| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 6, December 2023, pages 287-293
Cryocrystalglobulinemia Leading to Multi-Organ Failure in Chronic Lymphocytic Leukemia Achieving Complete Renal Recovery
Ashley Duntona, c , Shivi Jainb
aDepartment of Internal Medicine, Rush University Medical Center, Chicago, IL 60612, USA
bDepartment of Internal Medicine, Division of Hematology, Oncology, and Cellular Therapy, Rush University Medical Center, Chicago, IL 60612, USA
cCorresponding Author: Ashley Dunton, Department of Internal Medicine, Rush University Medical Center, Chicago, IL 60612, USA
Manuscript submitted October 30, 2023, accepted December 7, 2023, published online December 28, 2023
Short title: CCG Leading to Organ Failure in CLL
doi: https://doi.org/10.14740/jh1212
| Abstract | ▴Top |
Cryocrystalglobulinemia (CCG) is a rare and fatal subset of type I cryoglobulinemia that is classically associated with an underlying monoclonal gammopathy. Cryocrystalglobulins are created when immunoglobulins self-assemble into extracellular crystal arrays, which often leads to severe systemic hypoperfusion and occlusive vasculopathy that culminates in multi-organ failure. Most commonly, the resultant ischemia manifests as cutaneous lesions and renal insufficiency, which can progress to fulminant kidney failure requiring renal replacement therapy. CCG is commonly associated with lymphoproliferative disorders and is most frequently reported in the literature in context of plasma cell dyscrasias with minimal cases describing CCG secondary to other types of lymphoid neoplasms, especially those that attain complete organ recovery. We report a unique case of a patient who presented with multi-organ failure, including cryoglobulinemic glomerulonephritis (CryoGN) consistent with monoclonal gammopathy of renal significance (MGRS), who was found to have type I IgG kappa CCG due to chronic lymphocytic leukemia (CLL). With the assistance of plasmapheresis, hemodialysis, and clone-directed therapy, the patient achieved complete renal recovery. We highlight this uncommon entity to emphasize the clinical importance of early diagnosis and timely treatment given CCG’s significant morbidity and mortality.
Keywords: Cryocrystalglobulinemia; Cryoglobulins; Crystals; Chronic lymphocytic leukemia; Monoclonal gammopathy; Monoclonal gammopathy of renal significance; Renal recovery
| Introduction | ▴Top |
Cryoglobulins are immunoglobulins (proteins) that precipitate at cold temperatures (< 98.6 °F, < 37 °C). There are three types of cryoglobulins according to the Brouet classification [1]. Type I typically involves monoclonal immunoglobulins (MIg) (i.e., IgM, IgG, IgA) and is associated with lymphoproliferative disorders such as multiple myeloma, Waldenstrom macroglobulinemia, and chronic lymphocytic leukemia (CLL). Cryocrystalglobulinemia (CCG) is a rare variant of type I cryoglobulinemia in which MIg spontaneously self-assemble into extracellular crystalline arrays. These structures are characteristically eosinophilic, periodic acid-Schiff positive, and demonstrate non-birefringence under polarized light [2]. Cryoglobulins in the form of extracellular crystals are rarely reported in the literature and often confer a poor prognosis [3]. Unlike mixed cryoglobulinemias (types II and III) that involve complement-mediated inflammation leading to vasculitis, the deposition of crystallized paraproteins within vessels, as seen in CCG, induces ischemic hypoperfusion and an occlusive vasculopathy that culminates in multi-organ failure, often fatal. Most commonly, the resultant ischemia manifests as cutaneous lesions (such as purpura, livedo reticularis, ulcers, gangrene) and renal vasculopathy that can progress to fulminant kidney failure requiring renal replacement therapy [2]. CCG is most frequently reported in the literature in the context of plasma cell dyscrasias such as multiple myeloma and monoclonal gammopathy of unknown significance (MGUS), with little to no cases describing CCG secondary to other types of lymphoid neoplasms, such as CLL [4-6].
MIg produced by clonal proliferative B-cell and plasma cell disorders are highly nephrotoxic and often lead to renal impairment. Monoclonal gammopathy of renal significance (MGRS) is a highly heterogenous group of disorders categorized by the presence of MIg deposits in varying areas of the kidney. While MGRS includes monoclonal gammopathies with low tumor burden, in 2017 the International Kidney and Monoclonal Gammopathy Research Group (IKMG) updated the diagnosis to include patients with smoldering multiple myeloma and indolent lymphomas. MIg deposits are illustrated histologically on renal biopsy. A biopsy should be considered in patients with monoclonal gammopathy and renal impairment of unknown etiology. For suspected MGRS, a kidney biopsy should include immunofluorescence (IF) and electron microscopic (EM) studies to characterize the composition and organization of monoclonal deposits. MGRS is not restricted to plasma cell dyscrasias and has been reported in monoclonal protein-producing B-cell lymphomas and CLL [4]. Type I cryoglobulinemia is a rarer subtype of MGRS and typically manifests histologically as membranoproliferative glomerulonephritis with glomerular thrombi and microtubular deposits composed of monoclonal cryoglobulin. Renal manifestations are more common with IgG type I cryoglobulin than IgM variants [7, 8].
