| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 6, December 2023, pages 277-282
Refractory Pure Red Blood Cell Aplasia Secondary to Major ABO-Incompatible Allogeneic Stem Cell Transplantation Successfully Treated With Daratumumab
Clinton Wua , Pete Manchenb, Ariela Edelmanb, Muhammad Husnaina, c, Emmanuel Katsanisa, b, c, d, e, Deborah Fuchsb, Laura Stephensf, Sharad Khuranaa, c, g
aDepartment of Medicine, University of Arizona, Tucson, AZ, USA
bDepartment of Pathology, University of Arizona, Tucson, AZ, USA
cUniversity of Arizona Cancer Center, Tucson, AZ, USA
dDepartment of Pediatrics, University of Arizona, Tucson, AZ, USA
eDepartment of Immunobiology, University of Arizona, Tucson, AZ, USA
fDepartment of Pathology, University of California San Diego Health, La Jolla, CA, USA
gCorresponding Author: Sharad Khurana, University of Arizona Cancer Center, Tucson, AZ, USA
Manuscript submitted September 19, 2023, accepted October 30, 2023, published online December 9, 2023
Short title: Daratumumab in Allo-HSCT Induced PRCA
doi: https://doi.org/10.14740/jh1195
| Abstract | ▴Top |
Pure red cell aplasia (PRCA) is a rare hematologic phenomenon that is usually associated with inherited genetic mutations such as in Diamond-Blackfan anemia. However, due to the emergence of allogenic stem cell transplantation in the treatment of various malignant and non-malignant disorders, the incidence of PRCA has increased. PRCA following hematopoietic stem cell transplant (HSCT) is more commonly seen in the setting of a major ABO-incompatible transplant. Treatment of allo-HSCT induced PRCA can be initially supportive as it takes time for the bone marrow to fully recover. However, prolonged and/or failure of the bone marrow to recover, significantly increases patient’s risk of iron overload in the setting of frequent transfusions. Iron deposition can potentially lead to severe life-threatening multiorgan involvement which can be fatal. Therefore, earlier recognition and intervention with immunomodulators in patients who undergo frequent transfusions can be beneficial to mitigate this risk. Here, we present a case with severe transfusion-dependent PRCA following major ABO-incompatible allo-HSCT successfully treated with daratumumab.
Keywords: Pure red cell aplasia; Allogenic hematopoietic stem cell transplant; Daratumumab
| Introduction | ▴Top |
Pure red cell aplasia (PRCA) is a rare disorder with an estimated incidence of approximately one to two cases per million per year [1]. Defined by persistent anemia, reticulocytopenia, and a bone marrow devoid of erythroid precursors, PRCA is also a well-recognized complication of patients undergoing major or bidirectional ABO-incompatible allogenic hematopoietic stem cell transplants (allo-HSCT), where the incidence ranges from 7.5% to 30% [2]. As PRCA is mediated by the persistence of high anti-donor isohemagglutinins directed against erythroid precursors [3], patients undergoing major/bidirectional allo-HSCT with reduced intensity conditioning regimens are at increased risk of post-transplant PRCA.
Although PRCA can resolve spontaneously without intervention, many patients develop transfusion-dependent anemia. This may result in a significant need for long-term red blood cell transfusion support and iron overload-associated morbidity. While standardized PRCA treatment has yet to be established, corticosteroids and/or rituximab are conventionally employed as first-line therapy [4]. Additional modalities including erythropoietin analogues, plasmapheresis, anti-thymocyte globulin, bortezomib, and donor lymphocyte infusions have also been used with variable success [5, 6].
