| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 3, June 2024, pages 94-98
Non-Secretory Multiple Myeloma Associated With High-Risk Phenotype and Complex Cytogenetics Including t(8;22)
Rahim A. Jiwania, g, Joseph R. Liputa, Attah Abrahamb, Khaled Alhamadb, Mukta Kapdib, Renan Motac, Kayla Fortec, John R. McGillc, Jasper C. Acerd, Palgun Nisargae, Nicholas R. Jaegere, Santhosh Sadashivf, Prerna Mewawallaf
aDivision of Hematology and Medical Oncology, Allegheny Health Network, Pittsburgh, PA, USA
bDivision of Internal Medicine, Allegheny Health Network, Pittsburgh, PA, USA
cNeoGenomics Laboratories, Fort Myers, FL, USA
dThe Lake Erie College of Osteopathic Medicine, Seton Hill Campus, Greensburg, PA, USA
eDivision of Pathology and Laboratory Medicine, Allegheny Health Network, Pittsburgh, PA, USA
fDivision of Hematology and Cellular Therapy, Allegheny Health Network, Pittsburgh, PA, USA
gCorresponding Author: Rahim A. Jiwani, Division of Hematology and Medical Oncology, Allegheny Health Network, Pittsburgh, PA, USA
Manuscript submitted February 20, 2024, accepted April 23, 2024, published online June 28, 2024
Short title: NSM Associated With t(8;22)
doi: https://doi.org/10.14740/jh1248
| Abstract | ▴Top |
Multiple myeloma (MM) is a plasma cell dyscrasia which is typically characterized by identifiable paraprotein in the blood or urine. However, the minority of patients in whom paraprotein cannot be identified are designated non-secretory MM (NSM). Evaluation of treatment response is more difficult in these patients as paraprotein levels cannot be followed. A dearth of clinical trials including these patients exists because of an inability to measure response by classical serum and urine measurement mechanisms as well as seemingly decreased overall survival compared to secretory MM. NSM is subdivided into four subgroups: “non-producers”, “true non-secretors”, “oligosecretors” and “false non-secretors”. The “non-producers” phenotype is associated with more aggressive disease course. Translocations such as those involving the proto-oncogene c-MYC (chromosome 8) and the lambda light chain gene IGL (chromosome 22) - more commonly associated with Burkitt lymphoma - are rare in MM. We describe a 60-year-old male with NSM who was identified as having multiple high-risk features including complex cytogenetics and a non-producer phenotype, which are features not considered in conventional MM staging and risk stratification. This case highlights the need for awareness of phenotypes and cytogenetics associated with higher clinical risk that are not included in the revised International Staging System.
Keywords: Multiple myeloma; Cytogenetics; c-MYC
| Introduction | ▴Top |
Multiple myeloma (MM) is a hematologic malignancy defined by clonal proliferation of plasma cells within the bone marrow and constitutes 1-2% of all new cancer diagnoses in the United States each year [1]. In addition to proliferation of monoclonal plasma cells within the bone marrow, MM is typically characterized by the presence of monoclonal immunoglobulins in the serum and/or urine. Either the secreted proteins or the malignant plasma cells can lead to the end-organ damage seen in MM [2]. Quantifiable serum and/or urine monoclonal proteins and/or light chains are used to monitor disease activity and response to treatment. However, approximately 3% of MM patients have malignant cells that do not make or secrete immunoglobulins, lack evidence of immunoglobulin in serum or urine, and are diagnosed as non-secretory multiple myeloma (NSM) [3]. NSM patients may have slightly reduced overall survival (OS) compared to MM patients with secretory disease [4]. Providers must rely on advanced imaging and frequent bone marrow biopsies to determine treatment response, and this would require a restructuring of commonly accepted treatment response criteria. Due to this, there is a scarcity of randomized controlled data describing how NSM patients respond to novel therapeutic options [5]. Certain cytogenetic abnormalities have been identified as harboring a worse prognosis with a more aggressive disease course [6]. The fusion product t(8;22) - rarely seen in MM - may also be related to poor prognosis.
| Case Report | ▴Top |
Investigations
A 60-year-old male with no significant past medical history presented to his primary care physician with gradually worsening left-sided chest pain over multiple weeks. Physical exam was significant for point tenderness over the left anterior lower ribs and was managed with over-the-counter analgesic medications.
