| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 4, August 2023, pages 187-196
Leukostasis With Isolated Central Nervous System Involvement in Chronic Phase of Chronic Myelogenous Leukemia
Won Jin Jeona, Suhani Dalala, Jin Hyun Moona, Bowon Jounga, Michael Nguyenb, Dani Castilloc, Jessica Hudsond, Kiwon Parke, Ravi Raghavand, Mojtaba Akhtarib, Ami Patelb, f
aDepartment of Internal Medicine, School of Medicine, Loma Linda University, Loma Linda, CA, USA
bDivision of Medical Oncology/Hematology, Department of Internal Medicine, Loma Linda University Medical Center, Loma Linda, CA, USA
cDivision of Medical Oncology, City of Hope, Duarte, CA, USA
dDivision of Anatomic and Surgical Pathology, Loma Linda University Medical Center, Loma Linda, CA, USA
eDepartment of Pharmacy, Loma Linda University, Loma Linda, CA, USA
fCorresponding Author: Ami Patel, Division of Medical Oncology/Hematology, Department of Internal Medicine, Loma Linda University Medical Center, Loma Linda, CA 92354, USA
Manuscript submitted June 1, 2023, accepted July 11, 2023, published online August 8, 2023
Short title: Isolated Central Nervous System Leukostasis
doi: https://doi.org/10.14740/jh1150
| Abstract | ▴Top |
Chronic myelogenous leukemia (CML) is a hematologic malignancy with unique significance to the field of hematology and oncology, specifically due to the development of tyrosine kinase inhibitors (TKIs). CML often presents with nonspecific symptoms, and the quality of life in patients with CML has drastically improved as a result of TKIs. However, complications of CML including the risk of transforming into life-threatening blast crises continue to exist. Further, as most patients are asymptomatic in the chronic phase, patients often present with serious complications associated with noncompliance to TKIs. For example, central nervous system (CNS) manifestations of CML have been reported, both as the initial presentation of undiagnosed CML and as known complication of uncontrolled CML. Hyperleukocytosis is a manifestation of uncontrolled CML and leukostasis is a complication, occurring in cases of acute myeloid leukemia (AML). Here we present a rare case of leukostasis in a patient with known CML presenting on computed tomography (CT) as intracranial masses in the chronic phase. Our goal is to discuss this rare case of leukostasis in adult CML and describe its management.
Keywords: Chronic myelogenous leukemia; Hyperleukocytosis; Leukostasis; Central nervous system
| Introduction | ▴Top |
Chronic myelogenous leukemia (CML) occurs in about 0.7 to 1 in 100,000 individuals per year according to several European registries and involves patients in the ages of 40 to 70s [1]. CML initially presents with nonspecific symptoms of fatigue, loss of appetite, weight loss, abdominal fullness and pain. Laboratory studies often exhibit leukocytosis with left shift. As a result of leukocytosis, patients develop splenomegaly, thrombosis, or abnormal bleeding [2]. Interestingly, some patients remain asymptomatic for long periods of time in the chronic phase (CP). According to the World Health Organization (WHO) criteria, CML is staged by the number of immature myeloid cells, or blasts, present in bone marrow (BM) or peripheral blood. The development of tyrosine kinase inhibitors (TKIs) has improved survival rates from < 15% to about 87% [2]. Furthermore, the progression of CML from CP to blast phase, leading to acute leukemia, has decreased from 20% to 1-1.5% [3]. Still, patients present with manifestations of noncompliance to TKIs, and untreated CML is associated with significant morbidity and mortality. In this case report, we discuss the case of a patient with known CML who presented with headache and hyperleukocytosis with findings of intracranial lesions. Upon pathology evaluation, the lesions were found to be the sequalae of leukostasis.
| Case Report | ▴Top |
We present the case of a 25-year-old male with known diagnosis of CML complicated by a history of hyperleukocytosis requiring leukapheresis and hearing impairment who initially presented to an outside institution with headache and associated nausea with vomiting and was transferred to our institution for higher level of care. Initial computed tomography (CT) of the head demonstrated multiple bilateral intracranial lesions, correlating with the patient’s symptoms. Upon chart review, the patient had been diagnosed with CML about 10 years prior to his presentation and had been started on imatinib but reportedly was noncompliant. In addition, due to concern for resistance against imatinib, the patient had been started on dasatinib several years prior. The patient again reported he was not taking dasatinib and had unfortunately missed many of his appointments in hematology clinic.
Upon presentation, the patient’s laboratory findings were remarkable for leukocytosis with white blood cell (WBC) count of 4.95 × 1011/L, anemia with hemoglobin (Hgb) of 5.6 g/dL, and thrombocytopenia with platelet count of 1.31 × 1011/L. Upon initial examination, the patient was alert and oriented without focal neurologic deficits besides the known hearing impairment. Hematology and Oncology was consulted and given concern for leukostasis in the setting of hyperleukocytosis and brain lesions with the differential including myeloid sarcoma (MS), emergent leukapheresis was started with further cytoreduction by starting hydroxyurea 2,000 mg twice a day. The patient was also started on prophylactic allopurinol 300 mg daily with monitoring for tumor lysis syndrome (TLS) with daily complete metabolic panel, phosphorous, lactate dehydrogenase (LDH), and uric acid levels. By the time a dialysis line was placed for leukapheresis, the patient’s WBC had worsened to 6.46 × 1011/L but this improved to 6.05 × 1011/L after the first session of leukapheresis.
