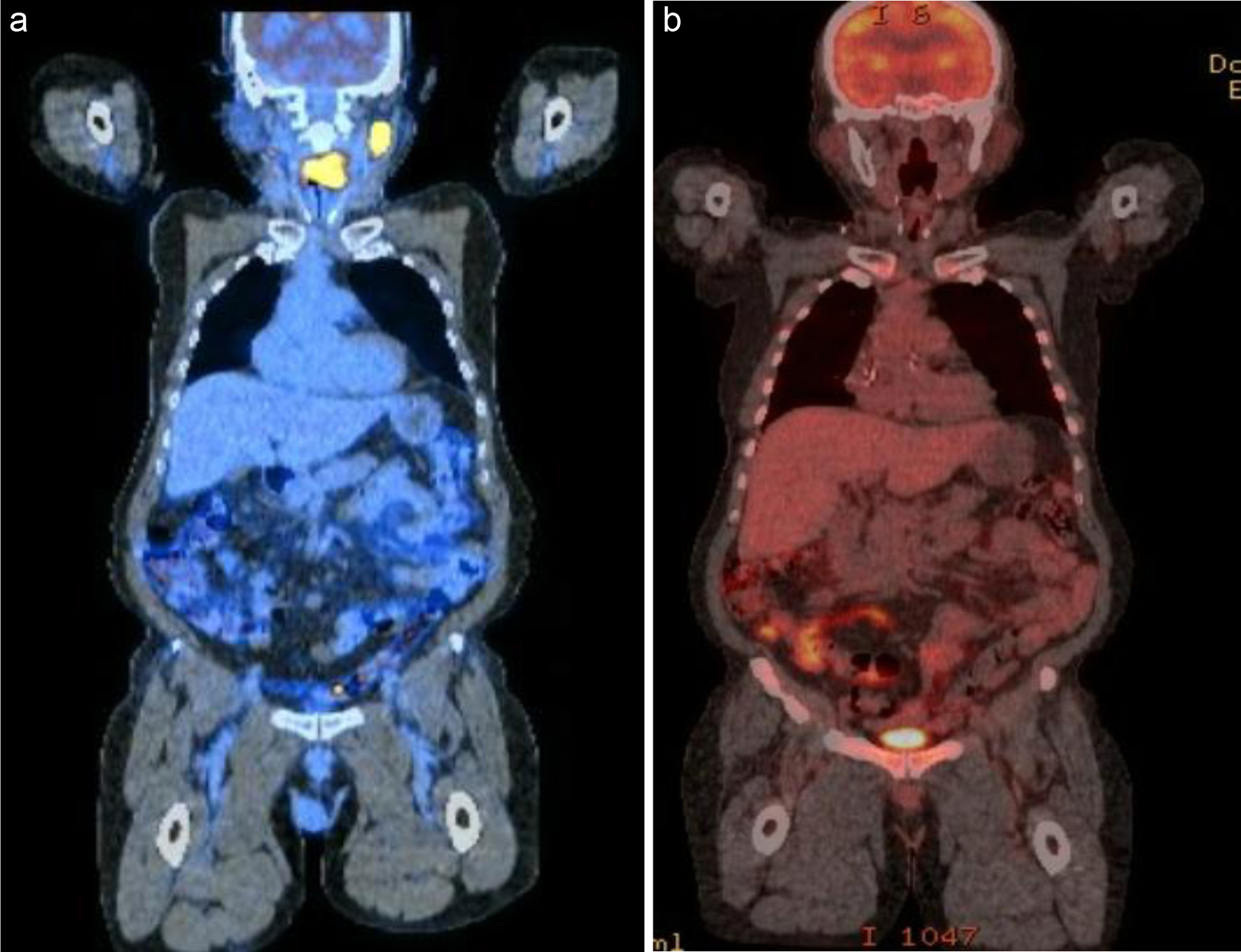 Click for large image | Figure 7. PET/CT scan showed a left tongue-base mass with intense FDG uptake, as well as lymphadenopathy in multiple levels of the left neck (a). He was treated with three cycles of R2-COP, and an interim PET scan showed a CMR (b). CMR: complete metabolic response; PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide. |
| Discussion | ▴Top |
Challenges in treating elderly DLBCL patients and the role of appropriate patient selection
DLBCL is the most common aggressive NHL seen in the elderly population [1]. There is paucity of data from randomized studies in elderly patients, as they are underrepresented in clinical trials, and treatment regimens for the elderly are often extrapolated from data pertaining to younger patients. Elderly DLBCL patients pose a unique therapeutic challenge due to their poor hematopoietic reserve, impaired mobility, comorbidities, malnutrition, and organ dysfunction; additionally, they are at a higher risk of drug toxicity. Anthracycline-based chemoimmunotherapy (R-CHOP) has the best curative potential and is the standard of care in young, fit patients [2]. Large database studies have shown that even older fit patients (> 80 years old) survive longer when treated with standard R-CHOP [8-10]; however, appropriate patient selection is critical. Frail individuals treated with anthracyclines are at a higher risk of treatment-related toxicity and have decreased survival [11, 12].
