| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 3, September 2014, pages 91-94
Anaplastic Large-Cell Lymphoma T Anaplastic Lymphoma Kinase-Positive Associated With Crohn’s Disease Undergoing Treatment With Azathioprine: A Case Report
Pamela Cristina Gaspara, Manoela Lira Reisa, Kaite Cristiane Peresb, Renata Cristina Messores Rudolf-Oliveiraa, Vanessa Mengattoc, Joanita Angela Gonzaga Del-Morald, Maria Claudia Santos-Silvaa, c, e
aProgram of Post Graduation in Pharmacy; Department of Clinical Analysis, Universidade Federal de Santa Catarina, Florianopolis, Brazil
bTechnical-Pedagogical Support From Management Course Pharmaceutical Assistance; Ministry of Health, Universidade Federal de Santa Catarina, Florianopolis, Brazil
cProgram of the Associated Professional Master Home Health Multidisciplinary, Universidade Federal de Santa Catarina, Florianopolis, Brazil
dDepartment of Clinical Medicine, Universidade Federal de Santa Catarina, Florianópolis, Brazil
eCorresponding Author: Maria Claudia Santos-Silva, Hospital Universitario Polydoro Ernani de Sao Thiago, Universidade Federal de Santa Catarina, Divisao de Analises Clinicas, Laboratorio de Oncologia Experimental e Hemopatias, Campus Universitario, s/n°, Trindade, Florianopolis, Santa Catarina 88040-900, Brazil
Manuscript accepted for publication December 20, 2013
Short title: Lymphoma Associated With Crohn’s Disease
doi: https://doi.org/10.14740/jh118w
| Abstract | ▴Top |
Anaplastic large-cell lymphoma (ALCL) is a subgroup of non-Hodgkin lymphoma (NHL) that the etiology is not totally elucidated. Hereditary, environmental, occupational and dietary factors are believed to be related with the development of NHL. The use of some immunomodulatory drugs such as azathioprine at the treatment of Crohn’s disease (CD) has been described in the literature causing NHL. We reported a clinical case of a possible association between NHL and Crohn’s treatment. Furthermore, ALCL is a rare subtype of NHL, thus, it has a difficult diagnostic. So in this study we also demonstrated clinical characteristics and laboratory findings that are helpful to rapid and accurate diagnostic of ALCL.
Keywords: Anaplastic large-cell lymphoma; Crohn’s disease; Non-Hodgkin lymphoma
| Introduction | ▴Top |
Anaplastic large-cell lymphoma (ALCL) is a subgroup of non-Hodgkin (NHL) malignant lymphoma, originated from T or natural killer cells that were initially described by Stein and co-workers in 1985 [1]. The lesion is characterized by proliferation of anaplastic large lymphoid cells with abundant cytoplasm that strongly express CD30 antigen [2]. There are two types of ALCL, the systemic type, which affects lymph nodes and other organs, and the cutaneous type, which affects mainly the skin. Depending on the expression of a protein known as anaplastic lymphoma kinase (ALK), patients with systemic ALCL are divided into two groups, ALK-positives and ALK-negatives [3]. The patients with ALK-positive respond better than other to chemotherapy, putting most patients in long-term remission or cure [4]. Studies reported that the etiology of this NHL may comprise hereditary, environmental, occupational and dietary factors. Furthermore, patients exposed to certain immunomodulatory drugs such as thiopurines, like azathioprine and its metabolite, 6-mercaptopurine, are related with a risk five times higher of lymphoproliferative disorder in comparison to those never exposed to it [5, 6]. These drugs are usually used to maintain remission in Crohn’s disease (CD), a chronic inflammatory condition affecting the gastrointestinal tract at any point from the mouth to the rectum [7]. Considering the disease rarity, the aim of this study was to report a case of ALCL associated with CD.