Management of CCG involves targeting the nephrotoxic MIg-producing clone involved in creating crystallized cryoglobulins with an objective to preserve renal function. This is accomplished by clone-directed therapy, aimed at the underling source of paraproteinemia, and is the mainstay of treatment of CCG secondary to a lymphoproliferative disorder. Similarly, the most effective treatment of MGRS and its associated monoclonal gammopathy is aimed at suppressing immunoglobulin production via clone-directed therapies with removal of serum proteins that can cause permanent kidney damage. Clearance of serum cryoglobulins is accomplished by plasma exchange with or without anticoagulation and is an indication for patients with acute vasculopathy prior to the initiation of clone-directed therapy [7, 9]. Because of the high risk of recurrence, patients with MGRS-related kidney diseases often require life-long hemodialysis for end-stage renal disease (ESRD) [10]. The initiation of clone-directed therapy is associated with favorable outcomes in patients with cryoglobulin glomerulonephritis (CryoGN) due to an underlying hematological condition [11]. First-line treatment regimens include bortezomib, rituximab, cyclophosphamide, and/or corticosteroids, which should be selected based on the underlying clonal process and degree of renal impairment [4, 7].
| Case Report | ▴Top |
Investigations
A 64-year-old man with a history of heart failure with preserved ejection fraction and hypertension presented with progressive dyspnea. Initial labs revealed worsening renal function, a serum creatinine level of 2.1 mg/dL (patient’s baseline: about 1.0 mg/dL), and pancytopenia (white blood cell count (WBC): 3,300/µL, hemoglobin: 7.8 g/dL, platelets: 118,000/µL). His lymphocyte count was low at 500/µL (reference range: 720 - 5,200/µL), which was suspected to be secondary to chronic prednisone use. He was also found to have an IgG kappa monoclonal gammopathy; quantification yielded 0.4 g/dL (reference value: 0 g/dL) with a kappa light chain level of 16.96 mg/dL (normal: 0.33 - 1.94 mg/dL). He also had low serum total complement levels (CH50 < 16, reference value: > 41 U/mL). Urine studies revealed proteinuria and hematuria. An echocardiogram showed a newly reduced left ventricular ejection fraction (LVEF) of 20-25% (previously 50%) and severe diffuse hypokinesis. Cardiac stress testing revealed a moderate-sized, severe defect in the basal to mid inferior wall consistent with infarct and peri-infarct ischemia. He was also intermittently in atrial fibrillation with rapid ventricular response.