More recently, emerging data have shown daratumumab, an anti-CD38 monoclonal antibody that targets plasma cells, to have encouraging efficacy in patients with refractory PRCA [7-12]. Due to the rarity of this complication, reports have been limited primarily to case studies and case series. Herein, we present another successful case of daratumumab in the treatment of major ABO-incompatible allogeneic-HSCT induced PRCA refractory to the use of conventional therapeutic options.
| Case Report | ▴Top |
A 61-year-old man presented with asymptomatic but progressive thrombocytopenia. Bone marrow (BM) aspiration/biopsy showed hypercellular marrow (about 99%) with dysmegakaryopoiesis, approximately 5% interstitial blasts, and absent iron stores. Cytogenetic analysis revealed an intermediate risk karyotype 47,XY,+8[11]/47,XY,+der(1;13)(q10;q10)[9]/46, XY[1] and fluorescent in situ hybridization (FISH) detected trisomy 8. Next-generation sequencing (NGS) identified ASXL1 (variant frequency 38%), NRAS (9%), U2AF1 (46%), ETV6 (49%), and FLT3 (40%). Based on the Revised International Prognostic Scoring System (IPSS-R), he had a score of 5 (trisomy 8 = 2, 5% blasts = 2, platelets < 50 × 103 = 1) which was most compatible with high-risk myelodysplastic syndrome (MDS) for which an allogeneic transplant was planned.
Pre-transplant, the patient was treated with four cycles of azacitidine and venetoclax. Since his restaging BM biopsy revealed increased blasts (7%) and persistent cytogenetic abnormalities, the patient subsequently received one cycle of CPX-351 (Vyxeos). He achieved morphologic remission as characterized by < 5% blasts. The patient then underwent transplantation with a peripheral blood stem cell (PBSC) graft from a 10/10 matched unrelated donor with major ABO incompatibility (recipient O+, donor A-).
A reduced intensity conditioning regimen was utilized with fludarabine 30 mg/m2 on days -6 to -2, anti-thymocyte globulin on days -3 to -1, and melphalan 140 mg/m2 on day -1. His PBSC graft was comprised of 6.0 × 106 CD34+ cells and 1.12 × 108 CD3+ T-cells. Graft-versus-host disease (GVHD) prophylaxis included methotrexate on days +1 (15 mg/m2), 3, 6, and 11 (10 mg/m2) as well as tacrolimus, which was initiated on day -2 with goal blood levels of 5 - 15 ng/mL. Neutrophil and platelet engraftment occurred on day +28. BM biopsy on day +30 was consistent with a complete remission as demonstrated by a hypocellular marrow with trilineage hematopoiesis, normal karyotype, negative minimal residual disease (MRD) assessment, negative MDS FISH, negative NGS panel, and donor chimerism of 99.67%.
The patient had slow red blood cell (RBC) engraftment with persistent anemia, however. He required type O blood transfusions approximately twice weekly to maintain hemoglobin levels of > 7 g/dL. Restaging BM biopsy at 3 months was MRD negative and showed no evidence of leukemia or dysgranulopoiesis, but there was evidence of persistent erythroid hypoplasia (Fig. 1a-c). There was no evidence of parvovirus in the marrow. Furthermore, his lactate dehydrogenase level was low, the direct antiglobulin test was negative with polyspecific antisera, and there was no laboratory or clinical evidence of hemolysis that may have contributed to persistent anemia. Anti-A isohemagglutinin titers were requested, which were performed manually by tube technique with group A1 cells at room temperature (for presumed immunoglobulin (Ig)M antibody) and by indirect antiglobulin test (for presumed IgG antibody). Although no pre-transplant baseline titers were available for comparison, the patient’s current anti-A IgG titer of 1:1,024 was considered elevated. Thus, the diagnosis of post-transplant PRCA was made. The patient was started on prednisone 1 mg/kg on day 96 (Fig. 2). Despite 2 weeks of treatment, his hemoglobin levels continued a downward trend, and he required ongoing transfusion support. He was then transitioned to weekly rituximab 375 mg/m2 on day +117 and completed a total of six doses, also without improvement in his transfusion requirements or his anti-A antibody titers. On day +146, after four treatments of rituximab, prednisone and tacrolimus doses were increased as he was experiencing worsening diarrhea, concerning for grade 2 acute GVHD. He was able to successfully taper off prednisone and tacrolimus on days +160 and +175, respectively, with resolution of acute lower gastrointestinal (GI) GVHD, but his transfusion-dependent anemia persisted. Day +180 BM showed persistent red cell aplasia (Fig. 3a-c).
