| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 1-2, April 2024, pages 34-38
Successful Treatment of Refractory Post-Transplant Lymphoproliferative Disorder With Chimeric Antigen Receptor T-Cell Therapy in a Heart Transplant Recipient
Katherine Hickmanna, Ryan Sweeneyb, Chelsea Petersonc, Kathleen Faringerc, Madeline Rileyd, Mark Bunkerd, Azam Hadie, Cyrus Khanc, Yazan Samhouric, f
aDrexel University College of Medicine, Philadelphia, PA, USA
bDepartment of Internal Medicine, Allegheny Health Network, Pittsburgh, PA, USA
cDivision of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
dDepartment of Anatomic and Clinical Pathology, Allegheny Health Network, Pittsburgh, PA, USA
eAdvanced Heart Failure and Transplant, AGH McGinnis Cardiovascular Institute, Pittsburgh, PA, USA
fCorresponding Author: Yazan Samhouri, Division of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA 15224, USA
Manuscript submitted February 12, 2024, accepted March 13, 2024, published online April 9, 2024
Short title: Treatment of PTLD With CAR T-Cell Therapy
doi: https://doi.org/10.14740/jh1211
| Abstract | ▴Top |
Post-transplant lymphoproliferative disorders (PTLDs) are opportunistic malignancies that complicate the success of hematopoietic stem cell or solid organ transplantation. These disorders often arise post-transplant due to the immunosuppression required for minimizing the risk of rejection of donor tissue. First-line treatment of these disorders includes limiting immunosuppression when permissible. Subsequent treatment includes the use of monoclonal anti-CD20 antibody (rituximab), and/or combination chemotherapy. Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment paradigm in many lymphoid malignancies. It is not approved for PTLD due to exclusion of PTLD patients from pivotal clinical trials. Also, its utilization post-transplant can be complex and multidisciplinary care is of utmost importance for successful administration of a potentially curative treatment. We present a 68-year-old patient with history of heart transplant for non-ischemic cardiomyopathy, diagnosed with PTLD that was refractory to treatment using current guidelines until successfully receiving CAR T-cell therapy.
Keywords: Post-transplant lymphoproliferative disorder; CAR T-cell therapy; Heart transplant
| Introduction | ▴Top |
Post-transplant lymphoproliferative disorder (PTLD) is a heterogeneous group of disorders in patients who undergo either solid organ or hematopoietic stem cell transplant due to immunosuppressive therapy [1]. There is a fine balance post-transplantation between immunosuppression to prevent graft rejection while maintaining sufficient cellular immunity to prevent PTLD [2]. Heart transplant patients have an estimated 0.9% risk of developing PTLD; this risk is about four times that of adult kidney transplant recipients [3]. Malignancy is the leading cause of death at 5 years post heart transplant [4]. One factor that is associated with developing PTLD is when both the donor and recipient are Epstein-Barr virus (EBV) positive. The dominant theory for development of PTLD is that immunosuppressive therapy leads to T-cell depletion allowing EBV-infected B cells to proliferate without regulation.
Chimeric antigen receptor (CAR) T-cell therapy is an emerging modality of treatment for B-cell malignancies including relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and B-cell acute lymphoblastic leukemia (B-ALL). CAR T-cell therapy was approved by the FDA in 2017 [5]. This therapy includes using autologous T cells collected via apheresis from the patient which are modified and expanded ex vivo. These cells are manufactured to have a CAR specific to the patient’s malignancy. Previous solid organ transplantation is a universal exclusion criterion in CAR T-cell therapy trials. There are a few reports of successful CAR T-cell therapy in refractory PTLD in heart transplants, but mostly in patients under age 25 [2, 6]. In this case report, we present a 68-year-old male patient with refractory PTLD, monomorphic DLBCL subtype, who successfully responded to CAR T-cell therapy.
| Case Report | ▴Top |
We present the case of a 68-year-old male who underwent orthotopic heart transplant in 2021 for a diagnosis of non-ischemic cardiomyopathy. He was diagnosed with dilated cardiomyopathy secondary to alcohol use 20 years prior. Post-transplant the patient was initially treated with an immunosuppressive regimen of tacrolimus, mycophenolate mofetil, and prednisone.