A comprehensive geriatric assessment (CGA) proposed by an Italian lymphoma group is an effective strategy to stratify patients by risk and select elderly DLBCL patients, who can be cured with an anthracycline-containing chemoimmunotherapy regimen [13, 14]. A simplified geriatric assessment scale (SGAS) is another helpful tool that has been validated to select patients for curative-intent therapy. SGAS is based on age, the Cumulative Illness Rating Scale for Geriatrics, activities of daily living (ADL), and instrumental ADL. The Elderly Prognostic Index (EPI) score incorporated the International Prognostic Index, SGAS, and hemoglobin level. EPI score risk categorizes patients into three groups: low, intermediate, and high risk, and it has been shown to predict survival [15]. Use of these standardized assessment scales can help clinicians avoid undertreating fit patients and overtreating frail patients.
Chemoimmunotherapy in elderly and role of lenalidomide
R-CHOP chemoimmunotherapy is the main pillar of DLBCL therapy; however, there is significant morbidity and TRM associated with this regimen in elderly patients. Coiffier et al investigated the R-CHOP regimen in elderly patients (median age: 69 years), and grade 3 - 4 cardiac toxicity was seen in 8% and TRM in 9.6% [2]. An attenuated chemoimmunotherapy regimen (R-mini-CHOP: doxorubicin 25 mg/m2, cyclophosphamide 400 mg/m2, vincristine 1 mg, and prednisone 40 mg/m2) was investigated in a single-arm phase 2 study of 149 patients aged ≥ 80 years with newly diagnosed DLBCL. The 2-year overall survival (OS) and progression-free survival (PFS) were 59% and 47%, respectively. In that study, 12 (8%) patients died from treatment-related complications [5]. Comparing the two studies, it is clear that TRM is unchanged despite reducing the dose of anthracyclines by 50%. Hence, in frail, elderly patients, there is an unmet need for novel anthracycline-free regimens that are well tolerated but do not compromise curative potential.
