| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 4, August 2023, pages 161-169
Could the Crosstalk Between Myeloid-Derived-Suppressor Cells and Regulatory T Cells Have a Role in Beta-Thalassemia?
Asmaa M. Zahrana, Omnia El-Badawyb, Eman R. Badawyc, Khalid I. Elsayhd, Eman F. Gadd, Khaled Saadd, e, j , Khalid Hashim Mahmoudf, Amira Elhoufeyg, h, Hamad Ghaleb Dailahi, Marwa Ghazalyd
aClinical Pathology Department, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
bMedical Microbiology & Immunology Department, Faculty of Medicine, Assiut University, Assiut, Egypt
cClinical Pathology Department, Faculty of Medicine, Assiut University, Assiut, Egypt
dPediatric Department, Faculty of Medicine, Assiut University, Assiut, Egypt
ePediatrics Department, Assiut University Children’s Hospital, Assiut, Egypt
fDepartment of Pediatrics, Faculty of Medicine, Shaqra University, Dawadmi, Saudi Arabia
gDepartment of Community Health Nursing, Faculty of Nursing, Assiut University, Assiut, Egypt
hDepartment of Community Health Nursing, Alddrab University College, Jazan University, Jazan, Saudi Arabia
iResearch and Scientific Studies Unit, College of Nursing, Jazan University, Jazan 45142, Saudi Arabia
jCorresponding Author: Khaled Saad, Pediatrics Department, Assiut University Children’s Hospital, Assiut, Egypt
Manuscript submitted May 14, 2023, accepted July 14, 2023, published online August 8, 2023
Short title: MDSCs and Tregs in Children With Beta-Thalassemia
doi: https://doi.org/10.14740/jh1149
| Abstract | ▴Top |
Background: Secondary iron overload, alloimmunization, and increased risk of infection are common complications in patients with transfusion-dependent thalassemia (TDT). Regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) play an essential role in preventing excessive immune response. This research aimed to study the interaction between Tregs and MDSCs in TDT patients and to evaluate the association of these cell types with disease severity.
Methods: This case-control study included 26 patients with TDT and 23 healthy, age- and sex-matched controls. All patients were investigated for complete blood count (CBC), serum ferritin, and flow cytometric analysis of peripheral blood to detect Tregs, MDSCs, and MDSC subsets.
Results: A significant increase was observed in the frequencies of Tregs and MDSCs, particularly monocytic MDSCs (MO-MDSCs), in TDT patients compared with controls. The frequencies of these cells showed a direct association with ferritin level and total leukocyte count and an inverse association with hemoglobin level. Furthermore, a positive correlation was observed between Tregs and each of the total MDSCs and MO-MDSCs.
Conclusions: Levels of Tregs and MDSCs increased in TDT and may probably have a role in suppressing the active immune systems of TDT patients.
Keywords: Myeloid-derived-suppressor cells; Regulatory T cells; β-thalassemia; Children
| Introduction | ▴Top |
Thalassemia is a genetic disorder in which there is a defect in hemoglobin production. Thalassemia major (TM) patients suffer from severe anemia and require regular blood transfusion [1, 2]. TM is a common health problem in Mediterranean countries, especially in Egypt. The most common complications of TM are iron overload, heart failure, recurrent infections, and alloimmunization from repeated blood transfusion [3].
Regulatory T cells (Tregs) are CD4+CD25+high cells that express the transcription factor forkhead box protein 3 (Foxp3) and form up to 5-10% of CD4+ T cells [4]. The primary function of Tregs is to induce and maintain peripheral tolerance, which plays a vital role in preventing excessive immune response and autoimmunity [5]. The increase in antigenic stimulation that occurs due to repeated blood transfusions may alter the levels of Tregs and Foxp3 in β-thalassemia [6]. Previous studies have shown that Tregs play a crucial role in modulating the magnitude and frequency of alloimmunization by suppressing the immune system [7].
Myeloid-derived suppressor cells (MDSCs) are innate immune cells that represent a heterogeneous group of immature myeloid cells at a specific differentiation stage that exhibit immunosuppressive effects. Normally, MDSCs are present in low numbers and increase in certain disease conditions, psychological stress, or natural aging [8, 9]. They include monocytic-myeloid-derived suppressor cells (MO-MDSCs), which are human leukocyte antigen - DR isotype (HLA-DR)-, CD11b+, CD33+, and CD14+, and polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs), which are HLA-DR-, CD11b+, CD33+, and CD15+ [7]. The main function of MDSCs is to suppress immune cells, mainly T cells, and to a lesser extent B cells and natural killer (NK) cells [10].
Previous studies have shown that chronic inflammatory conditions (such as those observed in thalassemia), persistent tissue damage (as in cancer), autoimmunity, and chronic infection increase the release of damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), and various cytokines that in turn increase the release of myeloid cells from the bone marrow (BM) and induce the immunosuppressive effects of MDSCs [11]. The possible role of MDSCs in immune dysregulation in hematologic malignancies, BM failure syndromes, and autoimmune disorders, and their potential as therapeutic targets, has encouraged their study in other hematological diseases [12].
Interaction between Tregs and MDSCs has been studied in various conditions such as tumors, allergic disorders, diabetes, and autoimmune diseases. Previous studies have shown that MDSCs could control de novo development and induction of Tregs [13]. The overlap of Treg and MDSC target cells indicates the significance and resilience of immune suppression under pathological conditions [14, 15]. This research aimed to study the interaction between Tregs and MDSCs in transfusion-dependent thalassemia (TDT) patients and to evaluate the association of these cell types with disease severity.
| Materials and Methods | ▴Top |
This case-control study was conducted on TDT patients at the Pediatric Hematology Unit at Assiut University Children’s Hospital, Assiut, Egypt. The study was conducted as per the Declaration of Helsinki, and the Faculty of Medicine Ethics Committee reviewed and approved the study (ethical approval No. 17300557). Written informed consent was obtained from the guardians of the participants.
