| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 3, June 2024, pages 79-85
Steady-State Versus Chemotherapy-Based Stem Cell Mobilization in Multiple Myeloma: A Single-Center Study to Analyze Efficacy and Safety
Nora Obajed Al-Alia, b, Laszlo Imre Pinczesa, b, Katalin Farkasa, b, Gyorgy Kerekesc, Arpad Illesa, b, Laszlo Varoczya, b, d
aDepartment of Hematology, Institute for Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
bDoctoral School of Clinical Sciences, University of Debrecen, Debrecen, Hungary
cIntensive Care Unit, Institute for Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
dCorresponding Author: Laszlo Varoczy, Department of Hematology, Institute for Medicine, University of Debrecen, H-4032 Debrecen, Hungary
Manuscript submitted February 26, 2024, accepted March 29, 2024, published online June 28, 2024
Short title: Stem Cell Mobilization in MM
doi: https://doi.org/10.14740/jh1256
| Abstract | ▴Top |
Background: High-dose chemotherapy followed by autologous hematopoietic stem cell support is recommended in the treatment of eligible multiple myeloma (MM) patients. The aim of this study was to compare the efficacy and safety of steady-state versus chemotherapy-based stem cell mobilization in our Hungarian patient population.
Methods: The subjects were 210 MM patients who underwent stem cell mobilization procedure between 2018 and 2022. Solo granulocyte colony-stimulating factor (G-CSF) was administered in 104 cases, while 106 patients received chemotherapy which was followed by G-CSF administration. We evaluated the ratio of successful mobilizations, the amount of collected stem cells, the incidence of infections and cost-effectivity in the two groups.
Results: In the steady-state group, there was a significantly higher need for plerixafor (45% vs. 13%, P < 0.001), unsuccessful stem cell mobilization was more frequent (11% vs. 3%, P = 0.024) and the mean amount of collected stem cells was lower (6.9 vs. 9.8 × 106, P < 0.001) than in the chemotherapy group. However, infections were less frequent (4% vs. 27%, P < 0.001) and the number of days spent in hospital was significantly lower (6 vs. 14 days, P < 0.001). Plerixafor was more frequently administered in those who had received lenalidomide or daratumumab than in those who had been treated with other regimens (41% vs. 23%, P = 0.007 and 78% vs. 23%, P < 0.001, respectively).
Conclusions: Steady-state mobilization is a safe method; however, the higher rate of plerixafor administration and unsuccessful attempts may question its superiority to chemomobilization.
Keywords: Multiple myeloma; Stem cell; Mobilization; Chemotherapy; Granulocyte colony-stimulating factor; Plerixafor
| Introduction | ▴Top |
Multiple myeloma (MM) is a malignant lymphoproliferative disorder characterized by clonal plasma cell expansion. It represents 1-2% of all cancers and accounts for 17% of the hematological malignancies. It occurs mainly in elderly people. Major complications include the development of lytic bone lesions, hypercalcemia, bone marrow failure and renal impairment. MM is considered to be an incurable disease; however, there has been a significant improvement in patients’ survival, due to the novel drugs that have been introduced recently [1]. The first-line therapy is usually a three- or four-drug combination regimen which includes a proteasome inhibitor, an immunomodulatory drug, steroid and daratumumab as an anti-CD38 monoclonal antibody. After the induction treatment, high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) should be considered in approximately half of the patients. Transplant eligibility is determined by the age and comorbidities [2, 3].
Stem cell mobilization procedure usually includes the administration of chemotherapy and granulocyte colony-stimulating factor (G-CSF). The gold standard is intermediate-dose (2 - 4 g/m2) cyclophosphamide, but other regimens, e.g., high-dose etoposide, cytosine-arabinoside or combinations (bortezomib, thalidomide and dexamethasone-cisplatin, adriamycin, cyclophosphamide and etoposide (VTD-PACE), etoposide, dexamethasone, ara-C, and cisplatin (EDAP)), are also applied. Recently, steady-state mobilization method can also be considered. The administration of G-CSF may reduce chemotherapy-associated toxicities and infectious complications. The International Myeloma Working Group (IMWG) recommends collecting at least 4 × 106 CD34+ cells/body weight kilogram (bwkg) for each AHSCT, and a double amount is required if a tandem or a second transplantation is planned [4].