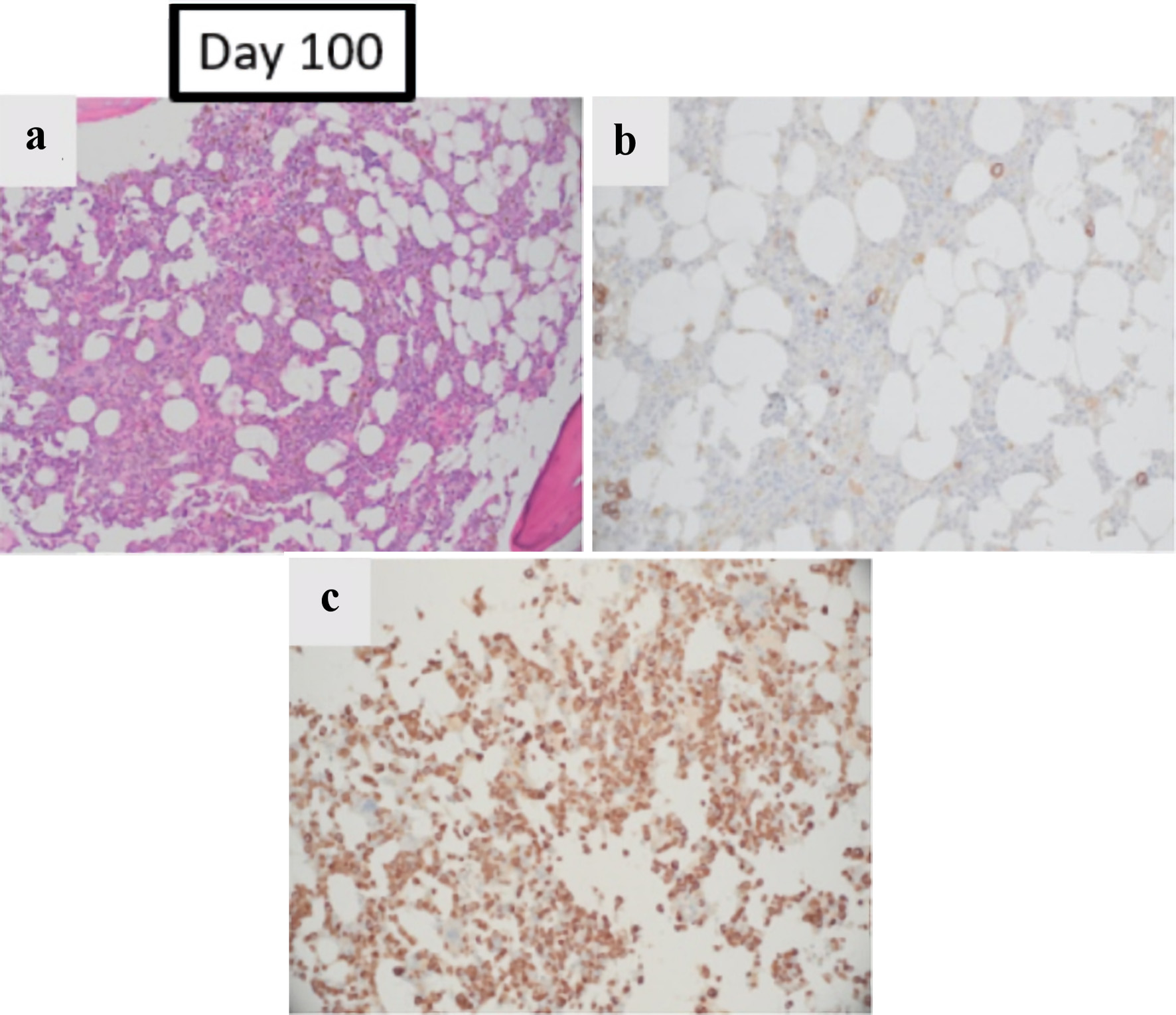 Click for large image | Figure 1. The effect of pure red cell aplasia on patient’s BM: BM obtained on day +100 post allo-HSCT, and prior to starting treatment for PRCA. (a) Hematoxylin and eosin stain (magnification: × 100) showing decreased erythroid elements. (b) E-cadherin stain which highlighted decreased erythroid precursors (< 5% of total cells) with limited colony formation (magnification: × 200). (c) Myeloperoxidase stain which showed marked predominance of maturing granulocytic precursors (magnification: × 200). Allo-HSCT: allogenic hematopoietic stem cell transplant; BM: bone marrow; PRCA: pure red cell aplasia. |
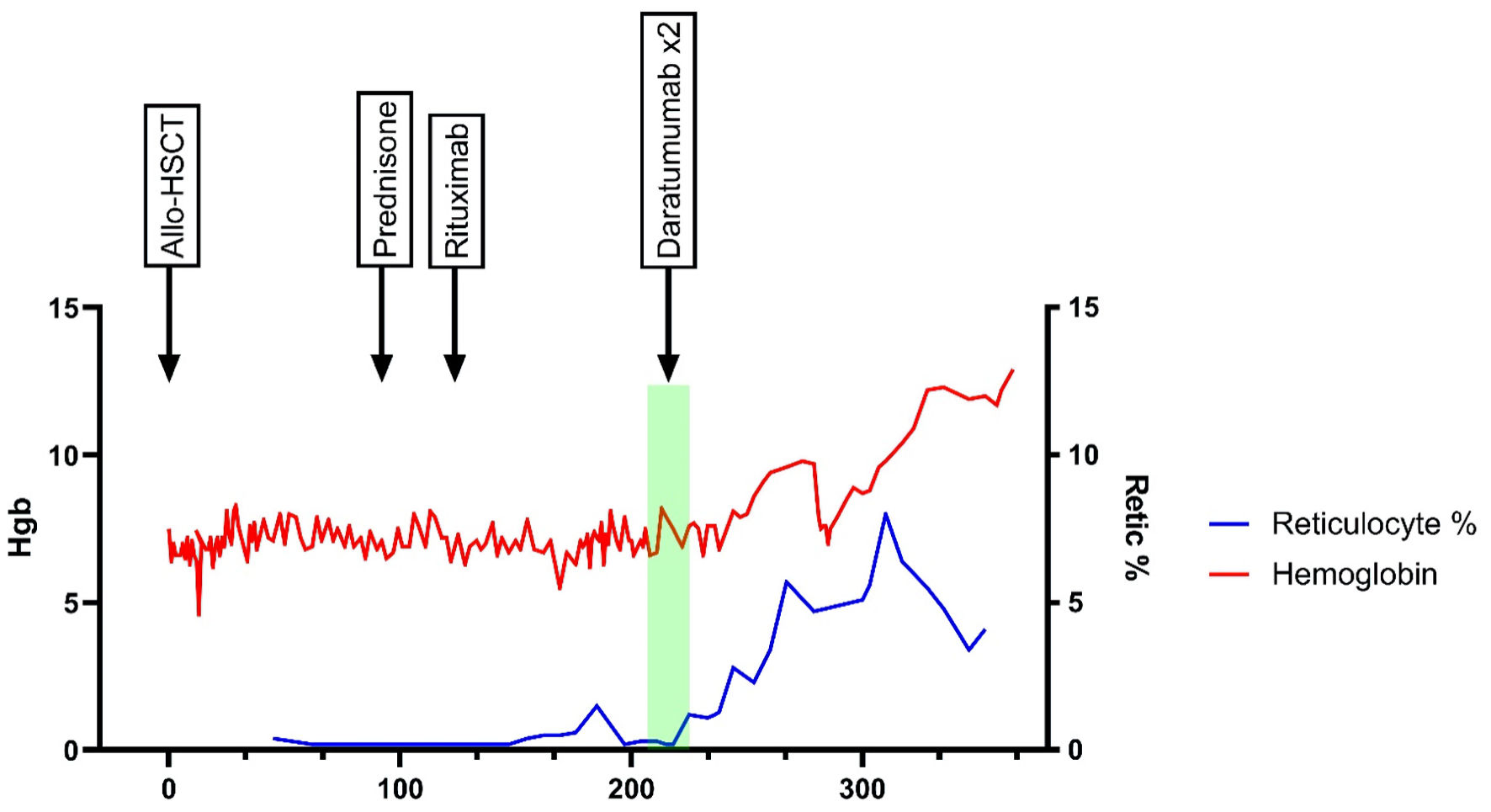 Click for large image | Figure 2. Chronological timeline of patient’s hemoglobin and reticulocyte percentage post allo-HSCT. It highlights the response with various modalities. Allo-HSCT: allogenic hematopoietic stem cell transplant; BM: bone marrow; Hgb: hemoglobin; PRCA: pure red cell aplasia; Retic: reticulocyte. |
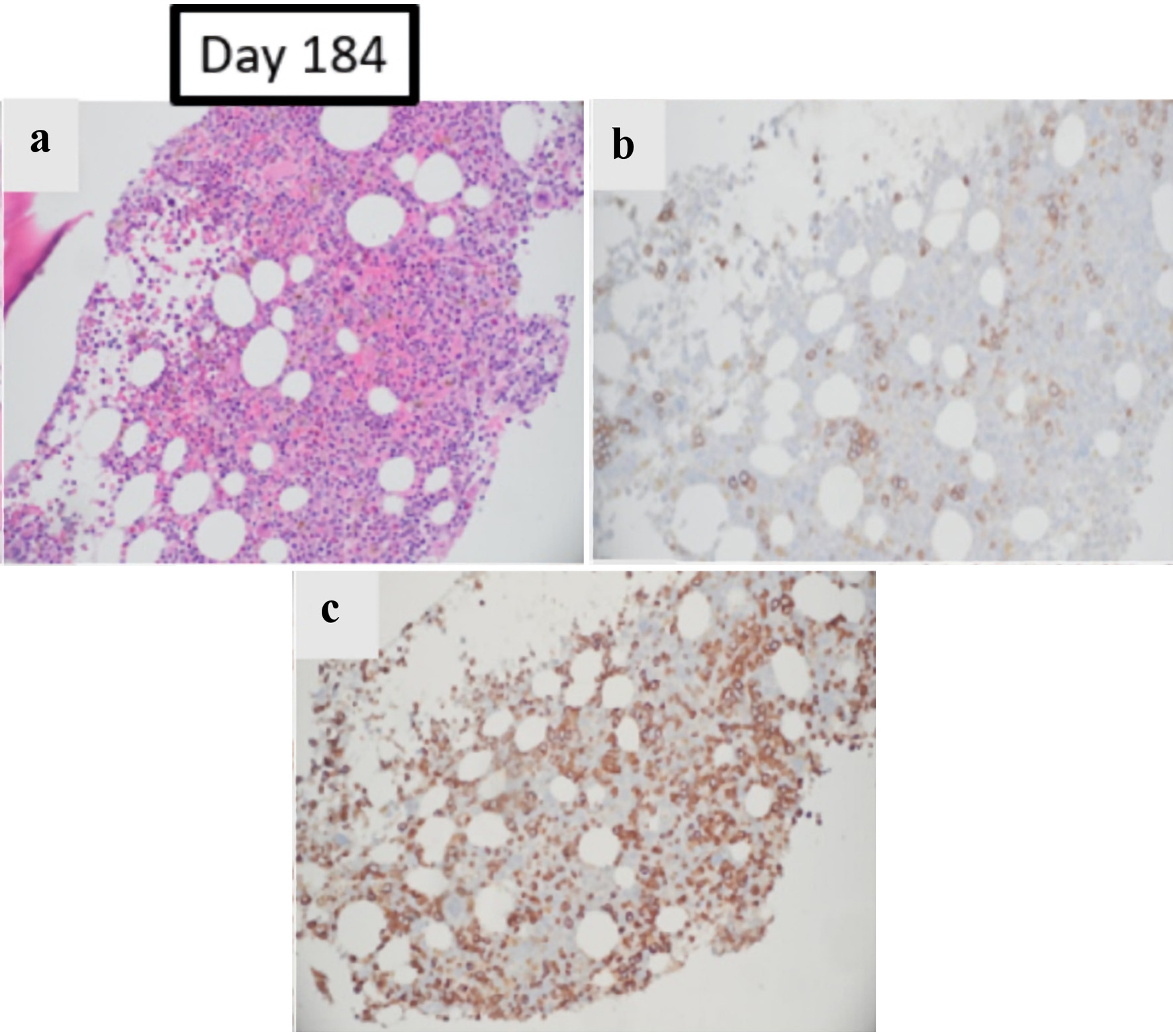 Click for large image | Figure 3. The effect of pure red cell aplasia on patient’s BM: BM obtained on day +184, after treatments with prednisone and rituximab. (a, b) Hematoxylin and eosin and E-cadherin stain re-demonstrating overall decreased erythroid elements accounting for < 10% of total cells and small colonies (magnification: × 200). (c) Myeloperoxidase stain continues to show predominance of maturing granulocytic precursors (magnification: × 200). |