Five months after transplantation, the patient presented to the hospital with abdominal pain and diarrhea. Infectious workup revealed Clostridium difficile (C. difficile) infection and persistent leukopenia from transplant immunosuppression. Due to low risk of rejection and concern for complications from immunosuppression, his mycophenolate was discontinued while tacrolimus and prednisone were continued. Imaging studies included computed tomography of the abdomen/pelvis (CTAP), which revealed new hypodense liver lesions. These findings were followed up with a liver magnetic resonance imaging (MRI) and ultrasound-guided biopsy. Liver biopsy showed EBV+ DLBCL consistent with monomorphic PTLD (Fig. 1a, b). Subsequently, he was started on rituximab 375 mg weekly and received four total infusions over the course of 4 weeks. After four cycles of weekly rituximab, positron emission tomography (PET)/CT was performed and revealed progression of disease with new lung involvement (Fig. 2a). Due to the lack of response demonstrated on imaging, his treatment was then escalated to rituximab, cyclophosphamide, etoposide, vincristine, prednisone (R-CEOP) regimen. Etoposide was used in place of anthracyclines to minimize cardiotoxicities due to post-heart transplant status.
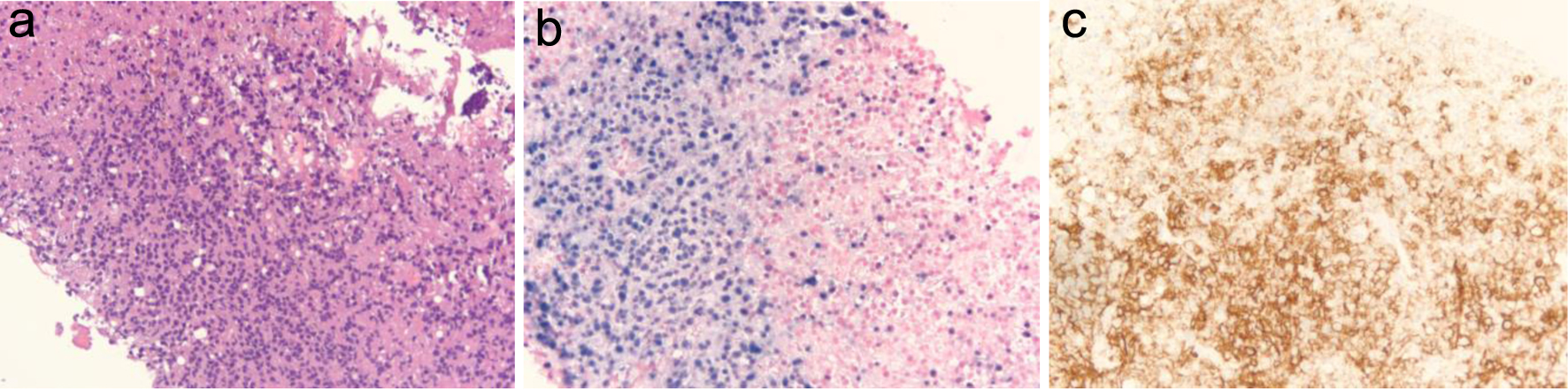 Click for large image | Figure 1. (a) Liver core biopsy showing monomorphic proliferation of neoplastic cells (at × 20 magnification). (b) Ebstein-Barr virus (EBV) stain showing positive nuclear staining of EBV-infected cells (at × 20 magnification). (c) CD20 stain showing positive membranous staining of B cells (at × 20 magnification). |
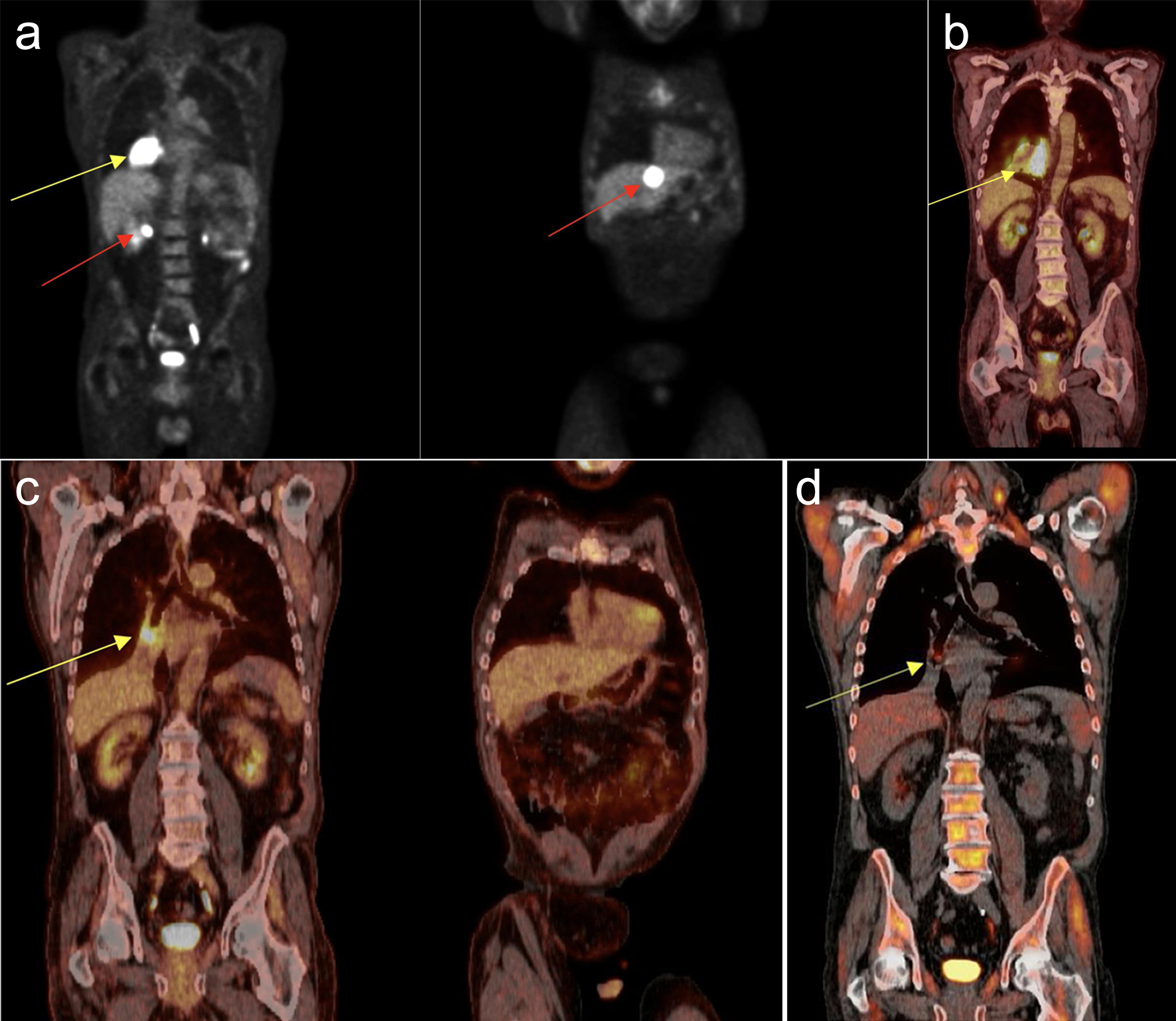 Click for large image | Figure 2. Positron emission tomography/computed tomography (PET/CT) over patient’s disease progression and treatment. (a) After rituximab: showing large mass in right lower lobe of the lung (yellow arrow) and lesions in segment 2 and 7 of the liver (red arrows). (b) After