Our aim was to compare the efficacy and safety of solo G-CSF versus chemotherapy-based stem cell mobilization in our Hungarian MM patients.
| Materials and Methods | ▴Top |
We collected data retrospectively from MM patients who underwent stem cell mobilization procedure in our institute between January 2018 and December 2022. Patients with plasma cell leukemia, solitary plasmacytoma and amyloidosis were excluded from the analysis. The clinical files were reviewed with particular reference to age, sex, clinical stage, previous treatment and response achieved, mobilization strategy, length of hospital stay, infectious complications and the amount of harvested stem cells. The International Staging System (ISS) and revised ISS (R-ISS) stages were determined using the IMWG criteria, if the relevant data were available. The way how fluorescence in situ hybridization (FISH) testing was performed varied, there was no consensus regarding the probes used, but those specific for 17p deletion, translocations (11;14), (4;14) and (14;16) and 1q amplification were generally part of the set. FISH results of unfavorable prognosis included t(4;14), t(14;16) and del(17p).
Response criteria (complete response (CR), very good partial response (VGPR), partial response (PR), no response (NR), and progressive disease (PD)) were also determined via the IMWG criteria.
All patients had undergone central venous catheter insertion before the stem cell mobilization process was initiated. Chemotherapy-based treatment included the administration of intermediate-dose cyclophosphamide (3 - 4 g/m2) or combination regimens (PACE). Filgrastim stimulation at the dose of 10 µg/kg/day was started if the patients’ absolute neutrophil count dropped off below 1,000/µL. Peripheral CD34+ cell counts were evaluated by flow cytometry method if leukocyte counts began to increase, and their number exceeded 5,000/µL. In terms of steady-state mobilization, patients received generic G-CSF 10 µg/kg/day subcutaneously for 4 days. On the fifth day, flow cytometry was performed to determine the peripheral stem cell count in the patients’ blood sample. In both groups, patients were administered plerixafor 24 mg/day subcutaneously if leukocyte count exceeded 5,000/µL and peripheral CD34+ cell count was in the 5 - 20/µL range. Stem cell harvesting procedure was launched if peripheral CD34+ stem cell count exceeded 20/µL. All collections were performed using the MNC program of the Spectra Optia apheresis system. The goal was to harvest at least 4 × 106 stem cells per bwkg for each autologous transplantation.
Categorical variables were given as their frequencies and percentages, while continuous variables with medians and ranges. The Kolmogorov-Smirnov test was used for evaluation of data normality. Discrete variables were compared using a Chi-square test, while analysis of variance (ANOVA) was used for the measurement of associations between patient characteristics and outcomes. Pearson’s correlation and t-test were used to assess whether the difference in the means of two variables reaches statistical significance. Binary logistic regression and multivariable logistic regression was used to determine which factors were significant in determining outcomes. The level of statistical significance was considered at P < 0.05. Post-hoc power analysis was used to assess statistical power. Statistical analyses were performed using SPSS26.0 (IBM Corp., Armonk, NY, USA).
Ethical issues
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research project was approved by the Regional Ethical Committee of the University of Debrecen (permission number: DEKK/RKEB/IKEB 6548-2023). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
| Results | ▴Top |
Patient characteristics
In that 5 years’ period, 210 patients underwent stem cell mobilization procedure. Their demographics were generally balanced. A moderate male predominance was observed (51.1%), while the average age was 61 years (range 32 - 75). One-hundred and six patients received chemotherapy-based protocols for stem cell mobilization, while 104 patients were administered G-CSF alone. The most commonly used chemotherapeutic mobilization protocol was cyclophosphamide monotherapy (84.9%) in patients also receiving MM-specific, PACE-based combination therapies (15.1%). There was no difference in the epidemiological, disease-related or treatment parameters between the two groups with different mobilization strategies. Patients’ main clinical features and induction therapies as well as their results are presented in Table 1.
 Click to view | Table 1. Patient Characteristics and Comparison of Different Stem Cell Mobilization Approaches |
Efficacy of stem cell mobilization
In the solo G-CSF group, there was a significantly higher need for plerixafor administration (45% vs. 13%, P < 0.001), unsuccessful stem cell mobilization was more frequent (11% vs. 3%, P = 0.024) and the mean amount of collected stem cells was significantly lower (6.9 vs. 9.8 × 106/bwkg, P < 0.001) than in the chemotherapy group. On the other hand, infections were less frequent (4% vs. 27%, P < 0.001) and the number of days spent in hospital was significantly lower (6 vs. 14 days, P < 0.001). There was no significant difference between the median days of apheresis (Table 1). Multivariable analysis identified chemotherapy + G-CSF stem cell collection protocol as a sole and independent risk factor for infectious complications (P = 0.001), while the solo G-CSF protocol (P < 0.001) and daratumumab exposure (P = 0.003) predicted requirement for subsequent plerixafor therapy (Table 2). No independent prognostic factor for mobilization failure was found.
