| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 1-2, April 2024, pages 46-51
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
Chun Li Yanga, Xi Chenb, Hui Jie Zhouc, Wan Chun Wua, Li Qun Zouc, d
aState Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China
bDivision of Radiotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
cDivision of Medical Oncology, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China
dCorresponding Author: Li Qun Zou, Division of Medical Oncology, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Manuscript submitted January 20, 2024, accepted March 25, 2024, published online April 9, 2024
Short title: PD-L1 Antibody in ENKTCL-LAHS
doi: https://doi.org/10.14740/jh1242
| Abstract | ▴Top |
Extranodal natural killer/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis (ENKTCL-LAHS) is a rare disease with poor prognosis. Currently, there are no well-established treatments for LAHS. Almost 50% of patients experience relapsed or refractory disease to anti-hemophagocytic lymphohistiocytosis (HLH) treatment, and the regimen for salvage therapy is limited. We report a case of ENKTCL-LAHS that was successfully treated with a programmed cell death ligand 1 (PD-L1) antibody (sugemalimab) alone and provide a literature review on existing ENKTCL-LAHS treatment options. A 31-year-old man with relapsed ENKTCL complicated by HLH was admitted to our hospital. Following the administration of the PD-L1 antibody sugemalimab, fever was resolved, Epstein-Barr virus (EBV) DNA copy number was negative, and HLH-related blood biochemical markers were decreased in the patient. Consequently, the patient achieved complete remission with a progression-free time (PFS) of 44 months. The prognosis of ENKTCL-LAHS is extremely poor, and the clinical treatment of ENKTCL-HLH is challenging. No previous reports exist regarding the use of PD-L1 antibodies in ENKTCL-LAHS treatment. This study is the first to report a patient with ENKTCL-LAHS treated with the PD-L1 antibody alone, who achieved a long PFS of 44 months. Our results suggest the effectiveness and safety of sugemalimab in the treatment of ENKTCL-LAHS; however, more clinical cases are required for validation. The PD-L1 antibody presents a novel treatment option for patients with ENKTCL-LAHS and warrants further clinical promotion.
Keywords: HLH; LAHS; NK/T lymphoma; PD-L1 antibody; Treatment
| Introduction | ▴Top |
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disease with a median survival of < 2 months when associated with malignancy [1, 2]. It is classified as primary or secondary HLH based on etiology. In 2019, more than 1,445 new cases of HLH were diagnosed in China, among which 13.8% were lymphoma-associated HLH (LAHS) cases [3]. Natural killer (NK)/T-cell lymphoma has been found to be the most common type of LAHS in a global as well as Japanese study [4, 5]. LAHS is currently managed as per the HLH-94 or HLH-2004 protocol [6, 7]. Recently, a prospective study reported that the 1-year overall survival (OS) rate is higher in non-malignancy-associated HLH than in malignancy-associated HLH after HLH-2004 treatment [8], indicating that a large number of malignancy-associated HLH patients experience relapsed or refractory disease after anti-HLH treatment. Programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) antibodies are widely used in cancer treatment, including extranodal natural killer/T-cell lymphoma (ENKTCL) [9, 10]. However, there are limited reports on the application of immunotherapy in ENKTCL-LAHS. Here, we report a case of relapsed/refractory (r/r) ENKTCL-LAHS that was successfully treated with sugemalimab alone, resulting in a durable response of 44 months.