Diagnosis
Due to lack of improvement, a chest radiograph was obtained 2 months later which showed a 2.1 × 1.9 cm lytic lesion in the lateral left seventh rib with a small left-sided pleural effusion. Computed tomography (CT) imaging revealed lytic lesions throughout the spine as well as on the left seventh rib and in the upper sternum. Additionally, a bone survey revealed further osseous disease in the proximal left humerus and re-demonstrated known lesions in the axial skeleton. Given the high suspicion for MM, serum and urine free light chain assays and serum protein electrophoresis with immunofixation were obtained which showed no evidence of a monoclonal protein. A 24-h urine electrophoresis and immunofixation was also negative for monoclonal protein. Chemistry panels including calcium, renal and liver function testing were all within normal limits. Complete blood counts showed mild normocytic normochromic anemia with a hemoglobin of 13.1 g/dL.
As the clinical suspicion for MM remained high due to lack of explanation of lytic lesions, a bone marrow biopsy was performed which revealed diffuse involvement by non-light chain restricted atypical plasma cells (Fig. 1).
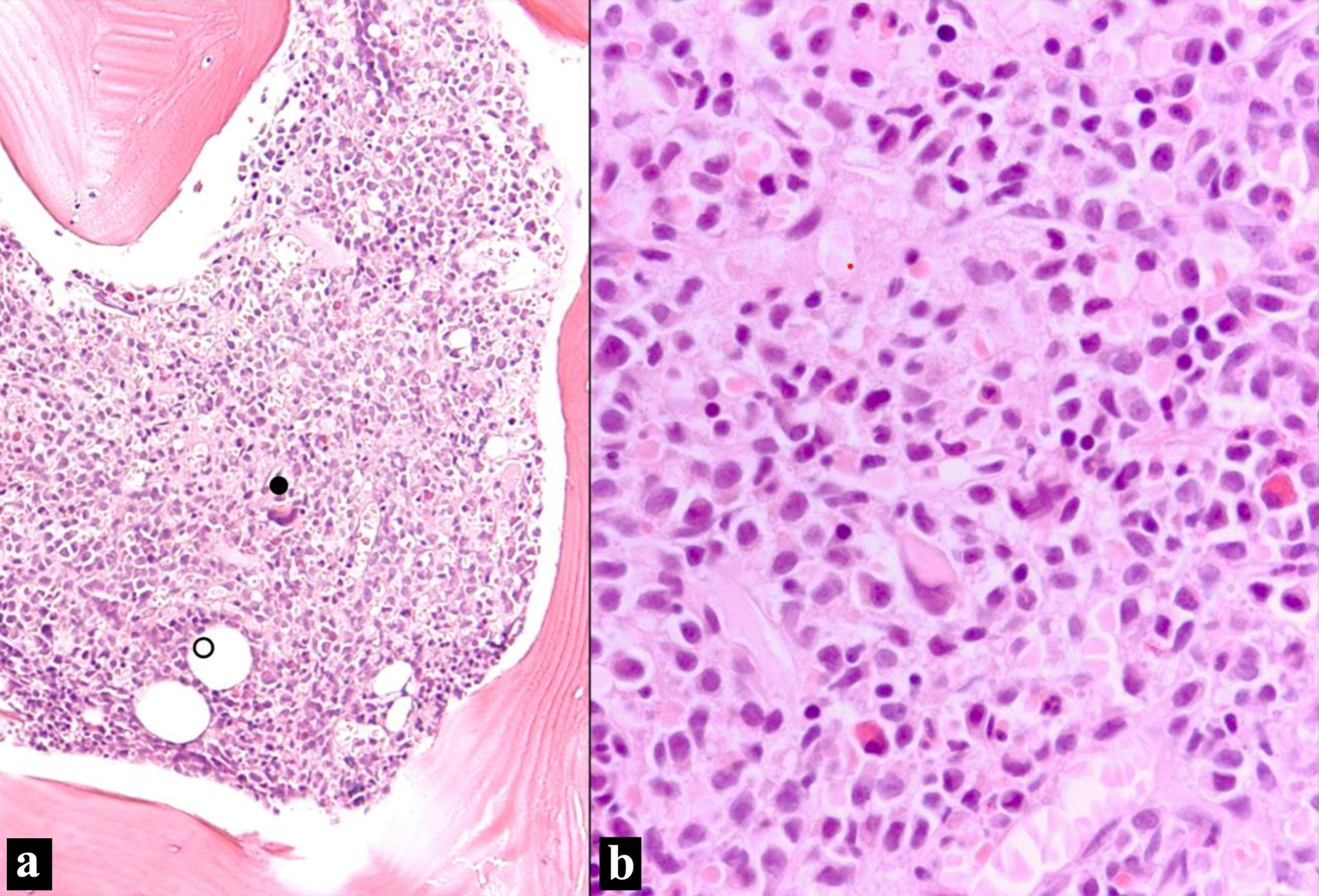 Click for large image | Figure 1. Bone marrow biopsy (H&E stain). (a) Bone marrow intertrabecular space diffusely infiltrated by sheets of non-cohesive cells. Residual bone marrow elements are scattered (• megakaryocyte; ○ adipocytes) (H&E, × 100 optical magnification). (b) Sheet of atypical plasma cells infiltrating with residual bone marrow elements. The plasma cells have eccentric nuclei with mild atypia and moderate eosinophilic cytoplasm with perinuclear hofs (H&E, × 400). H&E: hematoxylin and eosin. |