Given the hemorrhagic intracranial lesions, Neurosurgery was consulted and recommended serial repeat CT imaging without emergent surgical intervention. Repeat CT of the head without contrast showed hyperdense/hemorrhagic multifocal rounded lesions (largest lesion of 2.2 × 2.5 × 2.6 cm in the left frontal lobe and 2.3 × 2.0 × 2.2 cm in the right frontal lobe) throughout the bilateral cerebral hemispheres with surrounding vasogenic edema and mass effect (Fig. 1). Temporizing measures including steroids was initiated due to evidence of cerebral edema and mass effect. The patient’s serum WBC level peaked at 8.86 × 1011/L and trended down with continued hydroxyurea. However, due to hemodynamic instability, the patient was unable to tolerate further sessions of leukapheresis and developed acute worsening of mental status with Glasgow coma scale (GCS) of 6, and intubated for airway protection. Due to further increase in the WBC to 6.66 × 1011/L, the patient’s hydroxyurea dose was subsequently increased to 2,000 mg three times a day. Repeat CT imaging of the head revealed new and developing uncal herniation. The patient became comatose with the lack of brainstem reflexes and determined to be clinically brain dead and an autopsy was requested by the patient’s family.
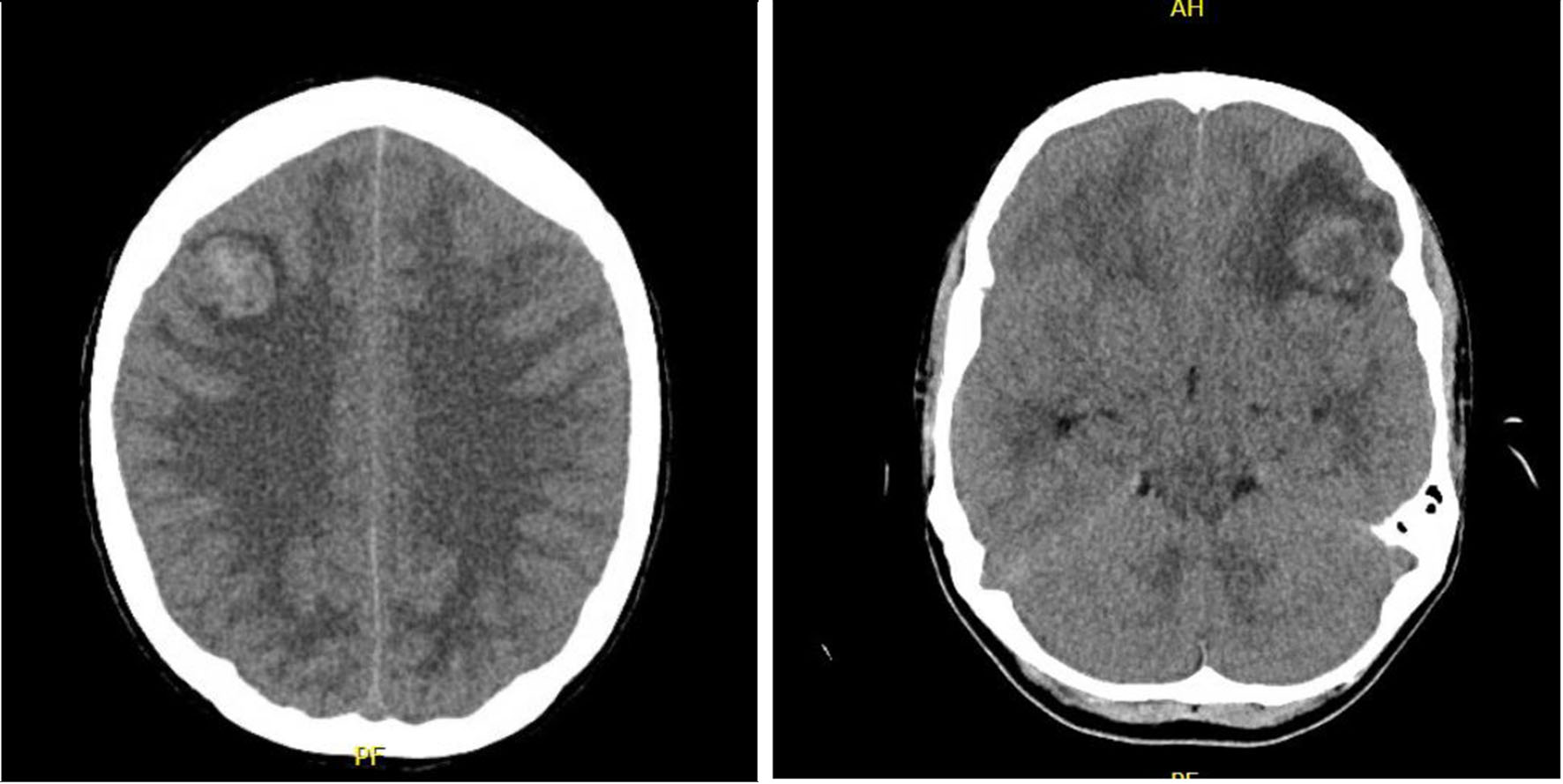 Click for large image | Figure 1. CT of head without contrast. Multiple lesions, prominently left frontal and right frontal lobe leukemic infiltrates with surrounding vasogenic edema and mass effect, concerning for a CNS manifestation of leukostasis. CT: computed tomography; CNS: central nervous system. |
The peripheral smear did not show any mutations associated with resistance against imatinib but otherwise demonstrated marked leukocytosis with left shift with only occasional blasts (1%) seen. In addition, flow cytometry was significant for 1.2% myeloblasts with no evidence of blast crisis, with findings consistent with the known CP of CML.