| Case Report | ▴Top |
A 31-year-old man with nasal obstruction and hyperpyrexia for 2 months was admitted to our hospital in May 2018. Clinical examination revealed splenomegaly without superficial lymphadenopathy. Several examinations were performed to aid the diagnosis. The complete blood test results were as follows: hemoglobin: 119 g/L; white blood cells: 2.34 × 109/L (neutrophils: 1.17 × 109/L); and platelets: 57 × 109/L. Blood biochemical test results were as follows: alanine transaminase (ALT): 96 IU/L; aspartate transaminase (AST): 69 IU/L; serum albumin: 34.1 g/L, triglycerides (TG): 4.8 mmol/L; and serum lactate dehydrogenase (LDH): 290 IU/L. Blood cultures under anaerobic and aerobic conditions tested negative at different time points. In addition, bone marrow aspiration and biopsy were performed to exclude hematological disorders, and the results were normal. Finally, nasal cavity biopsy via nasopharyngeal fiberscopy confirmed the diagnosis of ENKTCL, nasal type (Fig. 1). Additionally, the plasma Epstein-Barr virus (EBV) DNA copy number was 1.07 × 104 copies/L. F-18 fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) was used to determine the stage of the condition, revealing the following affected areas: bilateral nasal cavity, left tonsil, lung, and multisite bones (right zygomatic bone, bilateral humeral head, the second cervical vertebra, right collarbone, left scapula, manubrium, ilium, and the fourth sacral vertebrae) (Fig. 2).