 Click to view | Table 2. Univariate and Multivariable Logistic Regression Analyses of Disease Parameters, Associated With Infectious Complications, Plerixafor Use and Mobilization Failure |
We also investigated the impact of induction treatment to stem cell mobilization. Most patients received bortezomib-based triplets: VTD, bortezomib, lenalidomide and dexamethasone (VRD) or bortezomib, cyclophosphamide and dexamethasone (VCD). The amount of harvested stem cells was significantly lower in those patients who received lenalidomide-containing regimens than in those who were given lenalidomide-free induction therapy (6.6 vs. 9.3 × 106/bwkg, P < 0.001). Besides, plerixafor was more frequently used in the lenalidomide group (40.8% vs. 23%, P = 0.007). Also, there was a marked, but not significant difference in the ratio of unsuccessful mobilization attempts (11.3% vs. 4.3%, P = 0.056) (Fig. 1). However, lenalidomide exposure alone does not predispose to either plerixafor use or mobilization failure (Table 2). Eighteen patients were exposed to daratumumab before stem cell collection, which is only a relatively small portion of the whole population. Nevertheless, our preliminary data showed that daratumumab did not influence the efficacy of the stem cell harvesting procedure; however, the need for plerixafor administration was higher (77.8% vs. 24.5%, P < 0.001) (Fig. 1 and Table 2).
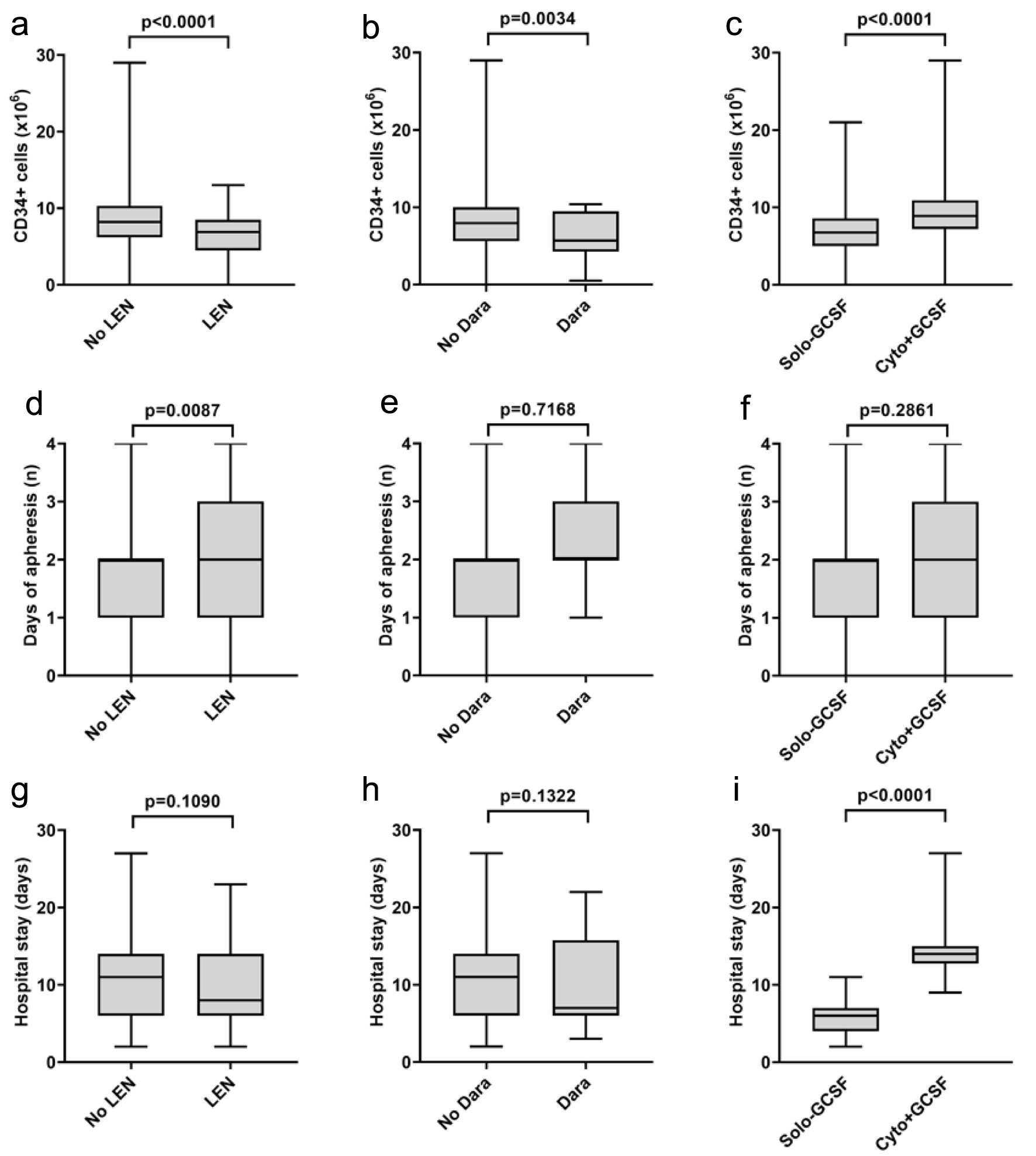 Click for large image | Figure 1. The effect of LEN, Dara and mobilization strategy on the amount of bone marrow stem cells collected (a-c), the number of days of apheresis (d-f) and the length of hospital stay (g-i). LEN: lenalidomide; Dara: daratumumab; G-CSF: granulocyte colony-stimulating factor. |
| Discussion | ▴Top |
The incorporation of novel drugs to the induction treatment may question the necessity of autologous stem cell transplantation even in young and fit MM patients. However, recent studies have reported that AHSCT significantly improves progression-free survival in patients who undergo the treatment compared to those who do not [5]. Therefore, international guidelines still recommend the administration of high-dose melphalan followed by autologous stem cell transplantation in the eligible population [6].
The method of stem cell mobilization depends on several factors, such as disease activity, the number of transplantations planned and the presence of risk factors that may predict poor mobilization potential [7]. Chemotherapy-based regimens include the administration of intermediate-dose cyclophosphamide, etoposide, cytarabine or combination therapies (e.g., PACE). This strategy can be recommended for heavily pretreated patients with active disease, as more potent mobilizing effects can be achieved. The beneficial effect of chemotherapy on the underlying disease during stem cell collection for patients with MM is a matter of debate [8]. Steady-state mobilization is accompanied with lower toxicities as only G-CSF is administered. Plerixafor, a selective and reversible CXCR4 inhibitor, can be added to the therapy if the