The neuropathology findings from the autopsy showed extensive microvascular and parenchymal involvement of the frontal lobe and brain stem by cells derived by the patient’s known CML with marked leukemic infiltration concerning for leukostasis (Fig. 2). Histology further showed hemorrhagic lesions involving brain parenchyma with marked left-shifted myeloid cells and leukemic infiltrates, and congestion of blood vessels with white blood cells. Overall, early ischemic changes were noted, indicative of a global ischemic event. Immunohistochemical staining was obtained for further characterization of the intracranial lesions and was positive for myeloperoxidase (MPO), CD43, and CD68, further consistent with known CML (Fig. 3). Further, the intracranial masses stained negative for CD117, CD45, CD34, CD3, and CD20.
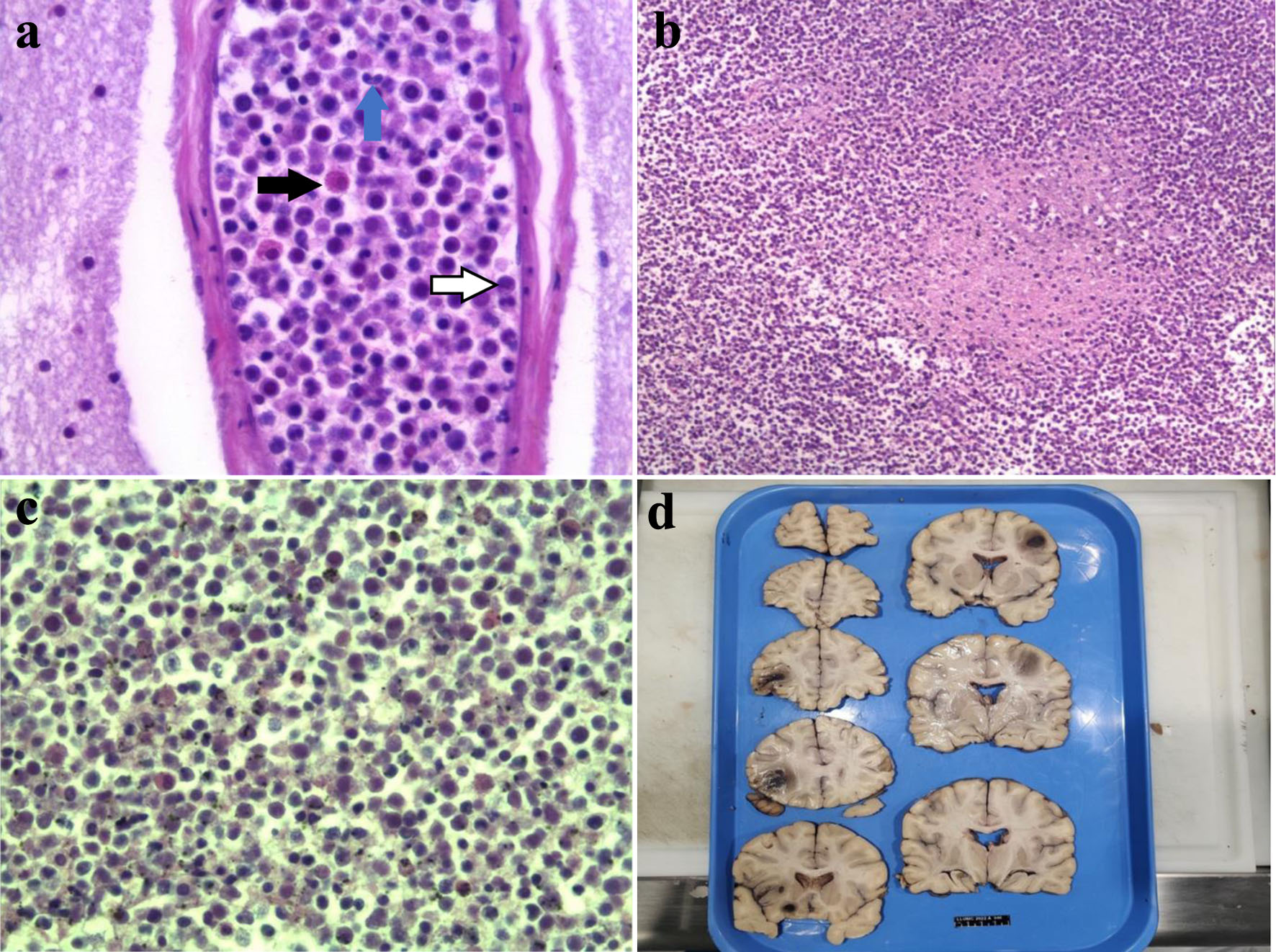 Click for large image | Figure 2. Autopsy images of hemorrhagic intracranial masses and pathology of mass lesions. (a) High power histology of the CML mass lesion in the brain. The black arrow represents an early myeloid cell (immature eosinophil), the blue arrow shows a neutrophil/band and the white arrow (with black outline) shows the monocytes. (b) Medium power histology of the CML mass with background of necrotic brain parenchyma. (c) High power histology of the