rituximab, cyclophosphamide, etoposide, vincristine, prednisone (R-CEOP) showing increase in size of the right lower lobe lesion (yellow arrow) now with central necrosis. Left lobe lesion in the liver resolved, but right lobe lesion remains. (c) After chimeric antigen receptor (CAR)-T cell therapy showing resolution of right lower lobe lesion with reduction in size of parahilar lesion (yellow arrow). Resolution of the liver lesions also noted. (d) Further reduction of the parahilar lesion 5 months post-CAR-T cell therapy (yellow arrow). |
He received two cycles of R-CEOP before presenting to the emergency department (ED) with neutropenic fever and respiratory failure. A bronchoscopy and bronchoalveolar lavage were done to further evaluate the etiology of his respiratory failure. The bronchoscopy revealed a near complete obstruction of the middle lobe bronchus and biopsy confirmed findings consistent with PTLD. Since the patient was now refractory to two lines of PTLD therapy, CAR T-cell therapy was considered. This idea was discussed at a multidisciplinary tumor board which concluded that despite off-label use, CAR T-cell therapy was the next best step to give the patient a chance of curative treatment. Additionally, this plan was brought to and considered by the heart transplant team who recommended a year from transplant pass before CAR T-cell therapy.
In order to allow time for a year to pass and the logistics of preparing therapy, he received two cycles of rituximab plus gemcitabine and oxaliplatin (GemOx) as a bridge to CAR T-cell treatment. The subsequent PET/CT showed further disease progression (Fig. 2b). Tacrolimus was discontinued and prednisone dose was increased 1 week prior to T-cell collection to optimize successful apheresis. Tacrolimus was restarted post apheresis until it was subsequently stopped again during lymphodepletion. Lymphodepletion was initiated with fludarabine and cyclophosphamide (Flu/Cy) prior to infusion of axicabtagene ciloleucel. The total time between apheresis and CAR T-cell infusion