| Case Report | ▴Top |
This report describes a case of a 37-year-old man, Caucasian, undernourished, smoker, not alcoholic, diagnosed in 2008 with CD and since then treated with azathioprine and mesalazine. In 2011, he presented cervical, axillary and femoral lymphonodemegaly. The enlargement of the axillary lymph nodes was noticed 6 months earlier, and the other ones 1 month earlier. The lymph node biopsy showed large cells, medium nucleocytoplasmic ratio, strong basophilic, vacuolated and agranular cytoplasm, pleomorphic nuclei, coarsely stippled chromatin pattern and two or three nucleoli (Fig. 1). The flow cytometric immunophenotyping of axillary lymph node cells showed 10% of large cells (high forward scatter (FSC)) with expression of CD4+, CD5+, CD25+, CD30+, CD43+ and CD45++ (Fig. 2), and the immunohistochemistry analysis showed expression of CD3+, CD5+, CD30+, CD45+ and ALK-1. Therefore, the combination of these findings is fairly diagnostic for an ALCL, ALK-positive. After that, the immunophenotyping of bone marrow and cerebrospinal fluid was made to determinate the stage of the lymphoma using the Ann Arbor staging. The immunophenotyping of the bone marrow showed that 0.01% of the cells expressed CD45++, CD25+, CD30+, CD5+, CD7+, CD45RO+, CD43+, and CD2+ and the immunophenotyping of the cerebrospinal fluid showed 0.6% of cells with the following phenotype CD45++, CD30+, CD25+ and CD5+. After all chemotherapy cycles, the patient was discharged and has been kept under ambulatory monitoring.
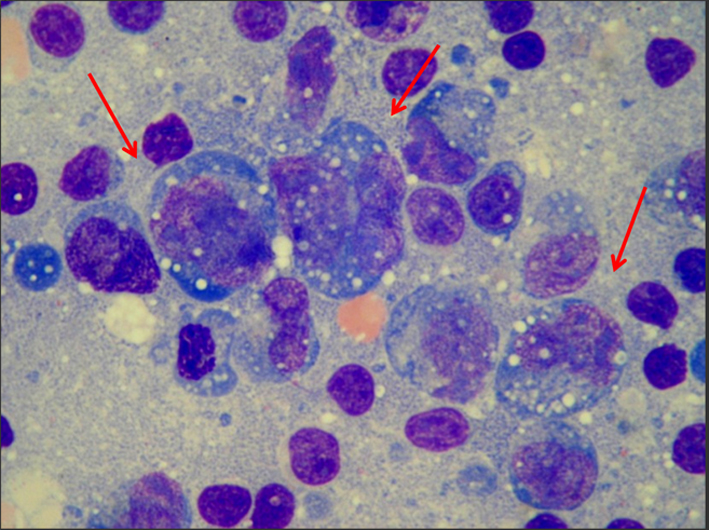 Click for large image | Figure 1. The lymph node biopsy showed large cells, medium nucleocytoplasmic ratio, strong basophilic, vacuolated and agranular cytoplasm, pleomorphic nuclei, coarsely stippled chromatin pattern and two or three nucleoli. |