The study included 26 patients with TDT and 23 healthy, age- and sex-matched children as controls. Patients received regular blood transfusions and chelation therapy. Patients with known cardiac, renal, infectious, inflammatory, or pulmonary diseases, diabetes, or newly diagnosed and non-TDT were excluded from the study. Any patient with a recent history of infection or immunosuppressive therapy (e.g., steroids) during 1 month prior to enrollment was also excluded.
All patients underwent history and physical examination, in addition to complete blood count (CBC), serum ferritin, and flow cytometric analysis of peripheral blood to detect Tregs (CD4+CD25+highFoxp3+), MO-MDSCs (HLA-DR-CD11b+CD33+CD14+), and PMN-MDSCs (HLA-DR-CD11b+CD33+CD15+).
Flow cytometric detection of Tregs
Tregs were enumerated using fluorescein isothiocyanate (FITC)-conjugated Foxp3 (IQ Product, the Netherlands), phycoerythrin (PE)-conjugated CD25 (Bioscience, CA), and peridinium-chlorophyll-protein (Per-CP)-conjugated CD4 (Becton Dickinson (BD) Bioscience, San Jose, CA). CD4 and CD25 (5 µL) were incubated with 50 µL of the blood sample for 15 min at 4 °C in the dark. Following incubation, red blood cells were lysed and washed with phosphate-buffered saline (PBS). The fixing solution was added, and the sample was incubated for 10 min. Cells were then washed with PBS and the permeabilizing solution, and 5 µL of Foxp3 were added; the sample was then incubated for 20 min at 4 °C. After one wash, the cells were resuspended in PBS and analyzed by FACSCalibur flow cytometry with CellQuest software (BD Biosciences, San Jose, CA). An isotype-matched negative control was used for each sample. Lymphocytes were detected according to their forward and side scatters. Then, CD4+ cells were gated. Total CD4+CD25+, CD4+CD25+low, CD4+CD25+high T cells, and CD4+CD25+highFoxp3+ Tregs were evaluated as percentages of the total CD4+ cells, as shown in Figure 1.
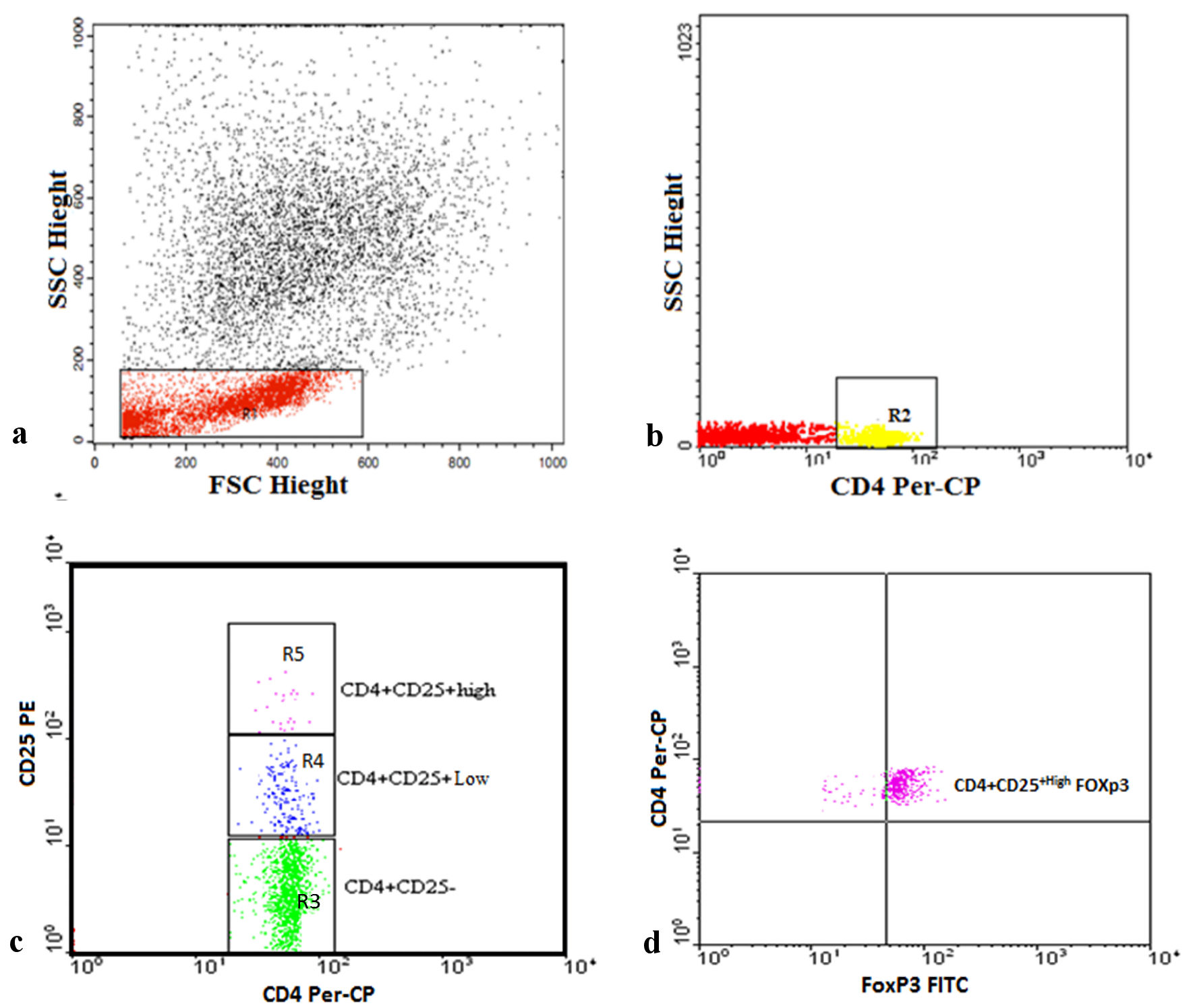 Click for large image | Figure 1. Representative plots showing flow cytometric detection of regulatory T cells in transfusion-dependent thalassemia (TDT) patients. (a) The lymphocyte population was defined on a forward scatter (FSC) and side scatter (SSC) histogram (R1). (b) The expression of CD4 on the lymphocytes population was detected, then CD4+ cells were gated (R2) for further analysis of CD25. (c) Three gates were drawn to define CD4+CD25- cells (R3), CD4+CD25+low cells (R4), and CD4+CD25+high cells (R5). (d) The percentage of CD4+CD25+highFoxp3+ cells (regulatory T cells) was then assessed. Foxp3: forkhead box protein 3; FITC: fluorescein isothiocyanate; PE: phycoerythrin; Per-CP: peridinium-chlorophyll-protein. |