tumor cells showing myeloid lineage including neutrophils and monocytes with myeloid precursors of various degrees of maturation. (d) Coronal sections of the patient’s brain which demonstrates several hemorrhagic masses. CML: chronic myelogenous leukemia. |
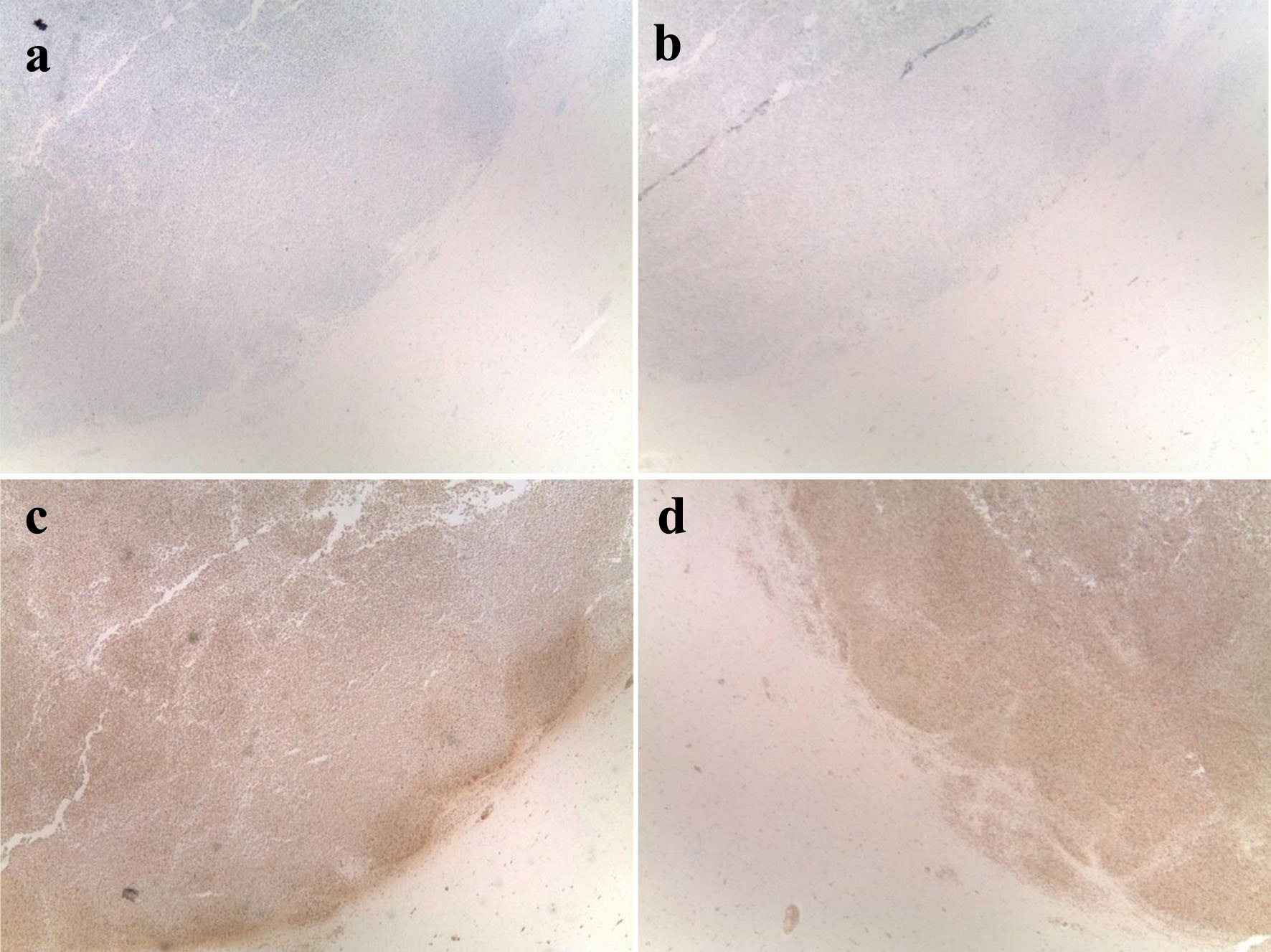 Click for large image | Figure 3. IHC staining of leukemic infiltrates in the frontal and brain stem lesions. IHC staining of the central nervous system lesions which was positive for MPO and CD68, consistent with known CML, and negative for CD20, confirming that the lesions do not originate from B-cell neoplasm. (a) IHC staining negative for CD20. (b) IHC staining negative for CD34. (c) IHC staining positive for MPO. (d) IHC staining positive for CD68. CML: chronic myelogenous leukemia; IHC: immunohistochemistry; MPO: myeloperoxidase. |
| Discussion | ▴Top |
CML arises from the fusion of the Abelson murine leukemia (ABL) gene with the breakpoint cluster region (BCR) gene in the Philadelphia chromosome, a translocation of chromosomes 22 and 9 [4]. This fusion creates the oncoprotein which stimulates the tyrosine kinase pathway and further activates downstream pathways including the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway. This leads to hematopoietic cell proliferation and resistance to apoptosis [4].