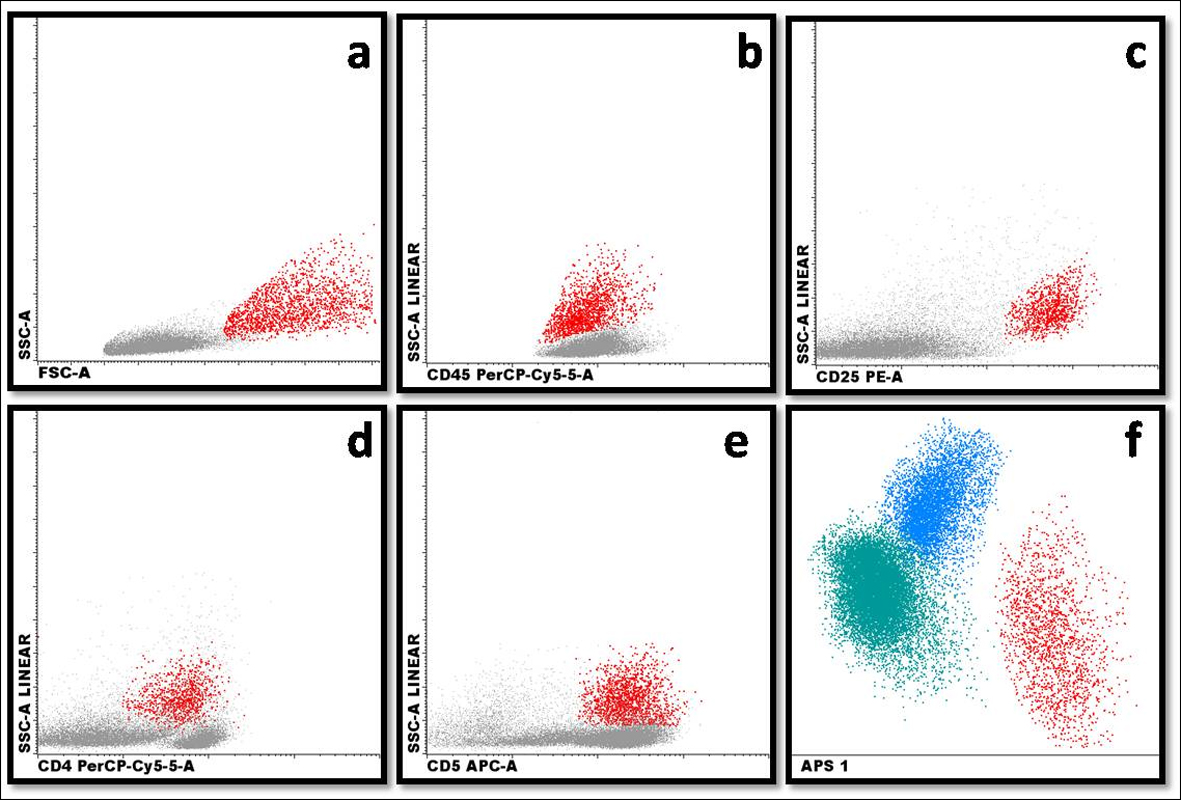 Click for large image | Figure 2. Demonstration of (a) large cells with high FSC × SSC light scattering (red population); (b) expression of CD45 in pathologic cells (red population); (c) expression of CD25 in pathologic cells (red population); (d) expression of CD4 in pathologic cells (red population); (e) expression of CD5 in pathologic cells (red population); (f) automatic population separator (APS): T lymphocytes (blue population), B lymphocytes (green population) and pathologic cells (red population). |
| Discussion | ▴Top |
CD is a chronic inflammatory condition that affects the gastrointestinal tract at any point from the mouth to the rectum. Patients may experience diarrhea, abdominal pain, fever, weight loss, abdominal masses and anemia [8]. CD diagnostic can be concluded by radiologic tests, endoscopy, colonoscopy or laparotomy [7, 9]. The most widely used therapy according to the literature consists in the association of drug to maintain remission of the disease, which includes immunomodulators (AZA), antiinflammatories (mesalazine) and antitumor (infliximab, adalimumab). CD patients may be at increased risk for the development of Hodgkin’s lymphoma (HL) or NHL, either through exposure to immunosuppressive medications or due to their underlying chronic inflammatory illness [10]. In literature, there are conflicting studies in relation to the possibility of developing NHL after treatment with immunomodulators/immunosuppressants. Studies conducted in France showed that the risk of lymphoproliferative diseases increases five times with the association of immunosuppressive drugs compared to patients not using this treatment [5]. A United Kingdom case-control study did not show significant overall risk of malignancy, but also report a positive association with lymphoma and utilization of immunosuppressant [11]. In order to clarify this paradigm, several studies must be performed yet.