primary mobilization seems to be ineffective [9]. However, several studies reported the superiority of chemotherapy-based mobilizations in terms of the harvested stem cell amounts and the number of successful attempts [10]. Formerly, intermediate-dose cyclophosphamide treatment followed by G-CSF was our first choice when we indicated stem cell mobilization in our MM patients. However, coronavirus disease 2019 (COVID-19) pandemic forced us to change our strategy, as the avoidance of therapies associated with possible infectious complications and long-term hospital stays were crucial. Therefore, we began to prefer steady-state mobilization from 2020. Our goal was to compare the efficacy and cost-effectivity of the two mobilization strategies in a retrospective way.
Half of our patient population received chemotherapy-based treatment, while the other half was administered G-CSF alone. In the chemotherapy group, the harvesting procedure was more effective considering the number of collected stem cells and the ratio of unsuccessful attempts. Our results indicate that chemotherapy produced a significantly higher harvest of the amount of stem cells than solo G-CSF. Both approaches provided a conveniently sufficient amount of product to store. Also, with both approaches, we were able to collect multiple times the minimum number of stem cells required for AHSCT by the IMWG guidelines [4]. Not surprisingly, hospitalization was significantly longer, and infectious complications were more frequent with chemomobilization. However, only 13% of the patients required plerixafor administration, while this ratio was 45% in the G-CSF group. These results are in line with what reported in previous studies [11, 12]. On the other hand, financing issues can be also taken into account. An Italian study has recently reported on 50 patients who underwent stem cell mobilization, and found that chemo-free strategy was associated with lower costs [13]. On the other hand, a Chinese group found no significant difference between the efficiency of steady-state and chemotherapy-based mobilization approaches, while the more frequent plerixafor use resulted in a high financial burden when no chemotherapy was administered [14].
We also investigated the impact of induction therapies administered before the stem cell harvesting procedures. Lenalidomide is known to have a negative effect on stem cell mobilization, and our results are also in accordance with this finding. Daratumumab administration has been recently approved in the first-line therapy of transplant-eligible patients [15]. Results from pivotal studies confirmed that patients who received daratumumab-containing triplets are more likely to require plerixafor support upon the stem cell collection [15, 16]. Our preliminary data also showed that previous daratumumab treatment did not influence the success rate of stem cell harvesting procedure; however, the need for plerixafor administration was significantly higher and the amount of collected stem cells was significantly lower than in those patients who were daratumumab-naive.