Flow cytometry revealed a population of CD38 and CD138 positive atypical plasma cells with aberrant expression of CD20 and CD117, without expression of CD19 or CD56. The plasma cells were negative for both surface and cytoplasmic light chain expression, supporting the diagnosis of NSM (Fig. 2).
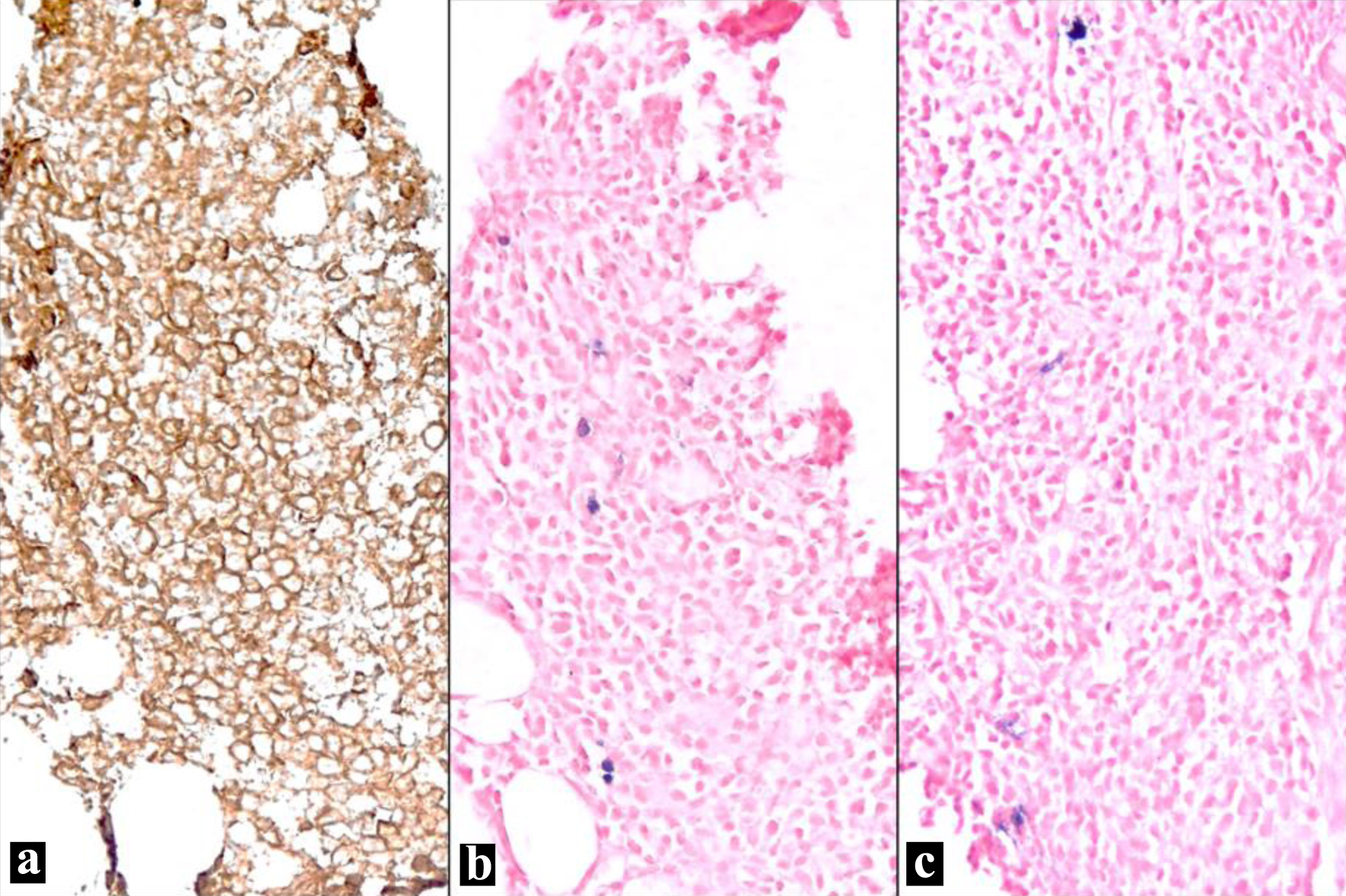 Click for large image | Figure 2. Bone marrow biopsy (IHC and ISH). (a) Plasma cells show strong membranous staining (CD138, IHC, × 100). (b) Few scattered positive cells (blue) represent residual uninvolved plasma cells; the atypical plasma cells appear negative (kappa light chain, ISH, × 100). (c) Few scattered positive cells (blue) represent residual uninvolved plasma cells; the atypical plasma cells appear negative (lambda light chain, ISH, × 100). IHC: immunohistochemical; ISH: in situ hybridization. |
Chromosome analysis revealed a complex karyotype in 6/20 cells characterized by deletions of chromosomes 7p and 20q, reciprocal translocations t(8;22) and t(10;22), and an unbalanced translocation between chromosomes 1 and 16 resulting in a der(16). In addition, fluorescence in situ hybridization (FISH) analysis confirmed the involvement of the MYC locus in the t(8;22)(q24.2;q11.2) translocation, as evidenced by MYC break apart probe (Fig. 3).
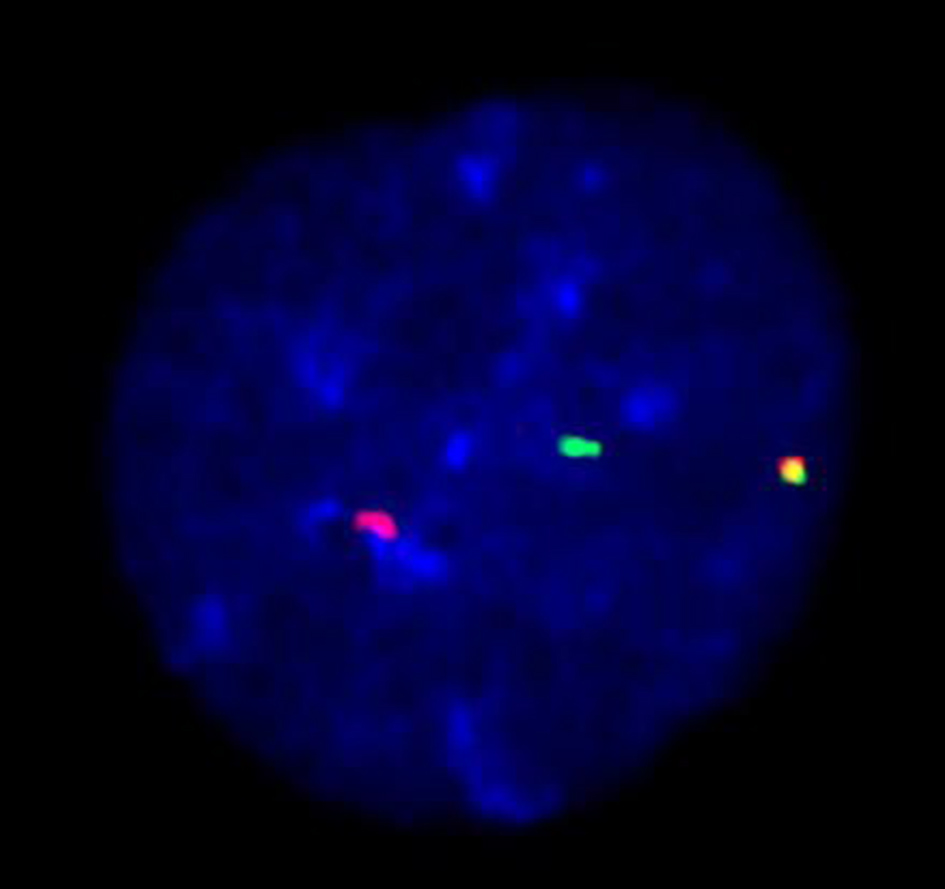 Click for large image | Figure 3. MYC break apart probe: t(8;22)(q24.2;q11.2) detected using dual color dual fusion FISH probe. FISH: fluorescence in situ hybridization. |