In this context, it was evaluated the case of a patient with CD that after approximately 3 years with immunomodulatory treatment developed ALCL, ALK-positive, a subtype of NHL. ALCL ALK-positive accounts in approximately 3% of adult NHL. It is most frequent in the first three decades of life and shows a male predominance (M:F ratio 1.5:1) [3]. This lymph proliferative disorder exhibits a wide morphological spectrum; however, in all morphological variations there is a proportion variable of Hellmark cells. These cells are characterized usually by large cells with abundant and pleomorphic cytoplasm, which can be clear, basophilic or eosinophilic. Their nuclei have a horseshoe shape and often have an eosinophilic region near the nucleus corresponding to the Golgi apparatus [3, 12-14]. Nuclear chromatin is mostly finely dispersed or aggregated with multiple small nucleoli and basophilic. In histological sections, depending on the plane section of tissue, some cells may appear to contain nuclear inclusions in the cytoplasm, but in reality they are invaginations of the nuclear membrane. The cells that have this characteristic are termed “Doughnut cells” [3]. It was possible to observe patient’s lymph node biopsy morphology and to analyze large cells, we found medium nucleocytoplasmic ratio, strong basophilic, vacuolated and agranular cytoplasm, pleomorphic nuclei, coarsely stippled chromatin pattern and two or three nucleoli. These morphology characteristics are consistent with descriptions of ALCL cells presented in the literature.
Approximately 60% of ALCL carry a translocation involving the ALK gene on chromosome 2p23, most commonly a t(2;5)(p23;q35) involving the nucleophosmin gene. These rearrangements result in overexpression of ALK fusion proteins, and their presence is associated with an excellent prognosis [4, 15, 16]. With the exception of the small cell variant, the majority of tumor cells in ALCL are by definition CD30+, and multiple studies have demonstrated variable positivity for a number of T cell and non-T cell-associated antigens, including CD2, CD3, CD4, CD8, CD25, CD43, CD45, CD45RO, CD56, CD13, CD15, CD33, HLA-DR, TIA-1, ZAP-70, and T-cell receptor molecules [3, 17]. The flow cytometric immunophenotyping of axillary lymph node showed that 10% of the large cells by the analysis of the FSC expressed CD4+, CD5+, CD25+, CD30+, CD43+, and CD45++ (Fig. 2) and the immunohistochemistry analysis showed expression of CD3+, CD5+, CD30+, CD45+ and ALK-1. The immunophenotyping was compatible of NHL anaplastic large T cells, which is characterized by pan T cell marker CD3 negative and positive for CD2, CD4 and CD5 markers. The cytotoxic T lymphocyte marker, CD8, usually is negative; pan leukocyte marker, CD45, positive in two-thirds of the cases; tumor cells variably positive for pan leukocyte CD45 marker and strongly positive CD25 marker [3]. Then, due to the association of clinical, morphology and immunophenotyping characteristics, it was possible to conclude that the reported case was an ALCL, ALK-positive.
Once diagnosed the disease, it is necessary to establish the staging of the lymphoma in order to evaluate the extent of disease and to determine the more appropriate protocol of treatment. The staging system Ann Arbor was developed in 1971 for HL. This system identifies the anatomical sites of involvement by lymphoma and classified the patients into four categories based on the extent of dissemination of the disease [18]. Despite the NHL have different characteristics of dissemination of HL, with frequent involvement of extranodal lymphatic growth and without contiguity, the system Ann Arbor system modified by Costwold remains as the method of choice also in the staging of NHL [19]. Then, after the diagnostic, the immunophenotyping of bone marrow and cerebrospinal fluid was made by flow cytometric to determinate the stage of the lymphoma by Ann Arbor staging. The immunophenotyping of the bone marrow showed 0.01% of cells expressing CD45++, CD25+, CD30+, CD5+, CD7+, CD45RO+, CD43+, and CD2+ and the immunophenotyping of the cerebrospinal fluid showed 0.6% of cells with the following phenotype CD45++, CD30+, CD25+ and CD5+. These results lead to stage 4 that indicates the presence of tumor cells located in large extranodal regions.