Flow cytometric detection of MDSCs
MDSCs were detected by using FITC-conjugated CD11b, PE-conjugated CD33, Per-CP-conjugated CD15, Per-CP-conjugated CD14, and allophycocyanin (APC)-conjugated HLA-DR (BD Biosciences). A blood sample (50 µL) was incubated with 5 µL each of CD33, CD11b, HLA-DR, and CD14 in one tube, and CD33, CD11b, HLA-DR, and CD15 in another tube for 20 minutes at 4 °C in the dark. Following incubation, red blood cell lysis and washing with PBS were performed. Then cells were suspended in PBS and analyzed using Cell Quest software on a FACSCalibur flow cytometer, as presented in Figure 2. An isotype-matched negative control was used for each sample. HLA-DR-negative cells were selected from the HLA-DR and side scatter histogram. The HLA-DR-negative cells were assessed for their expression of CD33 and CD11b to detect MDSCs (HLA-DR-CD33+CD11b+). Then, the MDSCs were assessed for their expression of CD15 and CD14 to detect PMN-MDSCs (HLA-DR-CD33+CD11b+CD15+) and MO-MDSCs (HLA-DR-CD33+CD11b+CD14+).
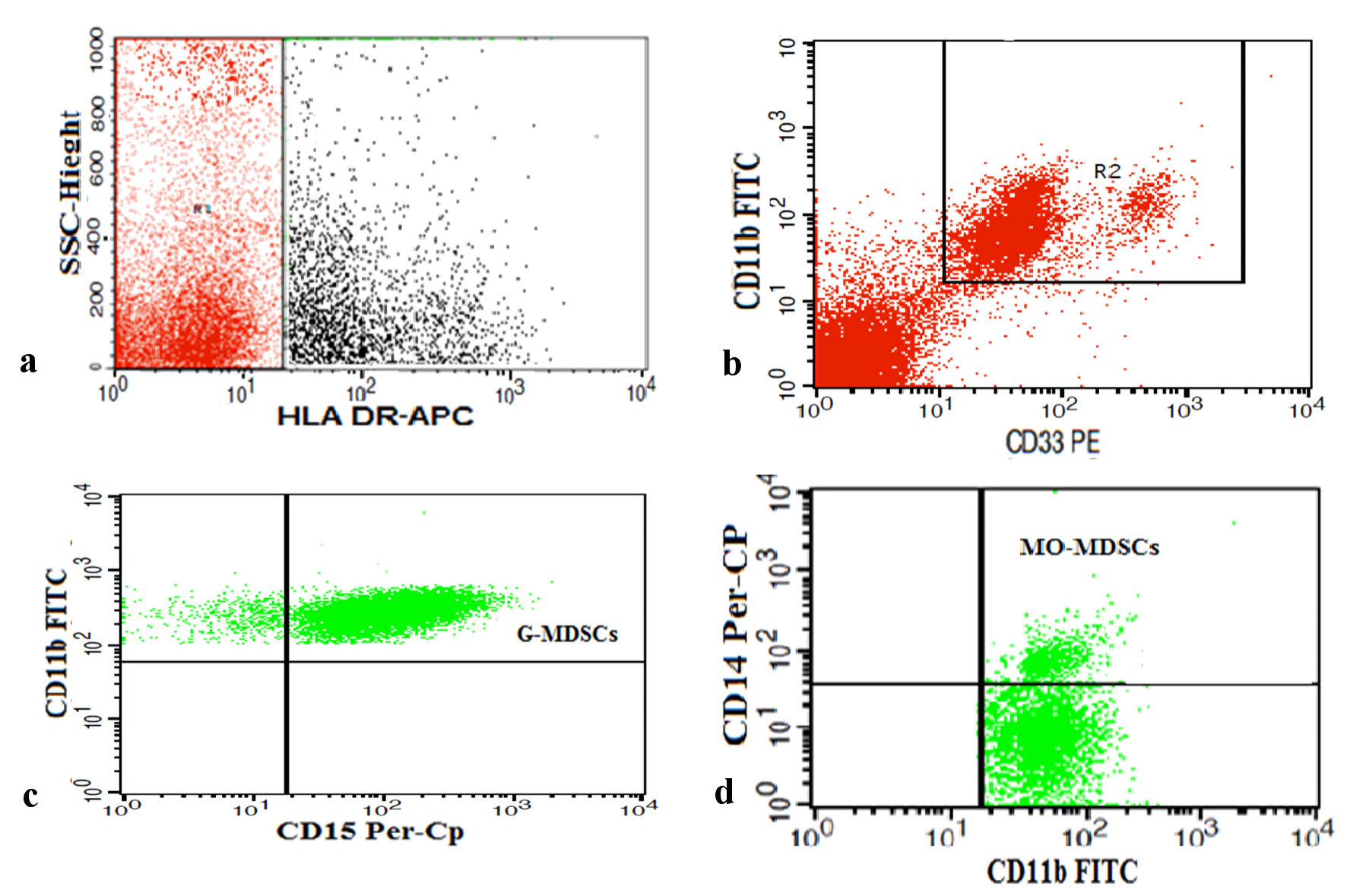 Click for large image | Figure 2. Representative plots showing flow cytometric detection of myeloid-derived suppressor cells (MDSCs) in transfusion-dependent thalassemia (TDT) patients. (a) HLA-DR negative cells (R1) were selected from HLA-DR and side scatter histogram. (b) HLA-DR negative cells were assessed for their expression of CD33 and CD11b to detect total MDSCs (R2) (HLA-DR-CD33+CD11b+). (c, d) Total MDSCs were assessed for their expression of CD15 and CD14 to detect polymorphonuclear MDSCs (PMN-MDSCs) (HLA-DR-CD33+CD11b+CD15+) and monocytic MDSCs (MO-MDSCs) (HLA-DR-CD33+CD11b+CD14+). HLA-DR: human leukocyte antigen - DR isotype; Foxp3: forkhead box protein 3; FITC: fluorescein isothiocyanate; PE: phycoerythrin; Per-CP: peridinium-chlorophyll-protein; SSC: side scatter. |
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS 22.0, IBM). Data were expressed as mean ± standard deviation (SD) or standard error (SE). The differences between groups were evaluated for statistical significance using the independent t-test. The correlation coefficient was generated by Pearson’s correlation. Statistical significance was defined as P < 0.05.