Serology at time of diagnosis revealed a β2-microglobulin of 5.5 mg/L (1 - 2 mg/L), serum albumin of 4.3 g/dL (3.2 - 4.6 g/dL), and lactate dehydrogenase (LDH) of 349 U/L (110 - 216 U/L). Findings were consistent with a stage III disease based on revised International Staging System (R-ISS).
Treatment
The patient was treated with lenalidomide, bortezomib, and dexamethasone induction therapy with daratumumab added after cycle 1. The addition of anti-CD38 monoclonal antibodies to standard triplet regimens has become the standard of care for high-risk transplant eligible MM patients [7]. Lenalidomide was discontinued after cycle 3 due to ocular toxicity. After four cycles of treatment, bone marrow biopsy showed a decrease in malignant plasma cell burden from almost 100% to 5% and he underwent an autologous peripheral blood stem cell transplant. Post-transplant, he was maintained on single agent bortezomib due to his inability to tolerate lenalidomide and the unclear role of daratumumab in the post-transplant maintenance setting [8]. Furthermore, bortezomib maintenance appears to improve survival in the post-transplant setting for high-risk MM patients when compared to thalidomide maintenance [9].
Follow-up and outcomes
As his disease cannot be monitored with serology, he has been followed with periodic bone marrow biopsies in addition to history, physical exam, laboratory evaluation and serial positron emission tomography CT (PET-CT) scans. Most recent PET-CT showed gross stability of all osseous lesions with no new or enlarging lesions. He is currently 29 months post-transplant and last bone marrow biopsy showed no morphologic increase in plasma cells with resolution of previously seen cytogenetic changes.