During this hospitalization, the patient was noted to have worsening ischemia of his right foot (Fig. 1) and left second and third digits (Fig. 2). Of note, he had previous amputations of his left fourth and fifth fingers due to a work-related injury. A skin biopsy of his right foot showed multiple fibrin microthrombi occluding medium-sized dermal blood vessels consistent with thrombotic vasculopathy. Ultrasound of the right lower extremity confirmed deep vein thrombosis (DVT) in the proximal peroneal, posterior tibial, and gastrocnemius veins. A computed tomography (CT) angiogram of the chest revealed a subacute pulmonary embolism (PE) in the right upper lobe. The patient was started on therapeutic heparin and transferred to our institution for further management.
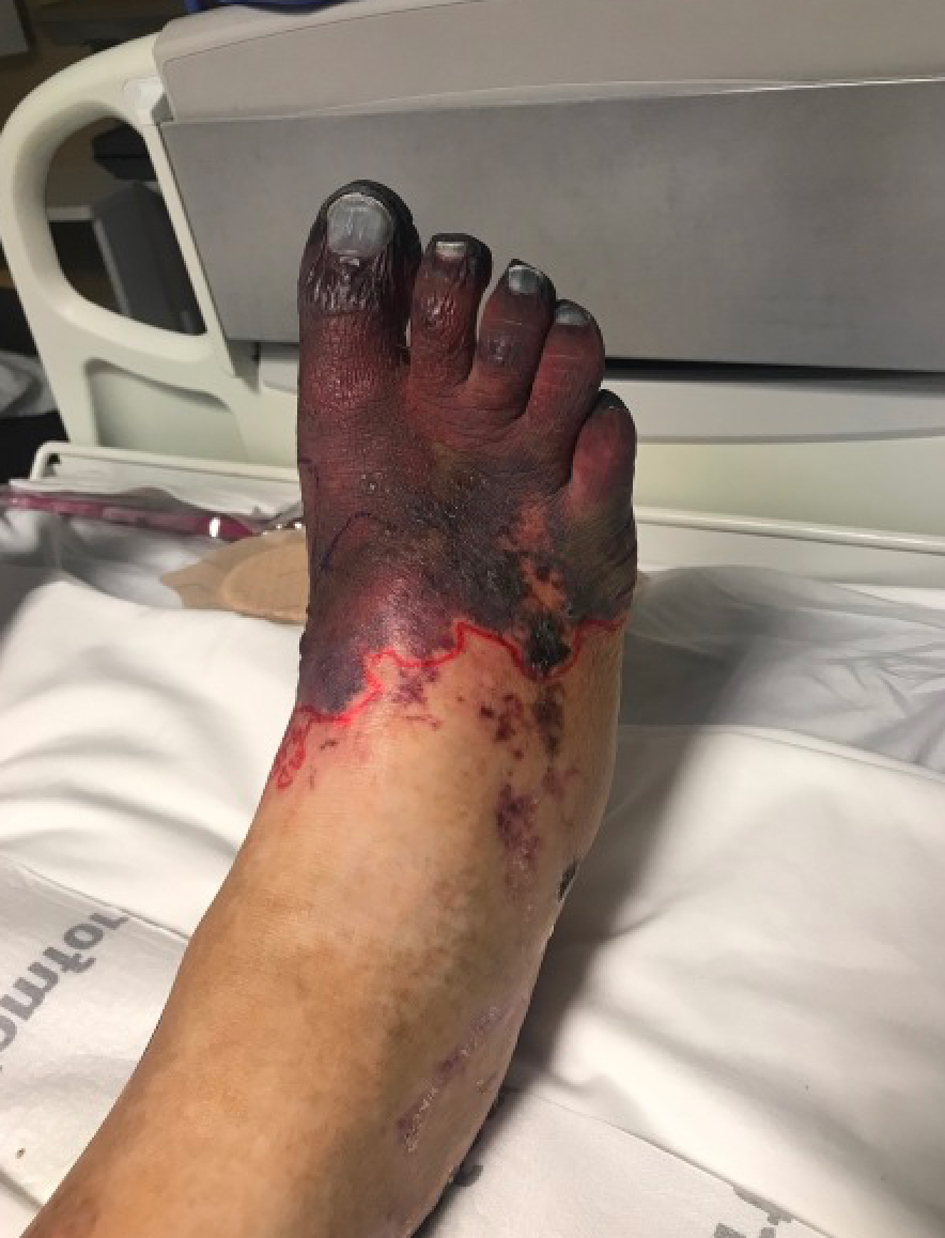 Click for large image | Figure 1. Marked gangrene (red) of the right foot. |
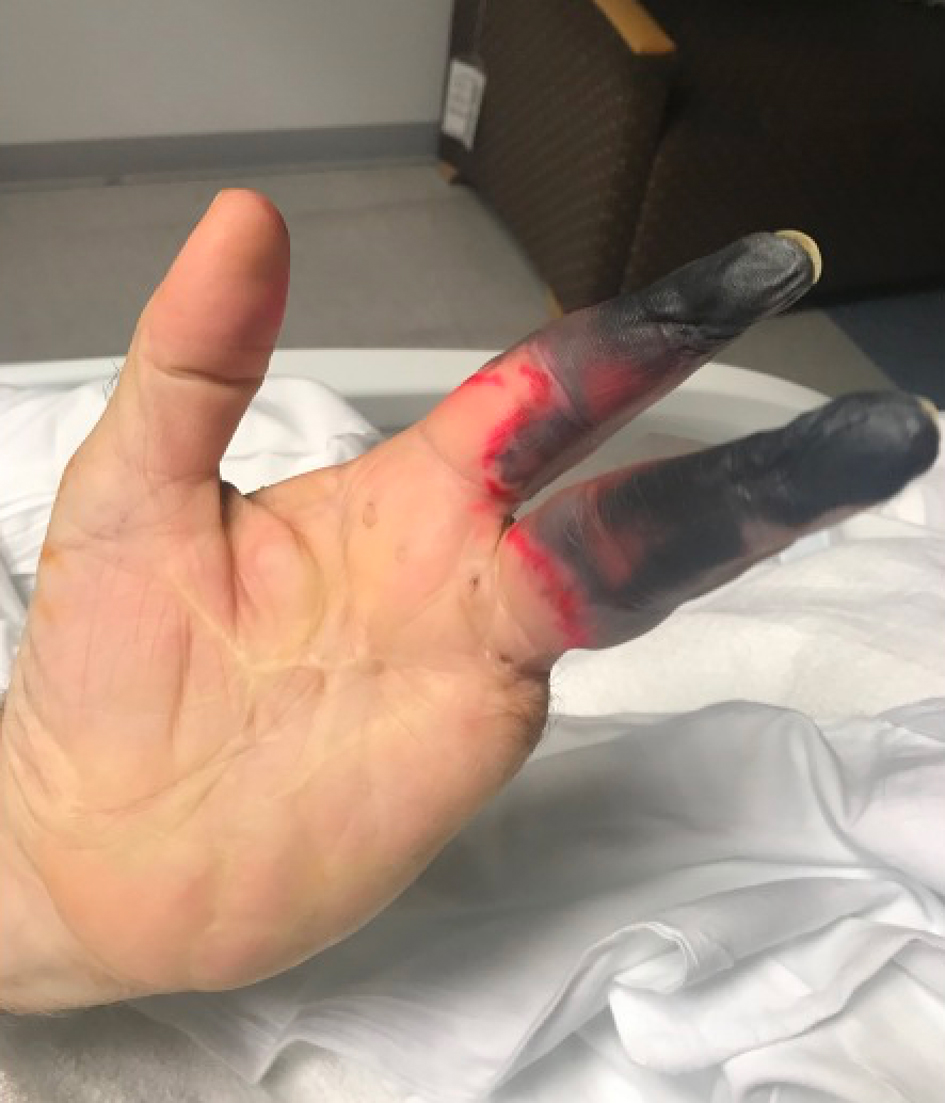 Click for large image | Figure 2. Marked gangrene (red) of the left second and third digits. |
Diagnosis
Due to progressive renal failure, the patient ultimately required hemodialysis and underwent a renal biopsy. This demonstrated a membranoproliferative glomerulonephritis pattern of injury with intracapillary pseudothrombi and focal arteriolar thrombosis that stained positive for IgG and kappa on light microscopy (Figs. 3 and 4). Ultrastructurally, global