TKIs, which inhibit the adenosine triphosphate (ATP) binding site of the ABL-BCR oncoprotein, revolutionized the treatment and prognosis of CML. Currently, the Sokal, Hasford (Euro), and European Treatment and Outcome Study (EUTOS) prognostic scores are utilized to risk stratify patients into low, intermediate, and high risk of disease progression prior to starting TKIs [2, 4, 5]. For patients with intermediate or high-risk scores, second-generation TKIs are preferred over first-generation TKIs [6]. Unfortunately, BCR-ABL dependent and independent mutations and noncompliance lead to TKI resistance [4].
In current literature, there are cases of central nervous system (CNS) complications, including leukostasis and MS, associated with CML (Table 1) [3, 7-31]. Leukostasis occurs in the setting of hyperleukocytosis, defined as serum WBC greater than 1.00 × 1011/L, which is a rare manifestation of several leukemias [32, 33]. Leukostasis is rare but most commonly associated with acute myeloid leukemia (AML) as an increased number of circulating leukemic blasts in the microvasculature cause vascular obstruction and tissue hypoxia [34-37]. The complications associated with the highest mortality risk of hyperleukocytosis are leukostasis, TLS and disseminated intravascular coagulation (DIC), with leukostasis being associated with a mortality rate of up to 40% [34, 38]. While the incidence of leukostasis is low in AML, leukostasis in patients with CML is extremely rare, occurring in the blast phase of CML compared to other phases [39]. A retrospective study of 256 pediatric patients with CML showed that 9.7% were diagnosed with leukostasis after developing symptoms such as headache, syncope, vision changes, and priapism [40]. In comparison, leukostasis in adults with CML has not been extensively studied due to its rare occurrence.
 Click to view | Table 1. A Review of the Cases of CNS Involvement in CML in Current Literature |
One proposed mechanism of leukostasis resulting in increased blood viscosity is the high fractional volume of leukocytes causing microvascular obstruction, ischemia, and endothelial dysfunction arising from damage to the vacular endothelium [41-43]. Patients often present with headache, dizziness, confusion, tinnitus, blurred vision, delirium, ataxia, and coma, although other systems can also be affected [37]. Leukostasis may present on CT or magnetic resonance imaging (MRI) as hemorrhagic changes and areas of cerebral edema [44].
When hyperleukocytosis is suspected, prompt cytoreduction is recommended. Hydroxyurea is a commonly used medication along with systemic therapy [45]. Another key component of treatment of hyperleukocytosis is leukapheresis. Leukapheresis involves the immediate removal of the excess leukocytes via blood cell separators. However, hypercalcemia and thrombocytopenia are common complications of leukapheresis, and hemodynamically unstable patients may not tolerate leukapheresis as it can cause significant vasovagal response [45, 46]. Cranial irradiation can also be considered, though it is not a standard component of emergent management of leukostasis [47]. In general, TKIs such as imatinib, dasatinib, and nilotinib should also be started in the acute setting along with leukapheresis [48]. Other key factors for management of leukostasis include monitoring fluid balance and checking serum uric acid, electrolytes including potassium and phosphate, and renal function due to possible TLS [38].
Given the rarity of leukostasis in CML, when patients present with intracranial masses, the diagnosis of MS should remain in the differential [49]. MS, also known as granulocytic sarcoma or chloroma, describes an extramedullary tumor associated with myeloid leukemia [49]. In both AML and CML, MS presents as a distinct tumor mass with myeloid blasts, with or without maturation, occurring at any site outside of the BM and often considered equivalent to the diagnosis of AML [49]. However, in CML, MS may present with less percentage of blast cells compared to MS in AML, as MS may arise in various phases of CML including the CP [50]. MS is most often seen in AML, and histology shows increased blasts often staining positive for MPO, CD34, and CD68 [49]. MS is usually associated with progression of disease and with extramedullary manifestations, commonly arising in the soft tissue, bone, peritoneum and lymph nodes [51, 52]. The progression of CML from the blast stage to the CP increases the risk of MS by 7-17% [9]. The rate of misdiagnosis of MS is as high as 75%, with common misdiagnoses including infection, tumors, and hemorrhage [53].