was 26 days. On day +3 post CAR T-cell therapy, he developed grade 1 cytokine release syndrome (CRS) with symptoms of fever treated with one dose of tocilizumab. He then developed confusion on day +4 with an immune effector cell-associated neurotoxicity syndrome (ICANS) score of 1, but CT of head, MRI of brain, and electroencephalogram (EEG) had no significant findings. He developed a slight tremor on day +10 and was evaluated by neurology who deemed it to be benign tremor most likely due to hypophosphatemia not CRS or neurotoxicity. He was discharged on day +24 and tacrolimus was restarted on day +30.
The PET/CT on day +100 (Fig. 2c) showed resolution of both hepatic and right lower lobe lesions as well as reduction in right parahilar mass. Subsequent PET/CT (Fig. 2d) 5 months post infusion showed further reduction of the parahilar mass and no new lesions.
| Discussion | ▴Top |
This presentation of a 68-year-old patient status post heart transplant with monomorphic DLBCL subtype PTLD who achieved remission with CAR T-cell therapy is, to the best of our knowledge, one of few examples of this treatment working in an older patient. The details of demographic information, regimen and outcomes of the other reported cases can be found in Table 1 [2, 6-8].
 Click to view | Table 1. Key Clinical Factors in Cases Utilizing CAR T-Cell Therapy in PTLD of Heart Transplant Patients |
When PTLD develops, the treatment strategy depends on pathological categorization as well as transplant type. PTLD can be classified using the 2017 World Health Organization (WHO) criteria and 2022 International Consensus classification (ICC) into four categories: non-destructive, polymorphic, monomorphic (B-cell, T-cell, natural killer (NK)-cell types), and classic Hodgkin lymphoma [9]. Since PTLD is associated with increased immunosuppression, the first-line treatment for most categories includes decreasing immunosuppressive therapy [10]. However, this increases the risk of donor tissue rejections and transplant failure.