subendothelial and intraluminal electron-dense deposits were with phagocytosed deposits. Many of these glomerular deposits displayed lattice-like substructures and some formed crystals in rod and rectangular shapes (Figs. 5 and 6). Together, these findings were suggestive of IgG kappa crystal cryoglobulinemic glomerulonephritis (CryoGN) type I with secondary vascular thrombosis consistent with an MGRS.
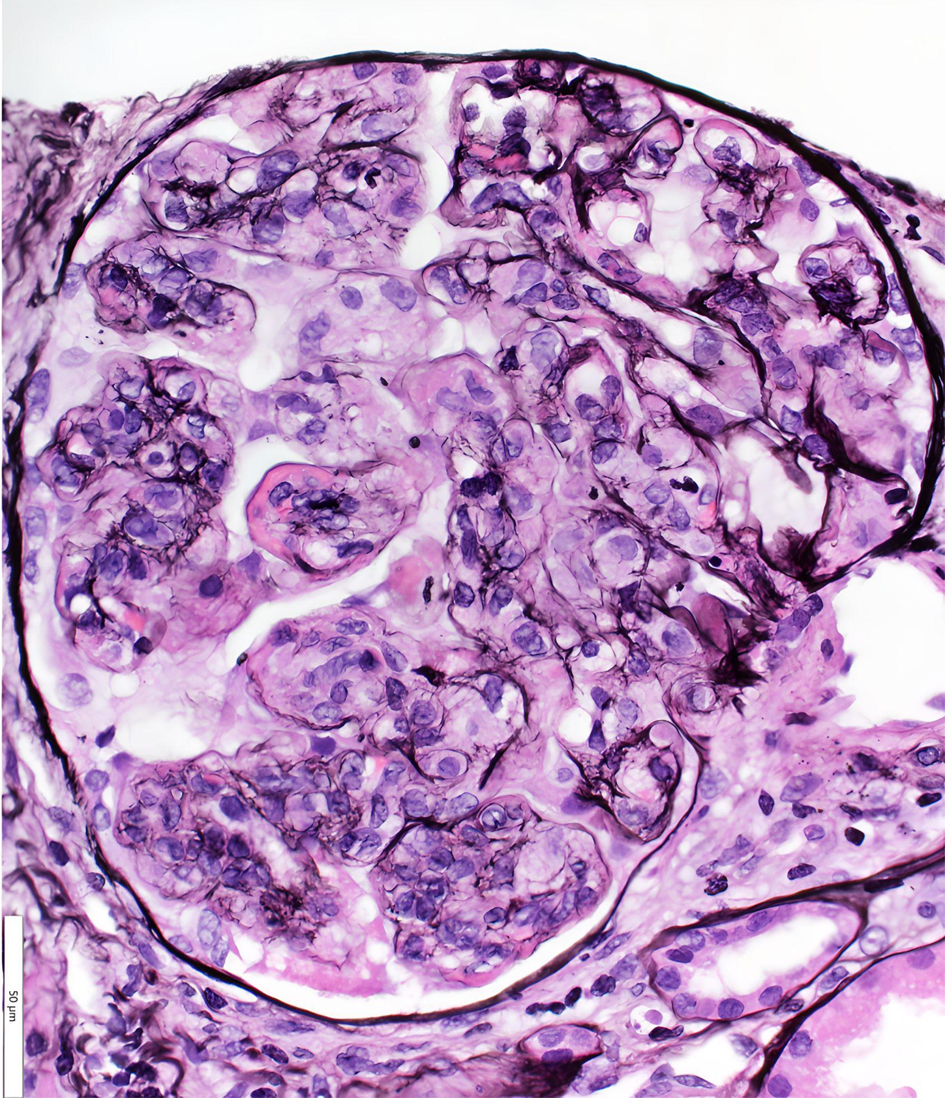 Click for large image | Figure 3. Light microscopy (hematoxylin and eosin stain): glomerulus with monocyte-rich, mesangial expansion and increase in matrix and cellularity consistent with membranoproliferative glomerulonephritis pattern of injury. |
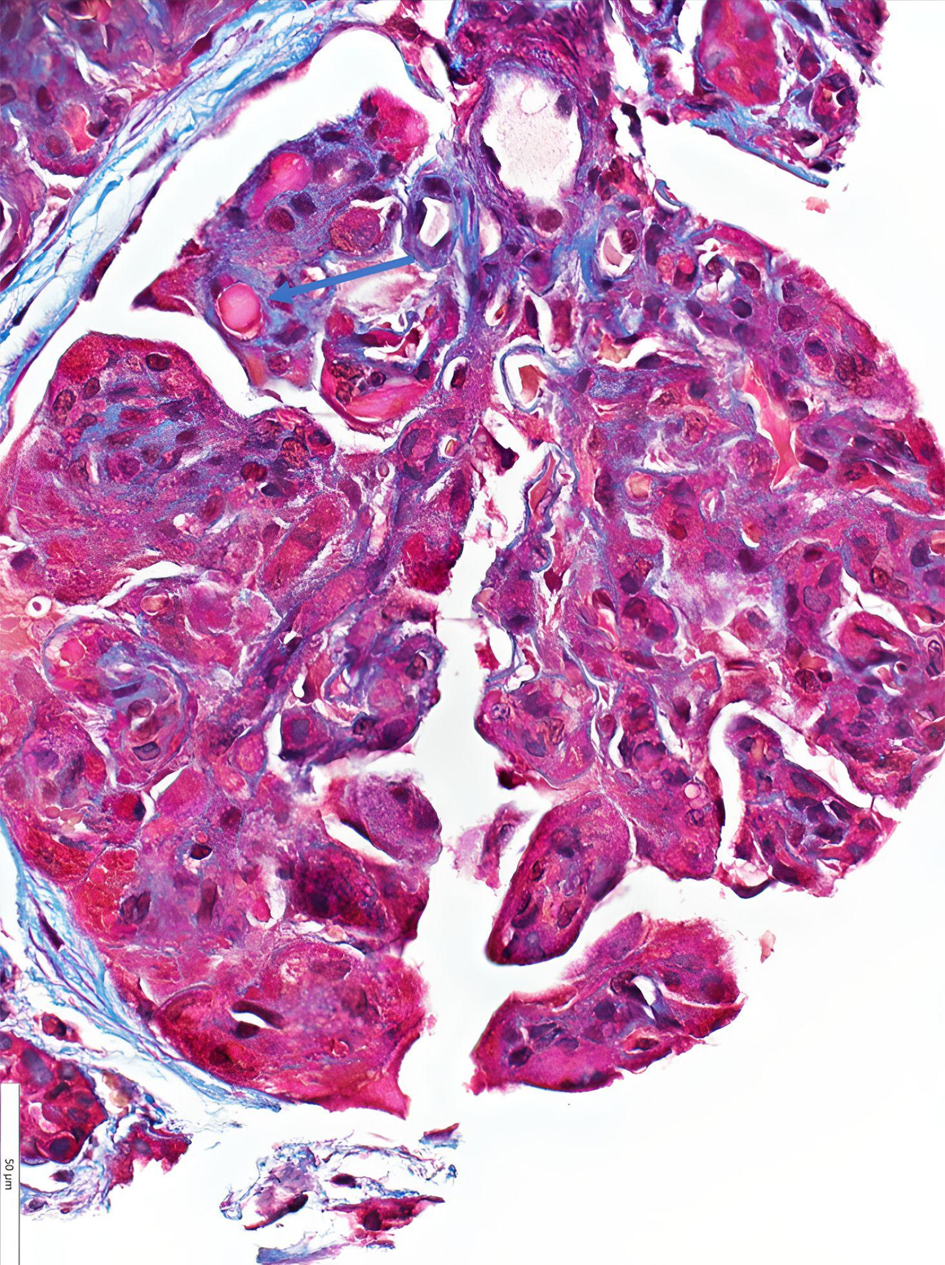 Click for large image | Figure 4. Light microscopy (hematoxylin and eosin stain): immune type/hyaline microthrombi denoted with blue arrow. |
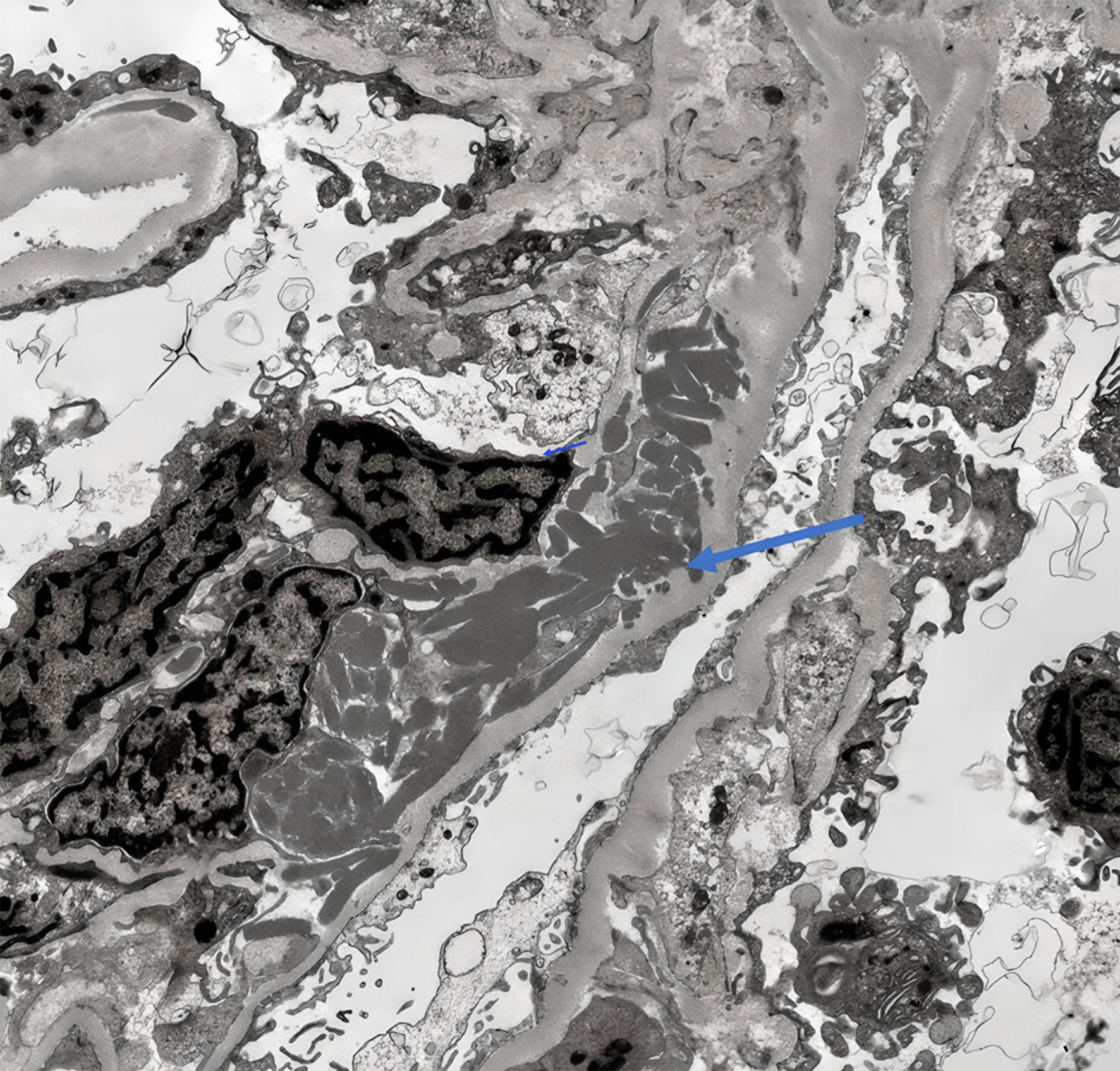 Click for large image | Figure 5. Electron microscopy showing subendothelial electron dense deposits denoted with blue arrow. |
 Click for large image | Figure 6. Electron microscopy showing electron-dense deposits forming crystals at higher power denoted with blue arrows. |