| Discussion | ▴Top |
The diagnosis of NSM first requires the diagnosis of MM, which is made more difficult due to the lack of light chain expression. MM can be established by various criteria, including: 1) ≥ 60% clonal plasma cell population in the bone marrow; and 2) ≥ 10% clonal plasma cells in the bone marrow or an extramedullary plasmacytoma with evidence of myeloma defining events including end-organ damage attributable to the neoplastic population. MM can then be subtyped, with NSM also requiring the absence of monoclonal protein in the serum and urine as well as negative serum free light chain study [2].
NSM constitutes around 3% of all cases of MM and can be further categorized into four subgroups: “non-producers”, “true non-secretors”, “oligosecretors” and “false non-secretors”. The non-producer subgroup is characterized by MM cells that are unable to synthesize any immunoglobulins whatsoever, while the non-secretor subgroup is characterized by MM cells that can produce protein but have defects in secreting immunoglobulins [3]. Oligosecretors or FLC-restricted MM is seen when MM cells only produce FLC in the absence of a heavy chain and the false non-secretor subgroup refers to MM cases where there are measurable intracellular immunoglobulins by immunofluorescence without measurable extracellular component by conventional testing. As our patient had no intracellular immunoglobulin detected nor any monoclonal heavy or light chains detected in serum, he would be classified as a non-producer [5].