| Results | ▴Top |
This study was conducted on 26 TDT patients with a mean age of 8.7 ± 5 years, and 54% were male. In the healthy control group, the mean age was 8.2 ± 6.3 years, and 52% of the participants were male. Demographic data and laboratory investigations of all patients are presented in Table 1. Results showed a significant increase in total leukocyte count (TLC) and ferritin than the healthy controls. However, there was a decrease in hemoglobin and platelet count.
 Click to view | Table 1. Demographic Data and the Laboratory Investigations of All Patients |
Levels of Tregs and MDSCs in patients and healthy controls
A comparison of the levels of Tregs and total MDSCs between TDT patients and controls is shown in Table 1. Patients with TDT had higher percentages of both CD4+CD25high T cells and CD4+CD25highFoxP3+ Tregs compared to controls. Moreover, total MDSCs, particularly the monocytic type (MO-MDSCs), were significantly higher in TDT.
The correlations between frequencies of Treg cells, MDSCs, and laboratory parameters
As illustrated in Figure 3, CD4+CD25highFoxP3+ Tregs were directly related to serum ferritin level (r = 0.5, P < 0.0001) and TLC (r = 0.6, P < 0.0001), and inversely related to hemoglobin level (r = -0.6, P < 0.0001). Similarly, total MDSCs showed a positive correlation with serum ferritin level (r = 0.7, P < 0.0001) and TLC (r = 0.5, P < 0.0001), and a negative correlation with platelet count (r = -0.4, P = 0.001) and hemoglobin level (r = -0.6, P < 0.0001) (Fig. 4). MO-MDSCs were positively associated with serum ferritin level (r = 0.7, P < 0.0001) and TLC (r = 0.4, P = 0.001), and inversely associated with hemoglobin level (r = -0.4, P = 0.005) (Fig. 5). Additionally, Tregs were positively correlated with total MDSCs (r = 0.3, P = 0.01) and MO-MDSCs (r = 0.3, P = 0.02) (Fig. 6).