Further serological evaluation was unrevealing including an autoimmune panel (anti-nuclear antibody, C3 and C4 levels, rheumatoid factor), anti-phospholipid antibody panel, lupus anticoagulant, cold agglutinin titer, human immunodeficiency virus (HIV), and hepatitis C. While his serum cryoglobulin was initially negative at the outside hospital, repeat serum cryoglobulin at our institution later returned positive at 5% (reference value: 0%). Peripheral blood flow cytometry demonstrated a kappa-restricted monoclonal B-cell population expressing CD19, CD5, and CD38 but negative for CD10, raising the concern of CLL. Bone marrow biopsy revealed nodular and interstitial lymphocytic aggregates composed of small lymphocytes highlighted by CD20 and negative for cyclin D1. Also noted were plasmacytoid lymphocytes and increased CD138-positive cells raising the concern for lymphoplasmacytic lymphoma; however, the specimen was negative for MYD88 mutation. Immunophenotyping showed a monoclonal B-cell population with kappa restriction and expression of CD5, CD19, dim CD20, CD22, partial CD23, CD38, and CD200. Review by our institution’s hematopathology confirmed mature B-cell lymphoma with a CLL phenotype comprising 30% of marrow cellularity. An extension study to assess lymph node burden was not performed. The diagnosis of type I crystal cryoglobulinemia secondary to IgG kappa monoclonal gammopathy in the context of underlying CLL was made.