The chemotherapy treatment was systemic and intrathecal Hyper-CVAD protocol (cyclophosphamide, vincristine, doxorubicin and dexamethasone). After all cycles, the patient was discharged and keeps monitoring ambulatory.
Acknowledgments
We acknowledge the CNPq and CAPES for financial support and fellowships.
Conflicts of Interest
All authors have no conflicts of interest.
| References | ▴Top |
- Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848-858.
pubmed - Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496-5504.
doi pubmed - Jaffe ES, Harris NL, Stein H, Campo E, Pileri SA, Swerdlow SH. World Health Organization International Classification of Tumours Hematopoietic and Lymphoid Tissues. 4th edition. New York: Springer-Verlag, 2008.
- Fornari A, Piva R, Chiarle R, Novero D, Inghirami G. Anaplastic large cell lymphoma: one or more entities among T-cell lymphoma? Hematol Oncol. 2009;27(4):161-170.
doi pubmed - Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, Hebuterne X, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617-1625.
doi - Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-alpha inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol. 2011;23(12):1150-1156.
doi pubmed - Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63(3):629-642.
doi - Wilkins T, Jarvis K, Patel J. Diagnosis and management of Crohn's disease. Am Fam Physician. 2011;84(12):1365-1375.
pubmed - Hidalgo LH, Moreno EA, Arranz JC, Alonso RC, Fernandez VMV. Entero-resonancia magnetica: revision de la tecnica para el estudio de la enfermedad de Crohn. Radiologia. 2011;53(5):1-13.
- El Mourabet M, Hashash JG, Sun NH, Issa M, Katz JA, Regueiro M, Barrie AM, 3rd, et al. Clinical course of Crohn's disease following treatment of lymphoma. Inflamm Bowel Dis. 2011;17(6):1265-1269.
doi pubmed - Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol. 2010;105(7):1604-1609.
doi pubmed - Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, Haralambieva E, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91(6):2076-2084.
pubmed - Delsol G, Brugieres L, Gaulard P, Espinos E, Lamant L. Anaplastic large cell lymphoma, ALK-positive and anaplastic large cell lymphoma ALK-negative. Hematology Meeting Reports. 2009;3(1):51-57.
- Ju E, Adigun C, Dunphy C, Gold S, Morrell DS. Anaplastic large cell lymphoma: an unusual presentation in a 7-year-old girl. Pediatr Dermatol. 2012;29(4):498-503.
doi pubmed - Borisch B, Yerly S, Cerato C, Schwaller J, Wacker P, Ozsahin AH, Brousse N, et al. ALK-positive anaplastic large-cell lymphoma: strong T and B anti-tumour responses may cause hypocellular aspects of lymph nodes mimicking inflammatory lesions. Eur J Haematol. 2003;71(4):243-249.
doi pubmed - Chang IW, Chen HK, Ma MC, Huang WT. Anaplastic large cell lymphoma with paraneoplastic leukocytosis: a clinicopathological analysis of five cases. APMIS. 2011;119(11):794-801.
doi pubmed - Kesler MV, Paranjape GS, Asplund SL, McKenna RW, Jamal S, Kroft SH. Anaplastic large cell lymphoma: a flow cytometric analysis of 29 cases. Am J Clin Pathol. 2007;128(2):314-322.
doi pubmed - Carbone A, Spina M, Gloghini A, Tirelli U. Classical Hodgkin's lymphoma arising in different host's conditions: pathobiology parameters, therapeutic options, and outcome. Am J Hematol. 2011;86(2):170-179.
doi pubmed - Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55(6):368-376.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