Lenalidomide, an oral immunomodulatory drug used either as a single agent or added to standard chemoimmunotherapy, has shown encouraging activity in preclinical studies [16]. Lenalidomide has diverse mechanisms of action [17]. It alters the tumor microenvironment and enhances natural killer (NK) cell proliferation and expansion [18]. Lenalidomide has been evaluated in the relapsed/refractory, frontline, and maintenance setting in DLBCL. A phase II Italian study of elderly patients (n = 23) with relapsed/refractory DLBCL treated with lenalidomide and rituximab achieved an overall response rate (ORR) of 35% and CR rate of 30% [19]. The phase II L-MIND study has shown durable remissions with lenalidomide and CD19 antibody in relapsed/refractory DLBCL [20]. A single-center retrospective study evaluating the real-world data of lenalidomide in relapsed/refractory DLBCL showed durable clinical activity regardless of the cell of origin [21]. In addition, Thieblemont et al investigated the role of lenalidomide maintenance in elderly patients with DLBCL and observed that there was PFS benefit with 24 months of maintenance compared to placebo [22]. However, the role of lenalidomide in frontline therapy has been controversial, with conflicting results from multiple studies. Multiple single-arm phase II studies have shown that addition of lenalidomide to the standard R-CHOP is safe, effective, and produces a durable response. Nowakowski et al investigated the addition of lenalidomide to standard R-CHOP in 64 patients with newly diagnosed DLBCL and observed an impressive ORR of 98% and CR rate of 80%. The 24-month PFS and OS were comparable between GCB and non-GCB subtypes, and it was thought that lenalidomide could overcome the adverse prognostic impact of the non-GCB subtype [23]. Castellino et al reported long-term outcomes from a phase II study (n = 112) of patients treated with R2-CHOP and showed an impressive 5-year PFS of 63.5% and OS of 75.4%, also suggesting that lenalidomide could overcome the negative prognosis of the non-GCB phenotype [24]. Two important randomized trials further explored the role of lenalidomide in DLBCL but yielded conflicting results. The ECOG-ACRIN 1412 trial (E1412) was a phase 2 trial of 280 patients with newly diagnosed DLBCL (stage II bulky to stage IV, both GCB and activated B-cell (ABC) subtypes), who were randomized to receive six cycles of R2-CHOP (n = 145) or R-CHOP (n = 135). The ORR and CR rates were similar in the R-CHOP and R2-CHOP arms. The R2-CHOP arm showed a 34% reduction in the risk of disease progression or death compared with the R-CHOP arm [25]. Despite the exciting data from the E412 trial, a larger phase III trial (Robust) failed to show an improvement in event-free survival or OS with the addition of lenalidomide to R-CHOP. The Robust trial included 570 patients with newly diagnosed stage II to IV ABC-subtype DLBCL, who were randomized to receive six cycles of R2-CHOP (lenalidomide was dosed at 15 mg/day on days 1 - 14 of a 21-day cycle) or standard-of-care R-CHOP21. The ORR and CR rates were comparable between the two arms. The 2-year OS rates were also similar in the two groups (79% for R2-CHOP and 80% for placebo/R-CHOP) [26]. The conflicting outcomes of the E1412 and Robust trials could be due to differences in lenalidomide dosing. Patients in the E1412 study were treated with a higher dose of lenalidomide (25 mg daily) than patients in the Robust study (15 mg). Also, the cell of origin could have played a role as well. The Robust trial included only patients with the ABC subtype, while E1412 study included patients with either GCB or ABC subtype DLBCL.
The addition of lenalidomide to an attenuated chemoimmunotherapy (R-mini-CHOP) regimen has also yielded negative results. A phase III randomized trial (SENIOR trial, n = 249) compared standard R-mini-CHOP with R2-mini-CHOP in DLBCL patients ≥ 80 years old. Lenalidomide was given at a dose of 10 mg daily on days 1 - 14 every 21 days. The 2-year PFS and OS were similar between the two treatment groups [6]. Hence, multiple phase III studies examining the addition of lenalidomide to standard R-CHOP have failed to show improvement in clinical outcomes. It is possible that differences in lenalidomide dosing could have contributed to the differences in outcomes. Both phase III studies (Robust and Senior trial) used a lower dose (10 or 15 mg) than the 25 mg used in the positive phase II studies. In our series of seven patients, most of them received the 25 mg dose. Also, it is possible that the immunosuppressive tumor microenvironment created by anthracyclines could be interfering with the efficacy of lenalidomide; this needs to be investigated further.