Since PTLD often involves B cells, rituximab can be used to target the CD20 antigen when lowering immunosuppression alone is unsuccessful [10]. If decreasing immunosuppression and rituximab alone do not result in remission of PTLD, treatment can be escalated to chemoimmunotherapy. If PTLD is refractory to both single agent rituximab and chemotherapy and the patient is EBV+, EBV-specific cytotoxic T lymphocytes (CTLs) can be considered [11].
CAR T-cell therapy is not FDA approved for PTLD but has been approved for DLBCL [7]. The trials for approval excluded patients on immunosuppressive therapy or prior solid organ transplant, therefore excluding PTLD patients [12]. There is one case of an 18-year-old female receiving CAR T-cell therapy for PTLD after heart transplant and achieving remission [2]. The medical team pursued similar treatment prior to CAR T-cell therapy except that she also received EBV CTLs. CAR T-cell therapy was pursued in our patient due to the biologic similarities of B-cell monomorphic subtype of PTLD to the approved indication of DLBCL. Axicabtagene ciloleucel is one of the CAR T-cell therapies currently approved to target the CD-19 antigen found on B cells, therefore it is plausible that current CAR T-cell therapies will be efficacious in similar B-cell-driven malignancies. Previously, autologous stem cell transplantation was considered a good option for refractory B-cell lymphomas. However, data from the ZUMA-7 trial showed the superiority of CAR T-cell therapy in comparison to standard treatment in terms of overall response rate and complete remission [13]. Additionally, the polatuzumab vedotin, bendamustine and rituximab (Pola-BR), loncastuximab tesirine, and tafasitamab/revlimid regimens were not considered a viable option for this patient due to his preexisting neuropathy [14]. Furthermore, these regimens are not considered curative, and the patient preference was to pursue a curative treatment. At the time of writing, the bispecific T-cell engagers, epcoritamab and glofitamab, have been approved (May 2024) [15]. However, the period of treatment for this patient was before that date.
In the post-transplant setting, one consideration with CAR T-cell therapy is the continuity of the patient’s immunosuppression [8]. A balance must be achieved between decreases in immunosuppression increasing risk of rejection and the current level of immunosuppression increasing chance of CAR T-cell failure. This is especially pertinent when the patient is on tacrolimus, a calcineurin inhibitor that blocks production of interleukin-2 (IL-2) and therefore T-cell proliferation. The key moments for changes in immunosuppressive regimens are prior to apheresis to ensure sufficient cells are collected for manufacturing, and during infusion back into the patient to ensure the CAR T-cell function does not get affected by immunosuppressants. To mitigate the risk of CAR T-cell failure in our case, tacrolimus was stopped 1 week before apheresis and then restarted 30 days post-infusion. These considerations were explained to the patient before treatment proceeded, and despite these risks the patient responded well to treatment and achieved remission.
Conclusion
With this example of CAR T-cell therapy being successful in an older patient with PTLD post heart transplant, we hope our case adds to the sparse literature about the use of CAR T-cell therapy post solid organ transplant and highlights the safety and feasibility of this treatment. Multidisciplinary care is of utmost importance to guarantee a successful administration of this potential curative approach. As there are limited guidelines on how to manage immunosuppression changes, this is an area to explore with future research.
Acknowledgments
None to declare.
Financial Disclosure
This case report was not funded.
Conflict of Interest
All authors have no conflict of interest.
Informed Consent
Informed consent has been obtained for treatment using CAR T-cell therapy, including that this use would be considered off label and did not have specific FDA approval. Patient has the same pathology for which CAR T-cell therapy was approved for.