Treatment and outcomes
The patient was treated with heparin, high-dose prednisone, and frequent plasmapheresis without further vascular complications. He underwent a right below the knee amputation and amputation of his left gangrenous digits. He received 10 sessions of plasmapheresis while admitted and continued receiving weekly sessions until acalabrutinib was initiated for CLL treatment. Repeat analysis of serum cryoglobulin was negative (0%). After approximately 6 months of clone-directed therapy with acalabrutinib and a prolonged prednisone taper, the patient achieved complete renal recovery with his serum creatinine returning to his baseline of 0.98 mg/dL. He remains on acalabrutinib.
Given the patient’s clinical instability and acute renal failure, further cardiac evaluation with percutaneous coronary intervention was deferred during hospitalization. Subsequent cardiac magnetic resonance imaging (MRI) showed global left ventricular hypokinesis with reduced biventricular systolic function, but no evidence to suggest infarct or fibrosis. His cardiomyopathy was thought to be non-ischemic and multi-factorial, possibly stress-induced (Takotsubo) or secondary to vascular/myocardial inflammation in context of CCG with a component of tachyarrhythmia. However, his cardiac function did not improve on repeat echocardiogram almost 1 year after hospitalization which demonstrated an LVEF of 20-25% and moderate diffuse hypokinesis. He was started on goal-directed medical therapy and had an inferior vena cava filter placed.
| Discussion | ▴Top |
Difficulty in diagnosis
Confirming the diagnosis of CCG may yield difficulties. Unlike general cryoglobulinemia, most cases of CCG do not have circulating cryoglobulin [4]. In fact, as demonstrated in our case, the patient initially had negative serum cryoglobulin at the outside institution despite the presence of ischemia, multi-organ dysfunction, and other clinical manifestations of CCG. Accordingly, in patients with CryoGN, seronegative cases account for 25% of the cases despite renal biopsy findings and are mostly kidney-limited diseases. Cryoglobulins can be negative in the presence of biopsy-confirmed cryoglobulinemia for multiple reasons including unintended cooling of the sample prior to analysis, low serum concentration, cyclical variation, or preferential precipitation in glomeruli with high dilution in the serum [12]. In our case, plasmapheresis may have impacted initial cryoglobulin detection. This underscores the importance of relying on clinical suspicion for timely diagnosis of CCG despite negative test results.
Our patient’s renal biopsy demonstrated crystal cryoglobulinemic glomerulonephritis type I (IgG kappa) with secondary vascular thrombosis consistent with an MGRS. Of note, the lattice-like structures and extracellular crystals (in rod and rectangular shapes) confirmed the presence of crystals. As seen in our case, patients with CCG may undergo extensive autoimmune and inflammatory serological evaluations, even biopsies, before a diagnosis is confirmed.
While type I cryoglobulinemia is known to be associated with lymphoproliferative disorders, association with CLL is a rare phenomenon. The presence of CCG and its resultant ischemic manifestations leading to a new diagnosis of CLL has not been reported in the literature. One paper written by Tang et al described an incidental finding of crystal cryoglobulins on peripheral blood smear of a patient with a history of CLL without clinical manifestations of CCG [13]. Another described a case of non-crystal cryoglobulinemia with CLL [14]. As such, it is difficult to compare these cases from a clinical and biological perspective to establish a common grounding. However, future comparison of these cases in the literature would be of high value.
Organ involvement
Cryoglobulinemic organ damage can occur by two different pathological mechanisms: accumulation of cryoglobulins and autoimmune-mediated vasculitis in small- to medium-sized blood vessels. It is suspected that our patient’s thrombosis (DVTs and PE) and peripheral gangrene are direct consequences of CCG, likely a combination of cryoglobulin accumulation and deposition leading to venous and arterial ischemic hypoperfusion. Yet, his cardiomyopathy was thought to be due to severe systemic inflammation rather than direct vascular ischemia induced by crystallized paraproteins. However, this is difficult to discern given cardiac catherization was not performed.
Controlling the clones
Definitive treatment of CCG secondary to a lymphoproliferative disorder involves directly targeting B-cell clonal proliferation to reduce the serum MIg level [10]. In our case, this was accomplished via acalabrutinib therapy. Correspondingly, treatment of MGRS-related kidney diseases secondary to monoclonal gammopathy is often mandatory and sometimes urgent to prevent renal deterioration and progression to ESRD. Recovery of renal function is possible with adequate hematological response. The literature suggests that a very good partial response (defined as a difference between involved and uninvolved free light chain < 4 mg/dL or > 90% reduction of the involved free light chain) is the minimum hematological response required for preservation of renal function, ultimately preventing progression to ESRD. Renal response to clone-directed therapies often occurs secondary to, and is further augmented by, achieving extremely strong hematological responses [12]. Yet, relapse with or without extrarenal manifestations should be anticipated. Even with plasmapheresis and clone-directed therapy, cases of CCG with renal involvement often have poor outcomes including eventual relapse and death [4, 14].