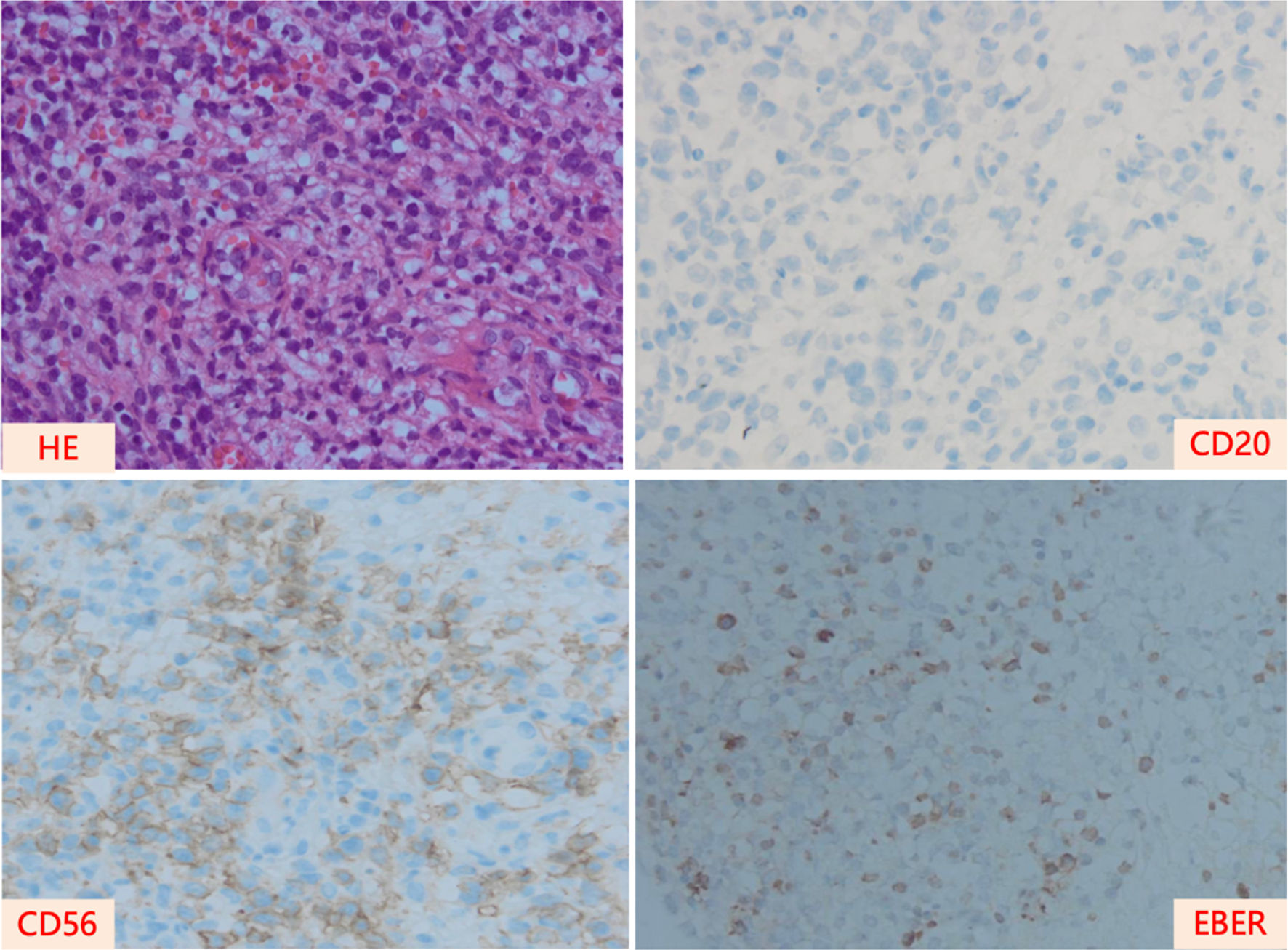 Click for large image | Figure 1. Pathological images of the nasal cavity of the patient at the time of diagnosis. HE: hematoxylin and eosin stain; EBER: EBV-encoded RNA. |
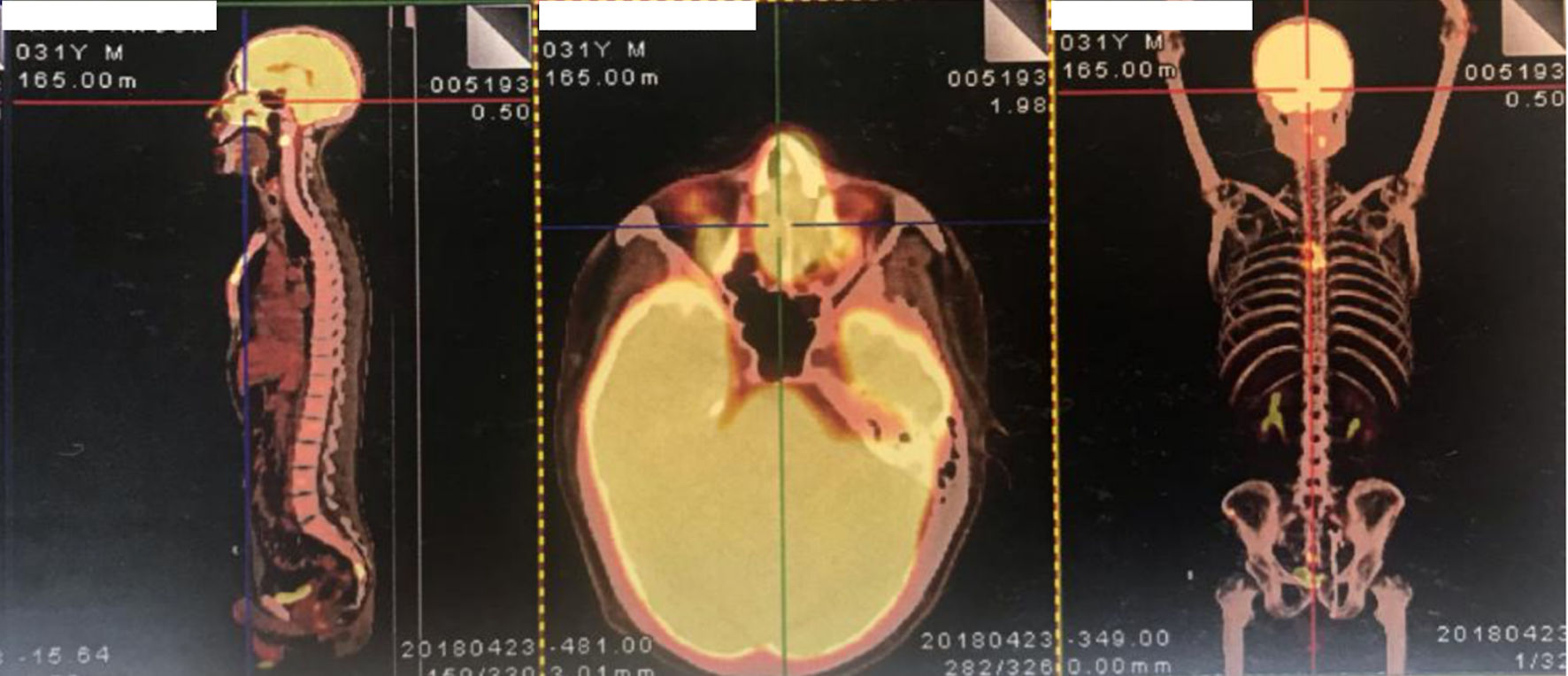 Click for large image | Figure 2. The PET/CT images of the patient at the time of diagnosis. PET/CT: positron emission tomography/computed tomography. |
To determine whether the patient had concurrent HLH, we examined the concentration of several biomarkers in his blood and found that ferritin level exceeded 2,000 ng/mL, peripheral blood NK cell percentage was 12.00%, and serum soluble CD25 (sCD25) was 5,699.0 IU/mL. Based on the above results and according to the HLH-2004 diagnosis criteria, the patient was diagnosed with ENKTCL-LAHS. We first initiated anti-HLH induction treatment according to the HLH-94 protocol. After two cycles, the hyperpyrexia symptoms disappeared, and the peripheral blood platelet count increased to the normal range. Blood biochemical markers (ALT, AST, and TG) also decreased. However, ferritin level was still ≥ 2,000 ng/mL, NK cell percentage was 6.5%, sCD25 was 2,056 IU/mL, and plasma EBV DNA copy number was 14.1 copies/mL, indicating that the treatment provided only a partial response.