The urgency to correct the patient’s PRCA intensified due to the significant secondary biochemical and liver iron overload observed on imaging. This was in the setting of multiple transfusions and the patient’s known HFE gene heterozygosity status. Therefore, the patient was started on off-label daratumumab 16 mg/kg/dose on day +209. After 2 weekly infusions, the patient had a noticeable improvement of his reticulocyte count percentage from 0.2% to 1.2%, which also correlated with a decrease in frequency of transfusion requirements. A repeat anti-A IgG titer remained elevated at 1:1,024 approximately 3 weeks after the last daratumumab dose. However, the titer was likely confounded by daratumumab interference on antibody testing, as red blood cells also express CD38, and reagent red cells were not treated with dithiothreitol to remove cell surface CD38. Irrespective of antibody titer measurements, there were noticeable improvements of his hemoglobin and reticulocyte measurements.
The patient ultimately achieved transfusion independence 1 month after the last treatment with daratumumab. His hemoglobin improved from a pre-treatment baseline of 6.6 g/dL to 8.1 g/dL and 10.4 g/dL 1 month and 3 months after his last dose of daratumumab, respectively. Thus, improvement in the patient’s anemia, recovery of reticulocyte counts, and resolution of transfusion requirements were all indicative of a response to daratumumab. To date, he remains transfusion independent with a 1-year post-transplant hemoglobin of 12.9 g/dL. Restaging BM was aspiculate with a limited core but showed hypocellular marrow with erythroid predominance. The patient remains on oral iron chelation for secondary iron overload.
| Discussion | ▴Top |
Despite the rarity of PRCA, the incidence of developing PRCA after allo-HSCT remains significantly high [2]. Patients undergoing allo-HSCT, especially with reduced intensity conditioning and/or major ABO-incompatibility, are at risk for delayed RBC recovery and PRCA [3]. Pathophysiologically, recipient plasma cells are likely to survive the reduced intensity cytotoxic agents and continue to secrete antibodies against incompatible blood type antigens [13]. In our case, our recipient was blood type O+ while the donor was blood type A-, thereby creating a major histocompatibility issue. The recipient continued to produce anti-A antibodies at elevated titers, which inhibited the proliferation and growth of erythroid precursors.
Management of persistent PRCA has traditionally revolved around the use of corticosteroids. With the introduction of novel immunotherapeutic and targeted agents for various hematological disorders, some of these agents have also been utilized in the management of PRCA, specifically, those refractory to corticosteroids with varying degrees of success. Rituximab, an anti-CD20 monoclonal antibody, was introduced for its ability to inhibit residual host B lymphocytes. In the most comprehensive review to date, Longval et al [6] retrospectively analyzed the use of rituximab in 17 cases of refractory PRCA. With a median duration of 71 days, transfusion independence only occurred in approximately half of the patients. Notably, the study did not find a significant reduction in PRCA period when comparing rituximab-treated patients to untreated patients (P = 0.62). Similarly, Griffith et al described several patients with PRCA following major ABO-incompatible HSCT who had persistent anti-donor isohemagglutinins despite converting to full donor B lymphocyte chimerism [13]. This suggests that host plasma cells may play a more dominant role than host B lymphocytes in the production of anti-donor isohemagglutinins. Additionally, bortezomib [14], a 26S proteasome inhibitor, and ibrutinib [15], a BTK inhibitor, have limited case reports or case studies demonstrating efficacy with approximately 4 weeks of treatment. However, these two agents have considerable risk profiles that may restrict their application for some patients, specifically in the post-transplant setting. Other treatment modalities described in the treatment of refractory PRCA cases have included erythropoietin-stimulating agents, donor lymphocyte infusion, complement inhibition, and other investigational agents [16].