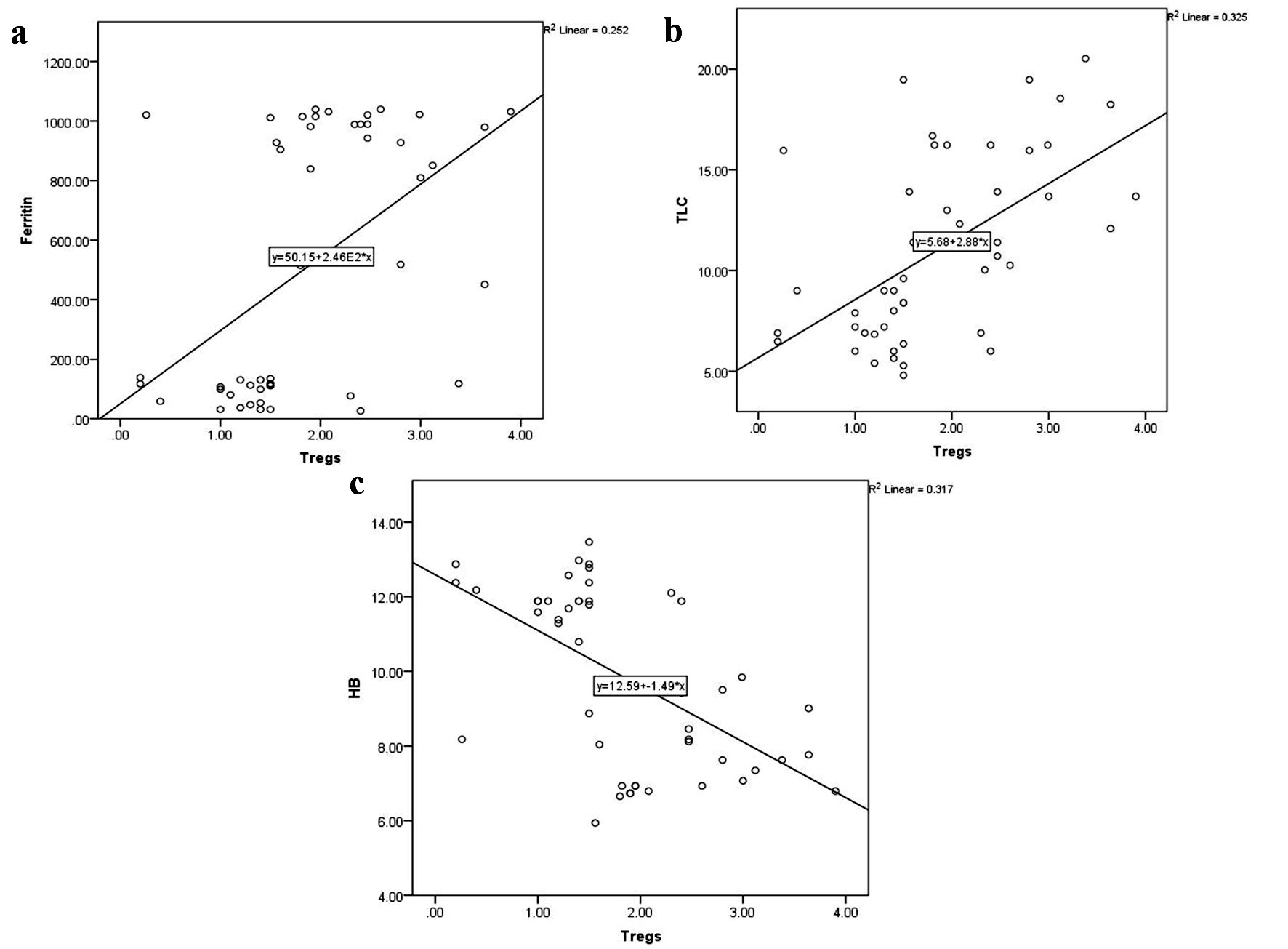 Click for large image | Figure 3. Correlations of CD4+CD25highFoxP3+ Tregs with serum ferritin level (a), total leukocyte count (TLC) (b), and hemoglobin (HB) level (c). Foxp3: forkhead box protein 3; Tregs: regulatory T cells. |
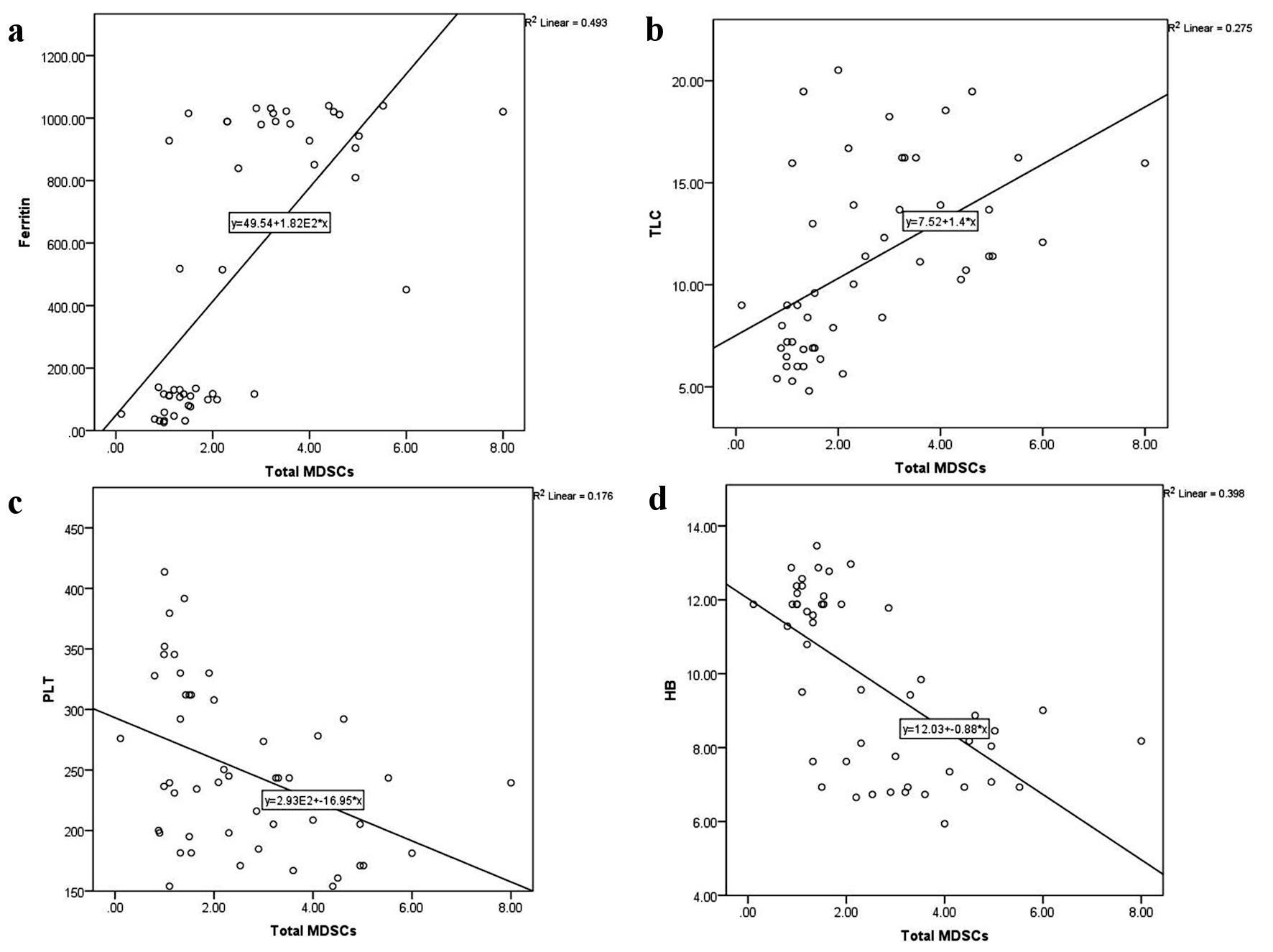 Click for large image | Figure 4. Correlations of total myeloid-derived suppressor cells (MDSCs) with serum ferritin level (a), total leukocyte count (TLC) (b), platelet (PLT) count (c), and hemoglobin (HB) level (d). |
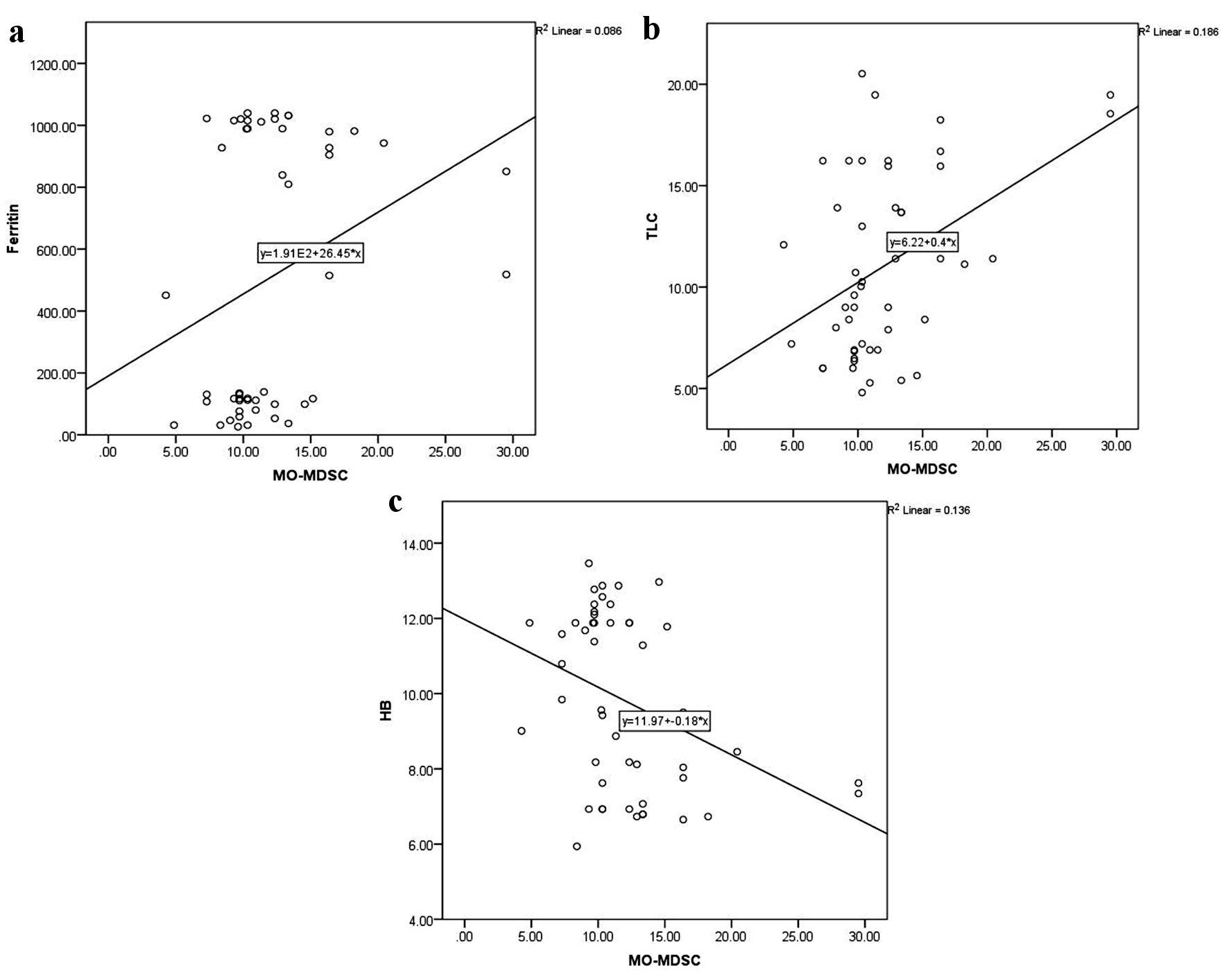 Click for large image | Figure 5. Correlations of monocytic-myeloid-derived suppressor cells (MO-MDSCs) with serum ferritin level (a), total leukocyte count (TLC) (b), and hemoglobin (HB) level (c). |
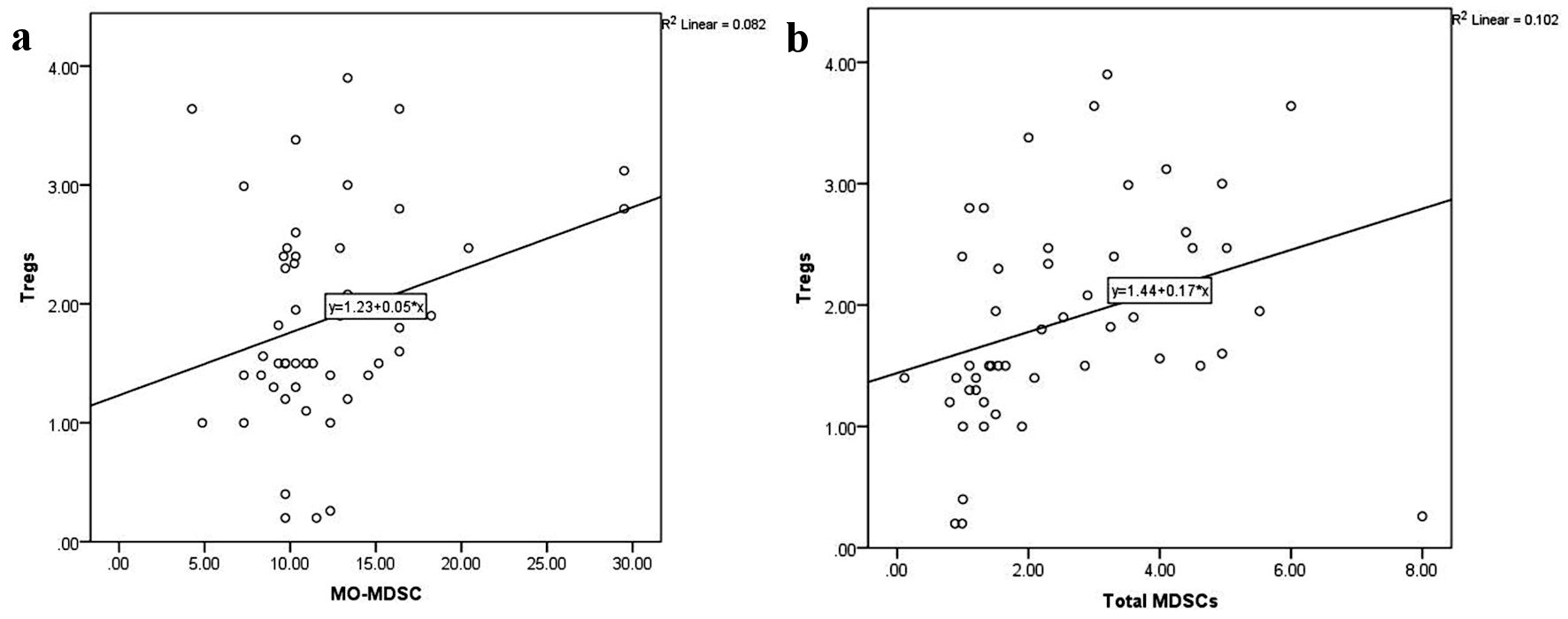 Click for large image | Figure 6. Correlations of CD4+CD25highFoxP3+ Tregs with monocytic-myeloid-derived suppressor cells (MO-MDSCs) (a) and total myeloid-derived suppressor cells (MDSCs) (b). Foxp3: forkhead box protein 3; Tregs: regulatory T cells. |
| Discussion | ▴Top |
β-thalassemia is one of the most common hereditary blood disorders. Regular transfusions, which remain the gold standard of therapy for β-thalassemia, are effective in managing symptoms. Although secondary iron overload is the main consequence of transfusion in TDT patients [16], alloimmunization and increased risk of infection represent other common complications. The pathogenesis of the disease is not fully understood [2]. Alloimmunization was found to depend on many factors, such as red blood cell (RBC) antigen discrepancy between donor and recipient, the immune status of the recipient, and the immunomodulatory effects of allogeneic blood transfusion [17]. The immune system is, therefore, a key determiner in the clinical features accompanying thalassemia.