Considering the relationship between ENKTCL and HLH, ifosfamide was administered to control lymphoma following cycles 3 and 4 of induction treatment. Subsequently, intravenous methotrexate (MTX) was administered to treat the lymphoma before cycles 5 and 6. After six cycles of introductory therapy for HLH, the patient achieved a partial response, with ferritin of 1,343 ng/mL, NK cell percentage 1.7%, and sCD25 730 IU/mL. The remaining two cycles were performed after delivering a cycle of the modified SMILE regimen (steroid, methotrexate, ifosfamide, l-asparaginase, and etoposide), resulting in a complete response to ENKTCL, as evidenced by PET-CT evaluation and undetectable EBV DNA copies; however, HLH was not completely treated, as intermittent fever continued to appear. Therefore, we initiated salvage treatment for HLH using the DEP regimen (docetaxel, epirubicin, and cisplatin) and continued the modified SMILE regimen. After two cycles of DEP and one cycle of SMILE, the patient’s fever persisted, and PET-CT showed lymphoma relapse. Additionally, EBV DNA copies, TG, and sCD25 were increased, indicating refractory HLH and ENKTCL. Subsequently, we recommended the patient to participate in a clinical trial evaluating the efficacy of CS1001 (a PD-L1 antibody, sugemalimab) monotherapy for relapsed or refractory ENKTCL. After four cycles of infusion, the patient showed no fever, tested negative for EBV DNA, and had decreased HLH-related markers, achieving a complete response for ENKTCL and HLH and a progression-free survival (PFS) of 44 months (Fig. 3).
 Click for large image | Figure 3. Treatment timeline of the patient. ENKTCL: extranodal natural killer/T-cell lymphoma; HLH: hemophagocytic lymphohistiocytosis; PR: partial response; CR: complete response; MTX: methotrexate; DEP regimen: docetaxel, epirubicin, and cisplatin; q3w: every three weeks; SMILE regimen: steroid, methotrexate, ifosfamide, l-asparaginase, and etoposide; PD: progression of disease; PD-L1: programmed cell death ligand 1. |
| Discussion | ▴Top |
ENTCL-LAHS is a rare disease. In our center, 13.3% of 533 ENKTCL cases have been identified as ENKTCL-LAHS [11], which is higher than previous reports [12, 13]. Additionally, most patients with LAHS are male, and the onset is concurrent with lymphoma at diagnosis [3], which were also observed in our present case. The prognosis of ENKTCL-LAHS is extremely poor and inferior to that of HLH [11]. In addition, there is a lack of effective biomarkers for evaluating the risk of ENKTCL-LAHS. Wen et al found that ECSIT V140A triggers the promotion of ENKTCL-LAHS [14]. In our case, we did not investigate the presence of any gene mutation because the HLH diagnosis was definite according to the HLH-2004 criteria.