Daratumumab was first described as an effective treatment for multiple myeloma given overexpression of CD38 on cell surface of myeloma-based plasma cells [17]. In the setting of allo-HSCT induced PRCA, daratumumab has been postulated to selectively target and suppress plasma cell clones responsible for alloimmunization. Some common features emerge when comparing various cases [7-12] utilizing daratumumab in post-transplant PRCA. Most patients were at least 6 months or more post-transplant with persistent packed red blood cell (PRBC) transfusion dependence. The diagnosis of PRCA was made given full donor chimerism, lack of or only mild-moderate GVHD, absence of active infection, elevated or rising isohemagglutinin titers, and patients had failed multiple prior conventional modalities. Lastly, most cases showed a brisk reticulocyte count response within one to three weekly dose(s) of 16 mg/kg of daratumumab. Our case follows a similar pattern as reported by Martino et al [7], Asawapanumas et al [8], and Rautenberg et al [9], whereby the patient maintained a durable response with only two doses of daratumumab. These observations suggest that only a short course daratumumab may be sufficient to induce a durable response if a brisk reticulocyte response is seen, and additional doses can be reserved for cases where inadequate responses are observed. However, the possibility or contribution of spontaneous recovery cannot be entirely excluded in our case, as PRCA often behaves as a self-limiting condition [4]. Of note, there are signals of other potential mechanistic effects of daratumumab, possibly related to its involvement in reducing CD38-positive immune suppressor cells, including regulatory T cells and natural killer cells [18-20]. For example, reports have associated post-HSCT daratumumab with low incidence of GVHD [18] and treatment of post-HSCT autoimmune-like hepatitis [21].
In conclusion, we present a case with severe transfusion-dependent PRCA following major ABO-incompatible allo-HSCT successfully treated with daratumumab. With the increased utilization of HSCT in both malignant and nonmalignant diseases, the incidence of PRCA may continue to rise. Our report reinforces the utility of daratumumab for treatment of refractory post-transplant PRCA. Future consideration of a prospective study is indicated to confirm the efficacy of this treatment.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
CW wrote the manuscript. PM and DF reviewed and obtained BM biopsies images. SK was involved in patient care, reviewed literature and mentored the implementation of the manuscript. LS was involved in patient diagnosis. LS and AE supported transfusion management. All authors reviewed, edited, and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PRCA: pure red cell aplasia; Allo-HSCT: allogenic hematopoietic stem cell transplant; BM: bone marrow; FISH: fluorescent in situ hybridization; NGS: next-generation sequencing; PBSC: peripheral blood stem cell; GVHD: graft-versus-host disease; MRD: minimal residual disease
| References | ▴Top |
- Nakazawa H, Sakai K, Ohta A, Fujishima N, Matsuda A, Hosokawa K, Nakamura F, et al. Incidence of acquired pure red cell aplasia: a nationwide epidemiologic analysis with 2 registry databases in Japan. Blood Adv. 2022;6(24):6282-6290.
doi pubmed pmc - Aung FM, Lichtiger B, Bassett R, Liu P, Alousi A, Bashier Q, Ciurea SO, et al. Incidence and natural history of pure red cell aplasia in major ABO-mismatched haematopoietic cell transplantation. Br J Haematol. 2013;160(6):798-805.