Author Contributions
Yazan Samhouri MD had full access to all the data and analysis in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. Concept and design: Yazan Samhouri MD. Drafting of the manuscript: all authors. Critical revision of the manuscript: all authors. Administrative and technical support: Yazan Samhouri MD. Supervision: Yazan Samhouri MD.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PTLD: post-transplant lymphoproliferative disease; EBV: Ebstein-Barr virus; CAR: chimeric antigen receptor; B-ALL: B-cell acute lymphoblastic leukemia; DLBCL: diffuse large B-cell lymphoma; R-CEOP: rituximab, cyclophosphamide, etoposide, vincristine, prednisone; GemOx: gemcitabine-oxaliplatin; EBV CTLs: Epstein-Barr virus cytotoxic T lymphocytes; Axi-cel: axicabtagene ciloleucel; Brexu-cel: brexucabtagene autoleucel; Lis-cel: lisocabtagene maraleucel
| References | ▴Top |
- Katz-Greenberg G, Ghimire S, Zhan T, Mallari K, Whitaker-Menezes D, Gong J, Uppal G, et al. Post-transplant lymphoproliferative disorders (PTLD)-from clinical to metabolic profiles-a single center experience and review of literature. Am J Cancer Res. 2021;11(9):4624-4637.
pubmed pmc - Dang BN, Ch'ng J, Russell M, Cheng JC, Moore TB, Alejos JC. Treatment of post-transplant lymphoproliferative disorder (PTLD) in a heart transplant recipient with chimeric antigen receptor T-cell therapy. Pediatr Transplant. 2021;25(5):e13861.
doi pubmed - Kotton CN, Huprikar S, Kumar D. Transplant infectious diseases: a review of the scientific registry of transplant recipients published data. Am J Transplant. 2017;17(6):1439-1446.
doi pubmed - Asleh R, Alnsasra H, Habermann TM, Briasoulis A, Kushwaha SS. Post-transplant lymphoproliferative disorder following cardiac transplantation. Front Cardiovasc Med. 2022;9:787975.
doi pubmed pmc - U.S. Food & Drug Administration. FDA approves CAR T-cell therapy to treat adults with certain types of large B-cell lymphoma. Fda.gov. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma.
- Oren D, DeFilippis EM, Lotan D, Clerkin KJ, Fried J, Reshef R, Fernandez H, et al. Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD. JACC CardioOncol. 2022;4(5):713-716.
doi pubmed pmc - McKenna M, Epperla N, Ghobadi A, Liu J, Lazaryan A, Ibrahim U, Jacobson CA, et al. Real-world evidence of the safety and survival with CD19 CAR-T cell therapy for relapsed/refractory solid organ transplant-related PTLD. Br J Haematol. 2023;202(2):248-255.
doi pubmed - Krishnamoorthy S, Ghobadi A, Santos RD, Schilling JD, Malone AF, Murad H, Bartlett NL, et al. CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder. Am J Transplant. 2021;21(2):809-814.
doi pubmed - Liu Y, Wang BC, Zuppan CW, Chau P, Fitts J, Chinnock R, Wang J. Relationship of post-transplant lymphoproliferative disorders (PTLD) subtypes and clinical outcome in pediatric heart transplant recipients: a retrospective single institutional analysis/experience of 558 patients. Cancers (Basel). 2023;15(3):976.
doi pubmed pmc - Shah N, Eyre TA, Tucker D, Kassam S, Parmar J, Featherstone C, Andrews P, et al. Front-line management of post-transplantation lymphoproliferative disorder in adult solid organ recipient patients - A British Society for Haematology Guideline. Br J Haematol. 2021;193(4):727-740.
doi pubmed - Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29-44.
doi pubmed - Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544.
doi pubmed pmc - Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654.
doi pubmed - Sehn LH, Hertzberg M, Opat S, Herrera AF, Assouline S, Flowers CR, Kim TM, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543.
doi pubmed pmc - U.S. Food & Drug Administration. FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma. Fda.gov. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-epcoritamab-bysp-relapsed-or-refractory-diffuse-large-b-cell.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