While large, randomized studies evaluating the prognosis and optimal treatment for NSM are lacking, the few studies comparing outcomes between non-secretory and secretory myeloma generally show similar outcomes between the two groups [10, 11], while at least one retrospective review found OS to be slightly worse in NSM patients [4]. However, when compared to other NSM patients, the non-producer subtype has been identified as having a markedly reduced progression-free survival (PFS) and decreased sensitivity to MM therapies [12]. A retrospective review of 30 patients with a new diagnosis of NSM over a 10-year period at the Mayo Clinic identified half the cohort of patients to have ISS stage III disease [4]. First-line therapy did not generally differ from age-matched controls with secretory MM. There was an increased propensity for these patients to harbor t(11;14) which is most commonly identified in mantle cell lymphoma [13]. This finding has clinical relevance due to high bcl-2 expression in MM patients with t(11;14) and the therapeutic benefit of bcl-2 inhibition in this subset of patients [4]. Further studies are needed to evaluate the efficacy of frontline MM therapies as well as the frequency and potential therapeutic implications of t(11;14) in newly diagnosed NSM patients.
Identifying patients with higher-risk disease is of utmost importance to determine the appropriate induction regimen. High-risk disease has been associated with increased risk of relapse, decreased PFS and OS. Prognostication and risk stratification of MM patients is typically performed using R-ISS which is based on cytogenetic profile using interphase FISH, and disease markers including β2-microglobulin, LDH, and albumin.
Our patient was diagnosed with R-ISS III disease based on elevated levels of β2-microglobulin and serum LDH, even without the presence of any high-risk cytogenetic abnormalities. Patients with stage III disease based on R-ISS have an estimated median PFS of 29 months [14].
Cytogenetic abnormalities play a significant role in prognostication systems for MM patients. The International Myeloma Work Group identified translocations t(4;14), t(14;16), t(14;20), del(17/17p), any non-hyperdiploid karyotype, del(13), gain of 1q and high-risk gene expression profiling as high-risk cytogenetic abnormalities. The presence of ≥ 3 cytogenetic abnormalities is considered complex cytogenetics and is associated with ultrahigh risk with an expected OS of < 2 years [6].
Translocations involving c-MYC on chromosome 8 and IGL on chromosome 22, in particular t(8;22)(q24.2;q11.2) is seen in approximately 5-10% of Burkitt lymphoma cases but is rare in MM [15]. Only 19 cases have been reported in literature of patients with this translocation. In all previously reported patients, t(8;22) was identified as a component of complex cytogenetic karyotype [16-24]. To our knowledge, this is the first reported case of a patient with t(8;22) with NSM.
Learning points
NSM is a rare subtype of MM which has four distinct phenotypic subgroups, one of which is the “non-producers”. We report a case of NSM with a non-producing phenotype and with t(8;22) as a part of complex cytogenetics. Despite R-ISS staging considering the patient to have stage III disease, the patient has multiple additional negative prognostic features including complex cytogenetics and the non-producing phenotype of NSM, suggesting a more inferior prognosis. Our patient has sustained complete response which may be attributed to aggressive frontline treatment and continued maintenance therapy. While complex cytogenetic abnormalities have been associated with worse outcomes in patients with MM, it remains unclear whether the presence of t(8;22) independently affects the prognosis of those patients. This case highlights the need for appropriate risk stratification of patients by identifying clinically higher risk phenotypes and incorporating all cytogenetic information.
Acknowledgments
The authors would like to thank Allegheny Health Network and NeoGenomics.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
RAJ and JRL wrote, edited and finalized the manuscript. AA, KA and MK conceptualized and performed background research. RM, KF and JRM provided formal analysis and writing regarding genetics. JCA wrote and edited the manuscript. PN and NRJ wrote and provided pathology images and analysis. SS and PM wrote, finalized and provided supervision for the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lam H, Musco K, Ahluwalia A, Zirlinger A. Colorectal carcinoma: a case of malignant fistula formation presenting as septic shock. Chest. 2021;160(4):A729.
doi - Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
doi pubmed - Hussain A, Almenfi HF, Almehdewi AM, Hamza MS, Bhat MS, Vijayashankar NP. Laboratory features of newly diagnosed multiple myeloma patients. Cureus. 2019;11(5):e4716.