Future directions
Older unfit/frail patients with high-risk features such as high IPI score, and hypoalbuminemia should prompt the physician to explore alternative chemotherapy-free or reduced intensity anthracycline free options. The substitution of anthracycline with etoposide (R-CEOP) is a reasonable frontline treatment option in anthracycline ineligible patients with conflicting results from studies. Moccia et al reported similar disease specific survival between R-CEOP versus R-CHOP with an acceptable TRM of 4% [27]. On the contrary, Puckrin et al reported a real-world multicenter study (n = 138) of DLBCL patients with cardiovascular comorbidities and outcomes were inferior with the R-CEOP regimen when compared to R-CHOP [28]. Similarly, a Dutch population-based study also showed inferior overall survival with R-CEOP compared to R-CHOP [29]. However, as with R-mini-CHOP, all these studies were done in fit patients.
Shah et al reported a small phase II study (n = 14) of split dosing of R-CHOP (full dose rituximab on day 1, 50% dose reduction of CHOP on days 1 and 15 of a 28-day cycle) for elderly unfit/frail DLBCL patients and the preliminary results appears promising [30].
We would like to highlight several exciting emerging targeted therapeutic options that are currently being explored in older/unfit DLBCL patients. Xu et al reported a small (n = 30) single institution phase 2 study of a novel chemotherapy-free RLI regimen (rituximab, lenalidomide 25 mg on days 1 - 10 and ibrutinib 560 mg on days 1 - 21) in older unfit/frail patients with DLBCL, and it showed encouraging results (CR -56% and 2-year OS of 66%) similar to the R-mini-CHOP regimen, but with no TRM [31]. In the Smart Start study, Westin et al showed durable responses in newly diagnosed non-GCB DLBCL achieving a high ORR of 86% after two cycles of RLI and CR of 94% after RLI-chemotherapy [32]. The Smart Stop is exploring a novel targeted therapy combination consisting of lenalidomide, tafasitamab, rituximab, and acalabrutinib for four cycles in non-GCB DLBCL followed by risk adapted chemoimmunotherapy for consolidation (NCT04978584).
The LOTIS-9 study (NCT05144009) is a novel phase 2 study exploring the role of loncastuximab tesirine, a CD19-directed antibody drug conjugate in combination with rituximab in older frail DLBCL patients (age > 80 years). Mosunetuzumab, a CD20XCD3 engaging bispecific antibody has shown promising results with durable CR in elderly unfit treatment-naive DLBCL patients [33]. Tafasitamab, a CD19-directed antibody in combination with lenalidomide and rituximab is being studied as a frontline treatment option in patients 80 years and older (NCT04974216). Preliminary safety data from the phase 3 POLAR BEAR trial (R-pola-mini-CHP versus R-mini-CHOP) in elderly or frail DLBCL patients has shown that the substitution of vincristine with polatuzumab vedotin in the R-mini-CHOP regimen has a manageable toxicity profile, and efficacy data are awaited [34].
Conclusions
The treatment of elderly patients with DLBCL continues to be a therapeutic challenge. Appropriate patient selection is important to achieve better clinical outcomes. Treating physicians should carefully weigh the risk of lymphoma-related death versus TRM when selecting a treatment regimen. EPI and SGAS are very helpful tools for patient selection that are linked to survival. Any chemoimmunotherapy regimen should be tailored to each individual patient based on the disease risk and geriatric assessment. The addition of lenalidomide to an anthracycline-free regimen appears to be safe, effective, and can achieve durable remissions in elderly unfit patients, who are ineligible for the standard of care mini-R-CHOP therapy. The treatment of elderly unfit/frail DLBCL patients is a rapidly evolving field with CD20XCD3 engaging bispecific antibody, CD19 antibodies and antibody drug conjugates holding great promise for a chemotherapy-free approach. However, the availability of these therapies to large segments of the world population remains a formidable challenge, to which an approach as the one described here may offer a viable alternative that needs to be explored further in larger prospective studies.
Acknowledgments
The authors would like to thank Ms. Virginia Mohlere for editorial assistance.