In this study, we measured the frequency of Tregs and MDSCs in TDT patients and evaluated their association with disease severity. Our results showed increased frequencies of Tregs and total MDSCS, particularly the MO-MDSCs, in TDT patients as compared with controls. Tregs are a component of the immune system that suppresses the immune response of other cells. Earlier studies reported higher Treg levels in TM patients compared with normal subjects [6, 17, 18]. Bozdogan et al [6] suggested that high levels of Tregs in TDT patients might be due to chronic antigenic stimulation from frequent blood transfusions that trigger Tregs to prevent alloimmunization. In line with our results, a significant positive correlation was observed between the Treg levels and ferritin concentration in thalassemia patients. Consequently, as ferritin levels increase, they can suppress the immune system by inducing Tregs in these patients [17].
MDSCs are functionally comparable to Tregs. Several studies have described a significant elevation in total MDSCs and MO-MDSCs in chronic inflammatory diseases and tumors [19, 20], representing an induced, core, anti-inflammatory mechanism to inhibit excessive immune cell activity. MDSCs negatively regulate immune function by suppressing the activity of T cells, NK cells, and B cells [21]; therefore, elevated MDSC levels in TDT patients could be implicated in these patients’ increased susceptibility to infection.
Little is known about MDSCs in TDT. Siriworadetkun et al [21] reported an increased level of MO-MDSCs in TDT patients, especially those who had undergone splenectomy, compared with healthy controls. Chronic inflammation may trigger the generation of MDSCs in the BM, causing MDSC expansion and accumulation in the circulation of TDT patients. In agreement with other studies, our TDT patients had high levels of inflammatory cells, indicated by a high TLC [22, 23]. Additionally, the direct relationship observed between TLC and both total MDSCs and MO-MDSCs may support the hypothesis that chronic inflammation triggers the BM to generate these cells.