ENKTCL-HLH treatment is clinically challenging. In LAHS, lymphoma is the cause of HLH, which progresses rapidly; however, the effective balance of treatment between lymphoma and HLH remains unclear. Song et al reported that etoposide-containing regimens should be considered in the initial therapy for LAHS, regardless of HLH-direct or lymphoma-direct treatment [15]. In the present case, we attempted to use a cross-treatment strategy to manage both HLH and lymphoma; induction therapy for HLH was administered in the first two cycles. Consequently, the patient achieved a complete response for lymphoma and a partial response for HLH. At present, HLH is treated with the HLH-94 or HLH-2004 regimen. HLH-94 was established based on a study involving mostly primary pediatric HLH cases with a 5-year OS rate of 54% [7]; however, the efficacy of these treatment regimens in HLH secondary to lymphoma is still uncertain.
Some studies have advocated a different and more targeted management approach for patients with secondary HLH; however, these were only case or case series reports, which are summarized in Table 1 [16-21]. In addition, Liu et al reported that programmed cell death protein 1 (PD-1) antibody alone could effectively control r/r EBV-associated HLH in adults, leading to 71.4% (5/7) of cases achieving CR and 57.1% (4/7) achieving EBV clearance [16]. Our patient achieved a complete remission and durable survival with PD-L1 antibody. Additionally, circulating EBV DNA was undetectable, indicating that PD-L1 antibody is a new approach for r/r ENKTCL-LAHS treatment. However, the potential mechanism underlying PD-L1 antibody treatment in ENKTCL-LAHS remains unclear. In ENKTCL, high PD-L1 expression is observed in most patients, suggesting that the PD-1/PD-L1 pathway plays a crucial role in immune evasion from cytotoxic T lymphocyte (CTL) recognition [22, 23]. In some tumors and chronic viral infections, CD8+ T cell exhaustion, characterized by high expression of PD-1, is observed. Blocking the PD-1/PD-L1 pathway can restore CD8+ T cell proliferation and normalize cytotoxic function, which can reduce the viral load and achieve long-term tumor control. Of note, some studies have reported the adverse effect of cytokine release syndrome with PD-1 antibody treatment in diseases, including HLH [24, 25]. In this case, we observed no severe side effects during treatment. Thus, in clinical practice, immunotherapy (PD-1 and PD-L1) for ENKTCL-LAHS requires caution and risk-benefit evaluation.
 Click to view | Table 1. Summary of the Targeted Drugs Applied in Adult sHLH |
Conclusions
We report a case of ENKTCL-LAHS that was successfully treated with PD-L1 antibody alone and achieved a PFS of 44 months. To the best of our knowledge, this is the first report on LAHS immunotherapy that provides a regimen for r/r ENKTCL-LAHS management. Furthermore, it is crucial to investigate the mechanisms underlying this condition to identify the beneficiary population.
Acknowledgments
We thank the patient for allowing us to share his medical history and clinical course.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Informed Consent
Written informed consent was obtained from the individual for the publication of any potentially identifiable data included in this article.
Author Contributions
Chun Li Yang drafted the manuscript and reviewed the literature. Xi Chen, Hui Jie Zhou, and Wan Chun Wu administered and followed up with the patient. Li Qun Zou supervised this study. All the authors have read and approved the final version of the manuscript.
Data Availability
The original contributions generated in the study are included in the article, and further inquiries can be directed to the corresponding author.
| References | ▴Top |
- Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, Allen CE, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: Relation to hemophagocytosis, characteristics, and outcomes. Cancer. 2016;122(18):2857-2866.
doi pubmed pmc - Setiadi A, Zoref-Lorenz A, Lee CY, Jordan MB, Chen LYC. Malignancy-associated haemophagocytic lymphohistiocytosis. Lancet Haematol. 2022;9(3):e217-e227.
doi pubmed - Posas-Mendoza TF, McLeod C, Davis W, Zakem J, Quinet R. Etiologies and management of haemophagocytic lymphohistiocytosis: is it time for an updated protocol and targeted treatments? Rheumatology (Oxford). 2021;60(6):2927-2933.
doi pubmed - Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516.