Immunophenotypic analysis, especially of the cerebrospinal fluid (CSF), and flow cytometry are helpful tools in accurate diagnosis and distinguishing leukostasis from MS [42]. In our patient, the infiltrates in the brain parenchyma were positive for MPO and CD68 and only occasional blasts (around 1%), which suggested leukostasis in the setting of the CP of CML. The negative CD20 staining additionally confirms that the intracranial masses were not derivative of a B-cell neoplasm. Further, the negative staining for CD34 is consistent with leukostasis in the setting of CML and not acute leukemia in the form of MS. The lack of increased blasts in the flow cytometry as well as BM biopsy showing hypercellular marrow with evidence of maturation and without significant increase in blast cells both further support the diagnosis of leukostasis over MS.
Conclusion
Leukostasis is a rare complication of hyperleukocytosis which manifests in the CNS or respiratory system. Here we described a rare case of leukostasis presenting with intracranial lesions and correlated neurologic symptoms in the setting of known CP of CML. Interestingly, the patient’s intracranial lesions were found to be leukostasis as there was no evidence of AML to point to MS or CML in the blast phase. Given the high mortality rate associated with leukostasis, patients presenting with suspected CNS manifestations of CML should be evaluated for leukostasis with the goal of prompt cytoreduction and leukapheresis as indicated upon initial diagnosis. Future studies should be directed at standardized surveillance strategies for patients with CML and development of prognostic scores to predict the development of CML, especially those in complete remission as a result of TKI response to first- and second-generation TKIs.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors do not report any conflict of interest.
Informed Consent
Unable to be obtained due to deceased patient and next-of-kin unavailable for consent.
Author Contributions
WJ, MN, and SD conceptualized the manuscript and contributed to the writing of the manuscript. JM, BJ, and DC provided critical review and contributed to the revision of the manuscript. KP provided their expertise in the discussion of pharmacotherapy for this manuscript. JH and RR contributed to the pathology details included in the manuscript. AM and AP provided critical review of the manuscript and their expert guidance on the topic. All authors approve of submission of this manuscript for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ABL: Abelson murine leukemia; AML: acute myeloid leukemia; ATP: adenosine triphosphate; BCR: breakpoint cluster region; BM: bone marrow; BMT: bone marrow transplant; CML: chronic myelogenous leukemia; CNS: central nervous system; CP: chronic phase; CR: complete remission; CT: computed tomography; DIC: disseminated intravascular coagulation; DLI: donor lymphocyte infusion; EUTOS: European Treatment and Outcome Study; GCS: Glasgow coma scale; HAD: homoharringtonine, cytarabine, daunorubicin; Hgb: hemoglobin; HSCT: hematopoietic stem cell transplant; hyperCVAD: cyclophosphamide, vincristine, doxorubicin, and dexamethasone; JAK: Janus kinase; LDH: lactate dehydrogenase; LP: lumbar puncture; MPO: myeloperoxidase; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; MS: myeloid sarcoma; RBCs: red blood cells; SCT: stem cell transplant; STAT: signal transducer and activator of transcription; TKIs: tyrosine kinase inhibitors; TLS: tumor lysis syndrome; WBC: white blood cell; WHO: World Health Organization
| References | ▴Top |
- Sampaio MM, Santos MLC, Marques HS, Goncalves VLS, Araujo GRL, Lopes LW, Apolonio JS, et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review. World J Clin Oncol. 2021;12(2):69-94.
doi pubmed pmc - Granatowicz A, Piatek CI, Moschiano E, El-Hemaidi I, Armitage JD, Akhtari M. An overview and update of chronic myeloid leukemia for primary care physicians. Korean J Fam Med. 2015;36(5):197-202.
doi pubmed pmc - Atilla E, Ataca P, Ozyurek E, Erden I, Gurman G. Successful bosutinib experience in an elderly acute lymphoblastic leukemia patient with suspected central nervous system involvement transformed from chronic myeloid leukemia. Case Rep Hematol. 2015;2015:689423.
doi pubmed pmc - Bhamidipati PK, Kantarjian H, Cortes J, Cornelison AM, Jabbour E. Management of imatinib-resistant patients with chronic myeloid leukemia. Ther Adv Hematol. 2013;4(2):103-117.
doi pubmed pmc - Aijaz J, Junaid N, Asif Naveed M, Maab R. Risk stratification of chronic myeloid leukemia according to different prognostic scores. Cureus. 2020;12(3):e7342.
doi pubmed pmc - National Comprehensive Cancer Network. Chronic Myeloid Leukemia (Version 1.2023). https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed October 31, 2022.