The relationship between Tregs and MDSCs has been widely studied. Previous research suggests that MDSCs release interleukin (IL)-10 and transforming growth factor β (TGF-β), which are critical for the induction of Tregs [24-26]. Furthermore, Tregs can enhance MDSC function and control their differentiation through a mechanism involving TGF-β [27]. An earlier study of the relationship between MDSCs and Tregs in transient hypogammaglobulinemia suggested that its pathogenesis is based on the interplay between the MDSCs and Tregs [28]. However, until recently, the association of MDSCs and Tregs had not been examined in TDT.
Our findings demonstrated that levels of both Tregs and MDSCs (particularly the MO-MDSCs) were significantly higher in the TDT group than in healthy controls. Increased levels of these cells also showed a direct association with ferritin level and TLC, and an inverse association with hemoglobin level. Furthermore, a positive correlation was seen between total Treg levels and both total MDSC and MO-MDSC levels.
Limitations of the study
The study has some limitations. Comparing the levels of Tregs and MDSCs in patients with newly diagnosed and non-transfusion dependent TDT patients with those receiving blood transfusion would have confirmed that the correlations observed between these cells and other clinical and laboratory parameters reflect the relations of these cells with disease severity, but not with the severity of repeated blood transfusion complications.
Conclusions
Levels of Tregs and MDSCs increased in TDT and may probably have a role in suppressing the active immune systems of TDT patients.
Acknowledgments
None to declare.
Financial Disclosure
The authors received partial funding for this work from the South Egypt Cancer Institute, Assiut University, Assiut, Egypt.
Conflict of Interest
None to declare.
Informed Consent
All caregivers of all participants have given their informed written consent.