doi pubmed - Sano H, Kobayashi R, Tanaka J, Hashino S, Ota S, Torimoto Y, Kakinoki Y, et al. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: a multicentre study. Br J Haematol. 2014;165(6):786-792.
doi pubmed - Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131.
doi pubmed - Trottestam H, Horne A, Arico M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577-4584.
doi pubmed pmc - Yoon SE, Eun Y, Huh K, Chung CR, Yoo IY, Cho J, Cho D, et al. A comprehensive analysis of adult patients with secondary hemophagocytic lymphohistiocytosis: a prospective cohort study. Ann Hematol. 2020;99(9):2095-2104.
doi pubmed - Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437-2442.
doi pubmed - Huang H, Tao R, Hao S, Yang Y, Cen H, Zhou H, Guo Y, et al. Sugemalimab monotherapy for patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (GEMSTONE-201): results from a single-arm, multicenter, phase II study. J Clin Oncol. 2023;41(16):3032-3041.
doi pubmed pmc - Li N, Jiang M, Wu WC, Wei WW, Zou LQ. How to identify patients at high risk of developing nasal-type, extranodal nature killer/T-cell lymphoma-associated hemophagocytic syndrome. Front Oncol. 2021;11:704962.
doi pubmed pmc - Jia J, Song Y, Lin N, Liu W, Ping L, Zheng W, Wang X, et al. Clinical features and survival of extranodal natural killer/T cell lymphoma with and without hemophagocytic syndrome. Ann Hematol. 2016;95(12):2023-2031.
doi pubmed - Li N, Zhang L, Liu J, Zhang J, Weng HW, Zhuo HY, Zou LQ. A clinical study of 21 patients with hemophagocytic syndrome in 295 cases diagnosed with nasal type, extranodal nature killer/T cell lymphoma. Cancer Biol Ther. 2017;18(4):252-256.
doi pubmed pmc - Wen H, Ma H, Cai Q, Lin S, Lei X, He B, Wu S, et al. Recurrent ECSIT mutation encoding V140A triggers hyperinflammation and promotes hemophagocytic syndrome in extranodal NK/T cell lymphoma. Nat Med. 2018;24(2):154-164.
doi pubmed - Song Y, Wang Y, Wang Z. Requirement for etoposide in the initial treatment of Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis. Br J Haematol. 2019;186(5):717-723.
doi pubmed - Liu P, Pan X, Chen C, Niu T, Shuai X, Wang J, Chen X, et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood. 2020;135(11):826-833.
doi pubmed - Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford). 2011;50(2):417-419.
doi pubmed - Keith MP, Pitchford C, Bernstein WB. Treatment of hemophagocytic lymphohistiocytosis with alemtuzumab in systemic lupus erythematosus. J Clin Rheumatol. 2012;18(3):134-137.
doi pubmed - He Y, Gao Y, Ping L, He H, Huang C, Bai B, Wang X, et al. The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis. J Cancer Res Clin Oncol. 2023;149(5):2017-2027.
doi pubmed - Song Y, Li X, He X, Zhou F, Du F, Wang Z, Chen S, et al. Dose-escalating ruxolitinib for refractory hemophagocytic lymphohistiocytosis. Front Immunol. 2023;14:1211655.
doi pubmed pmc - Dufranc E, Del Bello A, Belliere J, Kamar N, Faguer S, TAIDI (Toulouse Acquired Immune Deficiency and Infection) study group. IL6-R blocking with tocilizumab in critically ill patients with hemophagocytic syndrome. Crit Care. 2020;24(1):166.
doi pubmed pmc - Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, et al. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96(1):25-31.
doi pubmed - Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877-890.
doi pubmed - Sackstein P, Zaemes J, Kim C. Pembrolizumab-induced cytokine release syndrome in a patient with metastatic lung adenocarcinoma: a case report. J Immunother Cancer. 2021;9(7):e002855.
doi pubmed pmc - Del Bello A, Zakaroff AG, Meyer N, Delas A, Faguer S, Kamar N, Belliere J. Cytokine storm induced by a PD1 inhibitor in a renal transplant patient. Am J Transplant. 2021;21(7):2616-2618.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