doi pubmed pmc - Nandakumar B, Kumar SK, Dispenzieri A, Buadi FK, Dingli D, Lacy MQ, Hayman SR, et al. Cytogenetic features and clinical outcomes of patients with non-secretory multiple myeloma in the era of novel agent induction therapy. Clin Lymphoma Myeloma Leuk. 2020;20(1):53-56.
doi pubmed pmc - Dupuis MM, Tuchman SA. Non-secretory multiple myeloma: from biology to clinical management. Onco Targets Ther. 2016;9:7583-7590.
doi pubmed pmc - Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955-2962.
doi pubmed pmc - Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, Hulin C, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390(4):301-313.
doi pubmed - Moreau P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, Bene MC, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378-1390.
doi pubmed - Goldschmidt H, Lokhorst HM, Mai EK, van der Holt B, Blau IW, Zweegman S, Weisel KC, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32(2):383-390.
doi pubmed - Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
doi pubmed - Kumar S, Perez WS, Zhang MJ, Ballen K, Bashey A, To LB, Bredeson CN, et al. Comparable outcomes in nonsecretory and secretory multiple myeloma after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(10):1134-1140.
doi pubmed pmc - X P, Q Z, C H, et al. Non-producing Multiple Myeloma (MM) is a distinct subset of non-secretory MM characterized by high cyclin D1 expression and decreased progression free survival. Blood. 2013;122(21):1911.
- Jean-Loup Huret. t(11;14)(q13;q32) IGH/CCND1. Atlas Genet Cytogenet Oncol Haematol. 1998.
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869.
doi pubmed pmc - Chrystele Bilhou-Nabera, Eva van den Berg. t(8;14)(q24;q32) IGH/MYC t(2;8)(p12;q24) IGK/MYC 22)(q24;q11) IGL/MYC. Atlas Genetics Oncology. 2016.
- Kim H, Moon HW, Hur M, Yun YM, Park CM, Lee MH. Variant Burkitt-type translocation (8;22)(q24;q11) in plasma cell myeloma. Korean J Hematol. 2011;46(2):135-138.
doi pubmed pmc - Yamamoto K, Hamaguchi H, Nagata K, Taniwaki M. A variant Burkitt-type translocation (8;22)(q24;q11) in multiple myeloma. Report of a new case and review of the literature. Cancer Genet Cytogenet. 1998;104(2):98-103.
doi pubmed - Sole F, Woessner S, Acin P, Perez-Losada A, Florensa L, Besses C, Sans-Sabrafen J. Burkitt's type translocation in multiple myeloma. Leuk Res. 1994;18(9):671-673.
doi pubmed - Taniwaki M, Nishida K, Takashima T, Nakagawa H, Fujii H, Tamaki T, Shimazaki C, et al. Nonrandom chromosomal rearrangements of 14q32.3 and 19p13.3 and preferential deletion of 1p in 21 patients with multiple myeloma and plasma cell leukemia. Blood. 1994;84(7):2283-2290.
pubmed - Sawyer JR, Waldron JA, Jagannath S, Barlogie B. Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genet Cytogenet. 1995;82(1):41-49.
doi pubmed - Look RM, Lim SW, Schreck RR, Lee S, Fuerst MP, Lawrence GN, Kusuanco DA, et al. Burkitt translocation (8;22)(q24;q11) in a patient with multiple myeloma. Cancer Genet Cytogenet. 1995;82(2):100-102.
doi pubmed - Mugneret F, Sidaner I, Favre B, Manone L, Maynadie M, Caillot D, Solary E. Der(16)t(1;16)(q10;p10) in multiple myeloma: a new non-random abnormality that is frequently associated with Burkitt's-type translocations. Leukemia. 1995;9(2):277-281.
pubmed - Calasanz MJ, Cigudosa JC, Odero MD, Ferreira C, Ardanaz MT, Fraile A, Carrasco JL, et al. Cytogenetic analysis of 280 patients with multiple myeloma and related disorders: primary breakpoints and clinical correlations. Genes Chromosomes Cancer. 1997;18(2):84-93.
pubmed - Sugiyama A, Nakabayashi H, Kondo M, Tominaga T, Shinohara K. [Multiple myeloma with variant type translocation, t(8;22)(q24;q11.2)]. Rinsho Ketsueki. 2009;50(1):29-33.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