Author Contributions
KIE, KS, ERB, EFG, MG, and KHM designed the study, followed the patients, analyzed the data, and drafted the manuscript. AMZ and OE performed all laboratory investigations of the study. AE, HGD, and KS drafted the manuscript. All authors were involved in the critical analysis of the last version of the manuscript. All authors approved the manuscript as submitted and agreed to be accountable for all aspects of the work.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
BM: bone marrow; TDT: transfusion-dependent thalassemia; DAMPs: damage-associated molecular patterns; FITC: fluorescein isothiocyanate; Foxp3: forkhead box protein 3; MDSCs: myeloid-derived suppressor cells; MO-MDSCs: monocytic-myeloid-derived suppressor cells; NK: natural killer; PAMPs: pathogen-associated molecular patterns; PBS: phosphate-buffered saline; PE: phycoerythrin; Per-CP: peridinium-chlorophyll-protein; PMN-MDSCs: polymorphonuclear-myeloid-derived suppressor cells; TLC: total leukocyte count; Tregs: regulatory T cells
| References | ▴Top |
- Zahran AM, et al. Could the crosstalk between Mdscs and Tregs have a role in β-thalassemia? Research Square. 2021.
doi - Zahran AM, Nafady A, Saad K, Hetta HF, Abdallah AM, Abdel-Aziz SM, Embaby MM, et al. Effect of hydroxyurea treatment on the inflammatory markers among children with sickle cell disease. Clin Appl Thromb Hemost. 2020;26:1076029619895111.
doi pubmed pmc - Zahran AM, Saad K, Elsayh KI, Alblihed MA. Characterization of circulating CD4(+) CD8(+) double positive and CD4(-) CD8(-) double negative T-lymphocyte in children with beta-thalassemia major. Int J Hematol. 2017;105(3):265-271.
doi pubmed - Zahran AM, Saad K, Abdel-Raheem YF, Elsayh KI, El-Houfey AA, Aboul-Khair MD, Alblihed MA. Characterization of regulatory T cells in preterm and term infants. Arch Immunol Ther Exp (Warsz). 2019;67(1):49-54.
doi pubmed - Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43.
doi pubmed pmc - Bozdogan G, Erdem E, Demirel GY, Yildirmak Y. The role of Treg cells and FoxP3 expression in immunity of beta-thalassemia major AND beta-thalassemia trait patients. Pediatr Hematol Oncol. 2010;27(7):534-545.
doi pubmed - Bao W, Yu J, Heck S, Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood. 2009;113(22):5624-5627.
doi pubmed pmc - Hetta HF, Zahran AM, Mansor SG, Abdel-Malek MO, Mekky MA, Abbas WA. Frequency and Implications of myeloid-derived suppressor cells and lymphocyte subsets in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. J Med Virol. 2019;91(7):1319-1328.
doi pubmed - Palumbo GA, Parrinello NL, Giallongo C, D'Amico E, Zanghi A, Puglisi F, Conticello C, et al. Monocytic myeloid derived suppressor cells in hematological malignancies. Int J Mol Sci. 2019;20(21):1-14.
doi pubmed pmc - Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3-8.
doi pubmed pmc - Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150.
doi pubmed pmc - Younos IH, Abe F, Talmadge JE. Myeloid-derived suppressor cells: their role in the pathophysiology of hematologic malignancies and potential as therapeutic targets. Leuk Lymphoma. 2015;56(8):2251-2263.
doi pubmed - Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191(1):17-23.
doi pubmed pmc - Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105-115.
doi pubmed pmc - Politou M, Komninaka V, Valsami S, Kapsimali V, Pouliakis A, Koutsouri T, Panayiotakopoulos G, et al. The effect of transfusion on immune responses in thalassemia. Blood Cells Mol Dis. 2020;83:102425.
doi pubmed - Obermayer A, et al. A Lasso Cox score consisting of CTLA4+ regulatory T cells (Treg), monocytic myeloid derived suppressor cells (M-MDSC), and CXCR3+ CD8+ T cells: Association with adjuvant ipilimumab (ipi) survival outcomes in patients (pts) with high-risk melanoma. J Clin Oncol. 2023;41(6):e21557.
- Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly asian descent. Blood. 2000;96(10):3369-3373.
pubmed - Namazi Bayegi S, Ali Hamidieh A, Behfar M, Saghazadeh A, Bozorgmehr M, Tajik N, Delbandi AA, et al. Unbalanced T-cell subsets in pediatric patients with beta-thalassemia. Hum Immunol. 2023;84(3):224-234.
doi pubmed - Zahran AM, Saad K, Elsayh KI, Abdelmoghny A, Aboul-Khair MD, Sobhy A, Abdel-Raheem YF, et al. Myeloid-derived suppressor cells and costimulatory molecules in children with allergic rhinitis. Ann Otol Rhinol Laryngol. 2019;128(2):128-134.
doi pubmed - He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA, Kagan V, et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med. 2018;24(2):224-231.
doi pubmed pmc - Siriworadetkun S, Thubthed R, Thiengtavor C, Paiboonsukwong K, Khuhapinant A, Fucharoen S, Pattanapanyasat K, et al. Elevated levels of circulating monocytic myeloid derived suppressor cells in splenectomised beta-thalassaemia/HbE patients. Br J Haematol. 2020;191(3):e72-e76.
doi pubmed - Al-Dedah RM, et al. Biochemical and hematological study with the appreciation of some immunological parameters in thalassemia patients at Karbala province. J Pure Appl Microbiol. 2018;12(4):1965-1973.
- Elsayh KI, Mohammed WS, Zahran AM, Saad K. Leukocytes apoptosis and adipocytokines in children with beta thalassemia major. Clin Exp Med. 2016;16(3):345-350.
doi pubmed - Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123-1131.
doi pubmed - Zhang C, Wang S, Yang C, Rong R. The Crosstalk between Myeloid Derived Suppressor Cells and Immune Cells: To Establish Immune Tolerance in Transplantation. J Immunol Res. 2016;2016:4986797.
doi pubmed pmc - Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234-243.
doi pubmed - Lee CR, Kwak Y, Yang T, Han JH, Park SH, Ye MB, Lee W, et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis. Cell Rep. 2016;17(12):3219-3232.
doi pubmed - Sieminska I, et al. The level of myeloid-derived suppressor cells positively correlates with regulatory T cells in the blood of children with transient hypogammaglobulinemia of infancy. Cent Eur J Immunol. 2018;43(4):413-420.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