The main limitation of this work is the patients included in the study were recruited from only one hematological center. Moreover, daratumumab is still not widely available in the first-line therapy of MM; therefore, a relatively low number of patients could receive it as part of the induction treatment. We think that adding data of other centers as well as including more patients treated with daratumumab may make our results more remarkable.
Conclusions
Our study is the first that highlights the negative impact of daratumumab pretreatment on the efficacy of stem cell collection. Our results support that chemotherapy-based mobilization strategies are still cost-effective, despite the higher rate of infectious complications and longer hospital stays. The results presented here also suggest that the reduced potential for mobilization expected both with lenalidomide and daratumumab pretreatment and with solo G-CSF can be overcome by the administration of plerixafor. This recognition may motivate caretakers to choose a chemotherapy-free strategy with a lower risk of infections for the frail population, thereby bringing personalized medicine closer to MM patients.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
None of the authors had any conflict of interest.
Informed Consent
No informed consent was required for retrospective data collection.
Author Contributions
Conceptualization, formal analysis, data curation, visualization, and writing original draft preparation: NOA, KF and LV; treatment of the patients, investigation and resources: NOA, KF, LIP, GK, AI and LV; supervision: LV; writing review and editing: NOA, KF, LIP and LV. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, Tuazon S, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464-477.
doi pubmed - Bazarbachi AH, Al Hamed R, Malard F, Bazarbachi A, Harousseau JL, Mohty M. Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: an update. Blood Cancer J. 2022;12(3):47.
doi pubmed pmc - Kiss S, Gede N, Soos A, Hegyi P, Nagy B, Imrei M, Czibere B, et al. Efficacy of first-line treatment options in transplant-ineligible multiple myeloma: a network meta-analysis. Crit Rev Oncol Hematol. 2021;168:103504.
doi pubmed - Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, Bazarbachi A, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865-872.
doi pubmed - Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, Medvedova E, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387(2):132-147.
doi pubmed pmc - Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, Delforge M, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(dagger). Ann Oncol. 2021;32(3):309-322.
doi pubmed - Arora S, Majhail NS, Liu H. Hematopoietic progenitor cell mobilization for autologous stem cell transplantation in multiple myeloma in contemporary era. Clin Lymphoma Myeloma Leuk. 2019;19(4):200-205.
doi pubmed - Gentile M, Morabito F, Martino M, Vigna E, Martino EA, Mendicino F, Martinelli G, et al. Chemotherapy-based regimens in multiple myeloma in 2020. Panminerva Med. 2021;63(1):7-12.
doi pubmed - DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-5726.
doi pubmed - Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31-40.
doi pubmed pmc - Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43(8):619-625.
doi pubmed pmc - Wood WA, Whitley J, Moore D, Sharf A, Irons R, Rao K, Serody J, et al. Chemomobilization with etoposide is highly effective in patients with multiple myeloma and overcomes the effects of age and prior therapy. Biol Blood Marrow Transplant. 2011;17(1):141-146.
doi pubmed - Laszlo D, Marcacci GP, Martino M, Radice D, Rabascio C, Lucchetti B, Magaro A, et al. A comparison of chemo-free strategy with G-CSF plus plerixafor on demand versus intermediate-dose cyclophosphamide and G-CSF as PBSC mobilization in newly diagnosed multiple myeloma patients: An Italian explorative cost Analysis. Transfus Apher Sci. 2020;59(5):102819.
doi pubmed - Han X, Xiong Y, Huang X, Zheng G, Chen J, Guan F, He J, et al. Application of first-line steady-state mobilization vs. conventional chemotherapy mobilization for haematopoietic stem cell mobilization in newly diagnosed multiple myeloma patients. Clinics Oncol. 2022;7:1977.
- Chhabra S, Callander N, Watts NL, Costa LJ, Thapa B, Kaufman JL, Laubach J, et al. Stem cell mobilization yields with daratumumab- and lenalidomide-containing quadruplet induction therapy in newly diagnosed multiple myeloma: findings from the MASTER and GRIFFIN trials. Transplant Cell Ther. 2023;29(3):174.e1-174.e10.
doi pubmed - Kiss S, Gede N, Hegyi P, Nagy B, Deak R, Dembrovszky F, Bunduc S, et al. Addition of daratumumab to multiple myeloma backbone regimens significantly improves clinical outcomes: a systematic review and meta-analysis of randomized control trials. Sci Rep. 2021;11(1):21916.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