Conclusions
Definitive management of CCG secondary to a lymphoproliferative disorder and MGRS involves clone-directed therapy targeted at the underlying monoclonal gammopathy with an objective to preserve renal function. Plasmapheresis is used in acute systemic vasculopathy as a temporizing measure before clone-directed therapy is implemented. Even with adequate treatment, CCG with renal involvement often has poor outcomes with eventual relapse and death. We describe this unique case of CCG in context of CLL with multi-organ involvement, including acute renal failure, that achieves complete renal recovery following introduction of clone-directed therapy. Furthermore, we highlight this uncommon entity to emphasize the importance of early diagnosis with high clinical suspicion and timely treatment to prevent significant morbidly and mortality.
Acknowledgments
We thank Meloney Oliveira, MD and the Rush Department of Nephrology for providing their expertise and renal biopsy images. Seo-Hyun Kim, MD for providing pointers on the case. Kelsey Pandrangi, MD for grammar editing.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Informed Consent
Informed consent for publication from the patient was obtained.
Author Contributions
AD independently composed the manuscript with oversight from SJ, who provided review and guidance on the topic. AD revised the manuscript based on suggestions provided by SJ as well as those provided by Seo-Hyun Kim, Meloney Oliveira, Kelsey Pandrangi as mentioned above.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CCG: cryocrystalglobulinemia; CLL: chronic lymphocytic leukemia; CryoGN: cryoglobulinemic glomerulonephritis; CT: computed tomography; ESRD: end-stage renal disease; DVT: deep vein thrombosis; IKMG: International Kidney and Monoclonal Gammopathy Research Group; LVEF: left ventricular ejection fraction; MGUS: monoclonal gammopathy of unknown significance; MGRS: monoclonal gammopathy of renal significance; MIg: monoclonal immunoglobulins; PE: pulmonary embolism
| References | ▴Top |
- Kolopp-Sarda MN, Miossec P. Cryoglobulins: An update on detection, mechanisms and clinical contribution. Autoimmun Rev. 2018;17(5):457-464.
doi pubmed - Wee CLP, Lim JHL, Lee JSS. Cryocrystalglobulinemia - An uncommon cutaneous presentation of multiple myeloma and novel finding of transepidermal elimination of crystals. Am J Dermatopathol. 2021;43(12):e241-e244.
doi pubmed - Chan NC, Tang SS. Cryoglobulinemia presenting with crystals in peripheral blood. Blood. 2015;126(24):2642.
doi pubmed - Gilmore BA, Rodby RA, Cimbaluk D, Venugopal P, Patel P, Barton K, Hagen P, et al. When monoclonal gammopathy is of renal significance: A case study of crystalglobulinemia from Chicago Multiple Myeloma Rounds. Clin Lymphoma Myeloma Leuk. 2019;19(6):e251-e258.
doi pubmed - Dotten DA, Pruzanski W, Olin J, Brown TC. Cryocrystalglobulinemia. Can Med Assoc J. 1976;114(10):909-912.
pubmed pmc - Prasad B, Chibbar R, Muhammad S, Sethi S. A rare case of crystalglobulinemia. J Onco-Nephrol. 2019;3(1):53-57.
- Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, Drayson MT, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. 2013;122(22):3583-3590.
doi pubmed - Kousios A, Duncan N, Charif R, Tam FWK, Levy J, Cook HT, Pusey CD, et al. Autologous stem cell transplant for the treatment of type I crystal cryoglobulinemic glomerulonephritis caused by monoclonal gammopathy of renal significance (MGRS). Kidney Int Rep. 2019;4(9):1342-1348.
doi pubmed pmc - Papo T, Musset L, Bardin T, Bucki B, Jorgensen C, Dion E, Quillard A, et al. Cryocrystalglobulinemia as a cause of systemic vasculopathy and widespread erosive arthropathy. Arthritis Rheum. 1996;39(2):335-340.
doi pubmed - Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292-4295.
doi pubmed - Leung N, Bridoux F, Nasr SH. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384(20):1931-1941.
doi pubmed - Javaugue V, Valeri AM, Jaffer Sathick I, Said SM, Erdogan Damgard S, Murray DL, Klobucher T, et al. The characteristics of seronegative and seropositive non-hepatitis-associated cryoglobulinemic glomerulonephritis. Kidney Int. 2022;102(2):382-394.
doi pubmed - Tang C, Kumar P, Mackinlay N. Cryoglobulin crystals in a patient with chronic lymphocytic leukaemia. Pathology. 2018;50(6):670-671.
doi pubmed - Arora S, Levitan D, Regmi N, Sidhu G, Gupta R, Nicastri AD, Saggi SJ, et al. Cryoglobulinemia in a patient with chronic lymphocytic leukemia - A case report and review of literature of renal involvement in CLL. Blood Cells Mol Dis. 2016;60:7-11.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