Financial Disclosure
None to declare.
Conflict of Interest
Authors have no conflict of interest to disclose.
Informed Consent
Informed consents for publication from the patients were obtained.
Author Contributions
BY contributed to data collection and wrote the manuscript. AR is the treating physician and critically reviewed the manuscript for important intellectual content.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780-2795.
doi pubmed - Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242.
doi pubmed - Davis CC, Cohen JB, Shah KS, Hutcherson DA, Surati MJ, Valla K, Panjic EH, et al. Efficacy and tolerability of anthracycline-based therapy in elderly patients with diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15(5):270-277.
doi pubmed pmc - Carson KR, Riedell P, Lynch R, Nabhan C, Wildes TM, Liu W, Ganti A, et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J Geriatr Oncol. 2015;6(3):211-218.
doi pubmed pmc - Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, Coiffier B, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468.
doi pubmed - Oberic L, Peyrade F, Puyade M, Bonnet C, Dartigues-Cuilleres P, Fabiani B, Ruminy P, et al. Subcutaneous rituximab-MiniCHOP compared with subcutaneous rituximab-MiniCHOP plus lenalidomide in diffuse large B-cell lymphoma for patients age 80 years or older. J Clin Oncol. 2021;39(11):1203-1213.
doi pubmed - Hohloch K, Ziepert M, Truemper L, Buske C, Held G, Poeschel V, Chapuy B, et al. Low serum albumin is an independent risk factor in elderly patients with aggressive B-cell lymphoma: Results from prospective trials of the German High-Grade Non-Hodgkin's Lymphoma Study Group. EJHaem. 2020;1(1):181-187.
doi pubmed pmc - Chihara D, Westin JR, Oki Y, Ahmed MA, Do B, Fayad LE, Hagemeister FB, et al. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122(20):3145-3151.
doi pubmed - Huang HH, Ko BS, Chen HM, Chen LJ, Wang CY, Hsiao FY. Frontline treatments in extremely elderly patients with diffuse large B-cell lymphoma: a population-based study in Taiwan, 2010-2015. Immun Ageing. 2020;17:17.
doi pubmed pmc - Williams JN, Rai A, Lipscomb J, Koff JL, Nastoupil LJ, Flowers CR. Disease characteristics, patterns of care, and survival in very elderly patients with diffuse large B-cell lymphoma. Cancer. 2015;121(11):1800-1808.
doi pubmed pmc - Merli F, Luminari S, Rossi G, Mammi C, Marcheselli L, Ferrari A, Spina M, et al. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma. 2014;55(1):38-43.
doi pubmed - Wieringa A, Boslooper K, Hoogendoorn M, Joosten P, Beerden T, Storm H, Kibbelaar RE, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br J Haematol. 2014;165(4):489-496.
doi pubmed - Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F, Stelitano C, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma. 2015;56(4):921-926.
doi pubmed - Lin RJ, Behera M, Diefenbach CS, Flowers CR. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood. 2017;130(20):2180-2185.
doi pubmed pmc - Merli F, Luminari S, Tucci A, Arcari A, Rigacci L, Hawkes E, Chiattone CS, et al. Simplified geriatric assessment in older patients with diffuse large B-cell lymphoma: the prospective elderly project of the fondazione Italiana Linfomi. J Clin Oncol. 2021;39(11):1214-1222.
doi pubmed - Witzig TE, Nowakowski GS, Habermann TM, Goy A, Hernandez-Ilizaliturri FJ, Chiappella A, Vitolo U, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol. 2015;26(8):1667-1677.
doi pubmed - Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015;33(25):2803-2811.
doi pubmed pmc - Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, Wallace P, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140(1):36-45.
doi pubmed - Zinzani PL, Pellegrini C, Derenzini E, Argnani L, Pileri S. Long-term efficacy of the combination of lenalidomide and rituximab in elderly relapsed/refractory diffuse large B-cell lymphoma patients. Hematol Oncol. 2013;31(4):223-224.