- Jin M, Xuan C, Gao J, Han R, Xu S, Wang L, Wang Y, et al. Chronic myeloid leukemia extramedullary blast crisis presenting as central nervous system leukemia: A case report. Medicine (Baltimore). 2018;97(45):e13131.
doi pubmed pmc - Radhika N, Minakshi M, Rajesh M, Manas BR, Deepak Kumar M. Central nervous system blast crisis in chronic myeloid leukemia on imatinib mesylate therapy: report of two cases. Indian J Hematol Blood Transfus. 2011;27(1):51-54.
doi pubmed pmc - Abuelgasim KA, Alshieban S, Almubayi NA, Alhejazi A, Jazieh AR. An atypical initial presentation of chronic myeloid leukemia with central nervous system and lymph node blast crises. Case Rep Oncol. 2016;9(2):415-421.
doi pubmed pmc - Jain A, Gupta N. Isolated CNS blast crises in chronic myeloid leukaemia presenting as hypertrophic pachymeningitis and bilateral optic neuritis: A Case Report. J Clin Diagn Res. 2016;10(1):OE01-05.
doi pubmed pmc - Healey MA, Allendorf DJ, Borate U, Madan A. CNS involvement in a patient with chronic myeloid leukemia. Case Rep Hematol. 2021;2021:8891376.
doi pubmed pmc - Chiba A, Toya T, Mizuno H, Tokushige J, Nakamura F, Nakazaki K, Kurokawa M. Chronic myelogenous leukemia presenting with central nervous system infiltration, successfully treated with central nervous system-directed chemotherapy followed by allogeneic stem cell transplantation. Int J Hematol. 2018;108(6):640-646.
doi pubmed - Gomez J, Duenas V. Isolated central nervous system relapse in chronic myeloid leukemia. Case Rep Med. 2015;2015:232915.
doi pubmed pmc - Neumann M, Blau IW, Burmeister T, Tietze-Buerger C, Blau O, Gerbitz A, Uharek L, et al. Intrathecal application of donor lymphocytes in leukemic meningeosis after allogeneic stem cell transplantation. Ann Hematol. 2011;90(8):911-916.
doi pubmed - Park MJ, Park PW, Seo YH, Kim KH, Seo JY, Jeong JH, Kim MJ, et al. A case of isolated lymphoblastic relapse of the central nervous system in a patient with chronic myelogenous leukemia treated with imatinib. Ann Lab Med. 2014;34(3):247-251.
doi pubmed pmc - Kim HJ, Jung CW, Kim K, Ahn JS, Kim WS, Park K, Ko YH, et al. Isolated blast crisis in CNS in a patient with chronic myelogenous leukemia maintaining major cytogenetic response after imatinib. J Clin Oncol. 2006;24(24):4028-4029.
doi pubmed - Beyazit Y, Aksu S, Kekilli M, Haznedaroglu IC, Kilickap S, Goker H. Unusual extramedullary relapses under imatinib mesylate treatment in chronic myeloid leukemia. Am J Hematol. 2005;79(1):79-80.
doi pubmed - Gaur S, Torabi AR, Corral J. Isolated central nervous system relapse in two patients with BCR-ABL-positive acute leukemia while receiving a next-generation tyrosine kinase inhibitor. In Vivo. 2014;28(6):1149-1153.
pubmed - Rajappa S, Uppin SG, Raghunadharao D, Rao IS, Surath A. Isolated central nervous system blast crisis in chronic myeloid leukemia. Hematol Oncol. 2004;22(4):179-181.
doi pubmed - Bornhauser M, Jenke A, Freiberg-Richter J, Radke J, Schuler US, Mohr B, Ehninger G, et al. CNS blast crisis of chronic myelogenous leukemia in a patient with a major cytogenetic response in bone marrow associated with low levels of imatinib mesylate and its N-desmethylated metabolite in cerebral spinal fluid. Ann Hematol. 2004;83(6):401-402.
doi pubmed - Bujassoum S, Rifkind J, Lipton JH. Isolated central nervous system relapse in lymphoid blast crisis chronic myeloid leukemia and acute lymphoblastic leukemia in patients on imatinib therapy. Leuk Lymphoma. 2004;45(2):401-403.
doi pubmed - Johnson NA, Fetni R, Caplan SN. Isolated central nervous system relapse in patients with chronic myeloid leukemia on imatinib mesylate. Leuk Lymphoma. 2005;46(4):629-630.
doi pubmed - Matsuda M, Morita Y, Shimada T, Miyatake J, Hirase C, Tanaka M, Tatsumi Y, et al. Extramedullary blast crisis derived from 2 different clones in the central nervous system and neck during complete cytogenetic remission of chronic myelogenous leukemia treated with imatinib mesylate. Int J Hematol. 2005;81(4):307-309.
doi pubmed - Aichberger KJ, Herndlhofer S, Agis H, Sperr WR, Esterbauer H, Rabitsch W, Knobl P, et al. Liposomal cytarabine for treatment of myeloid central nervous system relapse in chronic myeloid leukaemia occurring during imatinib therapy. Eur J Clin Invest. 2007;37(10):808-813.
doi pubmed - Barlow A, Robertson M, Doig A, Stewart W, Drummond MW. Isolated central nervous system lymphoid blast crisis in chronic myeloid leukaemia in major molecular remission. Br J Haematol. 2008;142(3):327.
doi pubmed - Altintas A, Cil T, Kilinc I, Kaplan MA, Ayyildiz O. Central nervous system blastic crisis in chronic myeloid leukemia on imatinib mesylate therapy: a case report. J Neurooncol. 2007;84(1):103-105.