doi pubmed pmc - Kopko PM. Transfusion support for ABO-incompatible progenitor cell transplantation. Transfus Med Hemother. 2016;43(1):13-18.
doi pubmed pmc - Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142(4):505-514.
doi pubmed pmc - Marco-Ayala J, Gomez-Segui I, Sanz G, Solves P. Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question. Bone Marrow Transplant. 2021;56(4):769-778.
doi pubmed - Longval T, Galimard JE, Lepretre AC, Suarez F, Amiranoff D, Cazaux M, Kaphan E, et al. Treatment for pure red cell aplasia after major ABO-incompatible allogeneic stem cell transplantation: a multicentre study. Br J Haematol. 2021;193(4):814-826.
doi pubmed - Martino R, Garcia-Cadenas I, Esquirol A. Daratumumab may be the most effective treatment for post-engraftment pure red cell aplasia due to persistent anti-donor isohemagglutinins after major ABO-mismatched allogeneic transplantation. Bone Marrow Transplant. 2022;57(2):282-285.
doi pubmed pmc - Asawapanumas T, Chanswangphuwana C, Watanaboonyongcharoen P, Rojnuckarin P, Bunworasate U. Daratumumab as a frontline immunosuppression for pure red cell aplasia after major ABO-mismatched allogeneic hematopoietic stem cell transplantation. Leuk Res Rep. 2022;17:100314.
doi pubmed pmc - Rautenberg C, Kaivers J, Germing U, Haas R, Ackerstaff S, Hoffmann T, Kobbe G, et al. Daratumumab for treatment of pure red cell aplasia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2020;55(6):1191-1193.
doi pubmed - Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for delayed red-cell engraftment after allogeneic transplantation. N Engl J Med. 2018;379(19):1846-1850.
doi pubmed - Gangat N, Bleeker J, Lynch D, Olteanu H, Letendre L, Tefferi A. Daratumumab for treatment-refractory acquired idiopathic pure red cell aplasia. Haematologica. 2022;107(10):2523-2526.
doi pubmed pmc - Salas MQ, Alahmari A, Lipton JH. Successful treatment of refractory red cell aplasia after allogeneic hematopoietic cell transplantation with daratumumab. Eur J Haematol. 2020;104(2):145-147.
doi pubmed - Griffith LM, McCoy JP, Jr., Bolan CD, Stroncek DF, Pickett AC, Linton GF, Lundqvist A, et al. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005;128(5):668-675.
doi pubmed - Khan F, Linden MA, Zantek ND, Vercellotti GM. Subcutaneous bortezomib is highly effective for pure red cell aplasia after ABO-incompatible haematopoietic stem cell transplantation. Transfus Med. 2014;24(3):187-188.
doi pubmed pmc - Arslan S, Ali H, Mei M, Marcucci G, Forman S, Nakamura R, Stein A, et al. Successful treatment of refractory pure red cell aplasia in major ABO-mismatched allogeneic hematopoietic stem cell transplant with single agent Ibrutinib. Bone Marrow Transplant. 2022;57(5):830-833.
doi pubmed - Migdady Y, Pang Y, Kalsi SS, Childs R, Arai S. Post-hematopoietic stem cell transplantation immune-mediated anemia: a literature review and novel therapeutics. Blood Adv. 2022;6(8):2707-2721.
doi pubmed pmc - Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207-1219.
doi pubmed - Nikolaenko L, Chhabra S, Biran N, Chowdhury A, Hari PN, Krishnan A, Richter J. Graft-versus-host disease in multiple myeloma patients treated with daratumumab after allogeneic transplantation. Clin Lymphoma Myeloma Leuk. 2020;20(6):407-414.
doi pubmed pmc - van de Donk N. Immunomodulatory effects of CD38-targeting antibodies. Immunol Lett. 2018;199:16-22.
doi pubmed - Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384-394.
doi pubmed pmc - Epperly R, Santiago T, Morin CE, Patton K, Deyo J, Eshun J, Triplett B, et al. Targeting plasma cells with daratumumab aids in the treatment of post-transplant autoimmune-like hepatitis. Pediatr Blood Cancer. 2021;68(11):e29290.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