doi pubmed - Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, Nagy Z, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988.
doi pubmed - Rodgers TD, Baran A, Reagan PM, Casulo C, Zent C, Evans A, Burack R, et al. Efficacy of lenalidomide in high-risk diffuse large B-cell lymphoma. Br J Haematol. 2020;188(4):e33-e36.
doi pubmed - Thieblemont C, Tilly H, Gomes da Silva M, Casasnovas RO, Fruchart C, Morschhauser F, Haioun C, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473-2481.
doi pubmed - Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, Thompson CA, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251-257.
doi pubmed - Castellino A, Chiappella A, LaPlant BR, Pederson LD, Gaidano G, Macon WR, Inghirami G, et al. Lenalidomide plus R-CHOP21 in newly diagnosed diffuse large B-cell lymphoma (DLBCL): long-term follow-up results from a combined analysis from two phase 2 trials. Blood Cancer J. 2018;8(11):108.
doi pubmed pmc - Nowakowski GS, Hong F, Scott DW, Macon WR, King RL, Habermann TM, Wagner-Johnston N, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. J Clin Oncol. 2021;39(12):1329-1338.
doi pubmed pmc - Nowakowski GS, Chiappella A, Gascoyne RD, Scott DW, Zhang Q, Jurczak W, Ozcan M, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021;39(12):1317-1328.
doi pubmed pmc - Moccia AA, Schaff K, Freeman C, Hoskins PJ, Klasa RJ, Savage KJ, Shenkier TN, et al. Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines. Blood Adv. 2021;5(5):1483-1489.
doi pubmed pmc - Puckrin R, Ghosh S, Peters A, Stewart D. Inferior outcomes with R-CEOP for patients with diffuse large B-cell lymphoma and cardiovascular comorbidities. Leuk Lymphoma. 2022;63(3):583-590.
doi pubmed - Al-Sarayfi D, Meeuwes FO, Durmaz M, Issa DE, Brouwer RE, Beeker A, van Rhenen A, et al. R-CEOP as first-line treatment for anthracycline-ineligible patients with diffuse large B-cell lymphoma. Blood Cancer J. 2022;12(9):125.
doi pubmed pmc - Szabo A, Rajguru S, Devata S, Howard M, Pereira D, Endres M, Longo WL, et al. Phase II trial of split-dose R-CHOP for older patients with diffuse large B-cell lymphoma (DLBCL). Journal of Clinical Oncology. 2023;41(16_suppl):7554-7554.
doi - Xu PP, Shi ZY, Qian Y, Cheng S, Zhu Y, Jiang L, Li JF, et al. Ibrutinib, rituximab, and lenalidomide in unfit or frail patients aged 75 years or older with de novo diffuse large B-cell lymphoma: a phase 2, single-arm study. Lancet Healthy Longev. 2022;3(7):e481-e490.
doi pubmed - Westin J, Davis RE, Feng L, Hagemeister F, Steiner R, Lee HJ, Fayad L, et al. Smart start: rituximab, lenalidomide, and ibrutinib in patients with newly diagnosed large B-cell lymphoma. J Clin Oncol. 2023;41(4):745-755.
doi pubmed - Olszewski AJ, et al. Mosunetuzumab monotherapy continues to demonstrate promising efficacy and durable complete responses in elderly/unfit patients with previously untreated diffuse large B-cell lymphoma. Blood. 2022;140(Supplement 1):1778-1780.
doi - Jerkeman M, Leppa S, Hamfjord J, Brown P, Ekberg S, Ferreri AJM. Initial safety data from the phase 3 POLAR BEAR trial in elderly or frail patients with diffuse large cell lymphoma, comparing R-pola-mini-CHP and R-mini-CHOP. Presented at: European Hematology Association 2023 Hybrid Congress; June 8-11, 2023; Frankfurt, Germany. Abstract S227.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