doi pubmed - Lee KW, Song MK, Seol YM, Choi YJ, Shin HJ, Chung JS, et al. Isolated central nervous system blast crisis in chronic myeloid leukemia. Korean J Med. 2009;77(S2):S441-S444
- Isobe Y, Sugimoto K, Masuda A, Hamano Y, Oshimi K. Central nervous system is a sanctuary site for chronic myelogenous leukaemia treated with imatinib mesylate. Intern Med J. 2009;39(6):408-411.
doi pubmed - Thomas A, Stein CK, Gentile TC, Shah CM. Isolated CNS relapse of CML after bone marrow transplantation. Leuk Res. 2010;34(4):e113-114.
doi pubmed - Fuchs M, Reinhofer M, Ragoschke-Schumm A, Sayer HG, Boer K, Witte OW, Hochhaus A, et al. Isolated central nervous system relapse of chronic myeloid leukemia after allogeneic hematopoietic stem cell transplantation. BMC Blood Disord. 2012;12:9.
doi pubmed pmc - Nishimoto M, Nakamae H, Koh KR, Kosaka S, Matsumoto K, Morita K, Koh H, et al. Dasatinib maintenance therapy after allogeneic hematopoietic stem cell transplantation for an isolated central nervous system blast crisis in chronic myelogenous leukemia. Acta Haematol. 2013;130(2):111-114.
doi pubmed - Ali AM, Mirrakhimov AE, Abboud CN, Cashen AF. Leukostasis in adult acute hyperleukocytic leukemia: a clinician's digest. Hematol Oncol. 2016;34(2):69-78.
doi pubmed - Demir MK. A diagnostic dilemma in the emergency room: intracranial hemorrhages in acute myeloblastic leukemia with hyperleukocytosis. Acta Neurol Belg. 2020;120(2):487-489.
doi pubmed - Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, McCarthy LJ. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39(1-2):1-18.
doi pubmed - Macaron W, Sargsyan Z, Short NJ. Hyperleukocytosis and leukostasis in acute and chronic leukemias. Leuk Lymphoma. 2022;63(8):1780-1791.
doi pubmed - McKee LC, Jr., Collins RD. Intravascular leukocyte thrombi and aggregates as a cause of morbidity and mortality in leukemia. Medicine (Baltimore). 1974;53(6):463-478.
doi pubmed - Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM. Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev. 2012;26(3):117-122.
doi pubmed - Giammarco S, Chiusolo P, Piccirillo N, Di Giovanni A, Metafuni E, Laurenti L, Sica S, et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10(2):147-154.
doi pubmed - How J, Venkataraman V, Hobbs GS. Blast and accelerated phase CML: room for improvement. Hematology Am Soc Hematol Educ Program. 2021;2021(1):122-128.
doi pubmed pmc - Kurosawa H, Tanizawa A, Tono C, Watanabe A, Shima H, Ito M, Yuza Y, et al. Leukostasis in children and adolescents with chronic myeloid leukemia: Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer. 2016;63(3):406-411.
doi pubmed - Lichtman MA, Rowe JM. Hyperleukocytic leukemias: rheological, clinical, and therapeutic considerations. Blood. 1982;60(2):279-283.
pubmed - Algharras AA, Mamourian A, Coyne T, Mohan S. Leukostasis in AN ADULT WITh AML presenting as multiple high attenuation brain masses on CT. J Clin Diagn Res. 2013;7(12):3020-3022.
doi pubmed pmc - Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97(7):2121-2129.
doi pubmed - Koenig MK, Sitton CW, Wang M, Slopis JM. Central nervous system complications of blastic hyperleukocytosis in childhood acute lymphoblastic leukemia: diagnostic and prognostic implications. J Child Neurol. 2008;23(11):1347-1352.
doi pubmed pmc - Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125(21):3246-3252.
doi pubmed - Philip J, Sarkar RS, Pathak A. Adverse events associated with apheresis procedures: Incidence and relative frequency. Asian J Transfus Sci. 2013;7(1):37-41.
doi pubmed pmc - Chang MC, Chen TY, Tang JL, Lan YJ, Chao TY, Chiu CF, Ho HT. Leukapheresis and cranial irradiation in patients with hyperleukocytic acute myeloid leukemia: no impact on early mortality and intracranial hemorrhage. Am J Hematol. 2007;82(11):976-980.
doi pubmed - Raghuwanshi B, Chouhan S, Jain A. Leukocytapheresis in chronic myeloid leukemia with leukostasis. Cureus. 2020;12(12):e12375.
doi pubmed pmc - Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid sarcoma: presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk. 2017;17(5):263-267.
doi pubmed - Arzoun H, Srinivasan M, Thangaraj SR, Thomas SS, Mohammed L. The progression of chronic myeloid leukemia to myeloid sarcoma: a systematic review. Cureus. 2022;14(1):e21077.
doi pubmed pmc - Wilson CS, Medeiros LJ. Extramedullary manifestations of myeloid neoplasms. Am J Clin Pathol. 2015;144(2):219-239.
doi pubmed - Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013;3(4):265-270.
pubmed pmc - Liu J, Wang Y, Sun X, Liu S, Yu Z, Ji N, Sun S, et al. Lesions of the central nervous system in leukemia: Pathological and magnetic resonance imaging features at presentation in 14 patients. Oncol Lett. 2017;14(6):8162-8170.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


