| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 4, August 2024, pages 168-173
Breast Cancer With Release of Tumor Cells in Peripheral Blood Mimicking Acute Myeloid Leukemia
Arianna Gattia, g , Bruno Brandoa, Irene Cupparia, Nadia Violab, Lorenzo Brunettic, Michela Sampaolod, Sonia Morec, Doriana Morichettie, Laura Corvattaf
aHematology Laboratory and Transfusion Center ASST Ovest Milanese-Ospedale Legnano, Milan, Italy
bClinical Immunology Unit, Azienda Ospedaliero Universitaria delle Marche, Ancona, Italy
cDepartment of Hematology, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy
dLaboratory Medicine Unit, Azienda Ospedaliero Universitaria delle Marche, Ancona, Italy
eAnatomical and Histopathological Pathology Unit, Azienda Ospedaliero Universitaria delle Marche, Ancona, Italy
fDepartment of Medicine, Profili Hospital, Fabriano, Italy
gCorresponding Author: Arianna Gatti, Hematology Laboratory and Transfusion Center ASST Ovest Milanese-Ospedale Legnano, Milan, Italy
Manuscript submitted March 26, 2024, accepted June 19, 2024, published online July 18, 2024
Short title: BC With Peripheral Tumor Cells Mimicking AML
doi: https://doi.org/10.14740/jh1259
| Abstract | ▴Top |
A 75-year-old woman with a history of lobular breast adenocarcinoma treated with mastectomy and radiotherapy in 2021 and on maintenance hormone therapy, presented with asthenia and tremors. Laboratory tests showed leucocytosis, anemia and low platelet count, with increased serum calcium, lactate dehydrogenase and indirect bilirubin levels. Haptoglobin was decreased and renal function was normal. Peripheral blood smear showed red cell anisocytosis, many schistocytes and immature granulocytes. Furthermore, 15% of white cells displayed large size and atypical morphology. A macroangiopathic hemolytic anemia (MAHA) related to a de novo or recurring cancer was hypothesized, and total body computed tomography (CT) and 18F-FDG positron emission tomography (PET)/CT were undertaken. Only a slight FDG uptake was demonstrated in the spine, attributable to a reactive bone marrow due to MAHA. Then, to rule out a MAHA related to acute leukemia, a bone marrow aspirate and trephine biopsy were performed, with an extensive cell immunophenotyping. The first myeloid flow cytometry (FC) panel evidenced a large volume population of about 20%, expressing CD117 but negative for CD45 and CD34. All myeloid markers were negative. A more extensive panel was then used, including plasma cell and erythroid markers. Interestingly, the abnormal population resulted positive for CD138 and CD71 with negativity for CD38. A recent study reported that besides CD45 negativity, non-hematological neoplasms frequently express CD56, CD117, or CD138. Therefore, a panel for non-hematological markers including epithelial cell adhesion molecule (EpCAM) was carried out. This population resulted EpCAM positive and also expressed CD9, a breast cancer prognostic marker. Bone marrow smears revealed the presence of the same cells, and the immunohistochemistry analysis of bone marrow biopsy demonstrated the massive infiltration of breast cancer cells, expressing all epithelial markers identified at diagnosis. The FC analysis of the peripheral blood allowed the rapid characterization of a non-hematological neoplastic cell population, circulating at unusually high frequency and mimicking an acute myeloid leukemia. The FC detection of CD45-negative cell populations in peripheral blood, bone marrow or lymph node aspirate should prompt the setup of an immunophenotyping panel including EpCAM, CD9, CD56 and CD117, to allow for a rapid and accurate identification of ectopic malignant epithelial cells.
Keywords: Breast cancer; Macroangiopathic hemolytic anemia; Immunophenotyping; Flow cytometry; EpCAM
| Introduction | ▴Top |
Breast cancer (BC) is the most common malignancy among women in developed countries, with a mortality rate of 15%. Several studies have reported an increased incidence of acute myeloid leukemia (AML) after treatment of BC, with evidence of a dose-intensity relationship. Alkylating agents, topoisomerase II inhibitors and radiation therapy, fundamental to the treatment of BC, are the most likely contributors to increased risk of secondary AML [1, 2]. Another condition associated to BC is cancer-related microangiopathic hemolytic anemia (CR-MAHA) [3]. CR-MAHA is a paraneoplastic syndrome characterized by renal failure and/or neurological symptoms, increased schistocytes in the peripheral blood smear, negative direct antiglobulin test (DAT), hemolytic anemia with increased lactate dehydrogenase (LDH) level, increased indirect bilirubin, low haptoglobin and thrombocytopenia (< 150 × 109/L).
CR-MAHA is mostly associated with gastric cancer, followed by BC, prostate cancer, and lung cancer [4]. In literature, 51 cases of MAHA associated with BC are reported, most of them with poor outcome [5].
Here we describe the case of a patient with previous BC and a suspected CR-MAHA, however characterized by the detection of cells of unclear origin in the peripheral blood, at least initially suggesting a secondary AML.
| Case Report | ▴Top |
Investigations
A 75-year-old woman with a history of lobular breast adenocarcinoma (estrogen receptor (ER)-positive, progesterone receptor (PgR)-positive, human epidermal growth factor receptor 2 (HER2)-negative) treated with mastectomy and radiotherapy in 2021 and receiving hormone therapy, presented with asthenia and tremors. The full blood count showed leukocytosis (23.4 × 109/L with 56% neutrophils), anemia (Hb 72 g/L), macrocytosis (MCV 103 fL), slightly reduced platelet count (138 × 109/L) and increased reticulocytes (11%). Laboratory tests showed increased calcium level (13 mg/dL), LDH 600 U/L and indirect bilirubin (5 mg/dL), with undetectable haptoglobin (< 0.1 g/L). Tumor markers were negative. No coagulation abnormalities were documented. No changes of fibrinogen level were present, and no fibrinogen degradation product (FDP) was detected. ADAMTS13 testing was not carried out. DAT test was negative and renal function was normal. Peripheral blood smear showed marked red cell anisocytosis, a remarkable proportion of schistocytes (15% of erythrocytes), increased erythroblasts (3% of nucleated cells) and immature granulocytes (promyelocytes, myelocytes and metamyelocytes (Fig. 1a)). Furthermore, a significant number of large mononuclear cells of unclear lineage (15% of nucleated cells) were also detected (Fig. 1b).
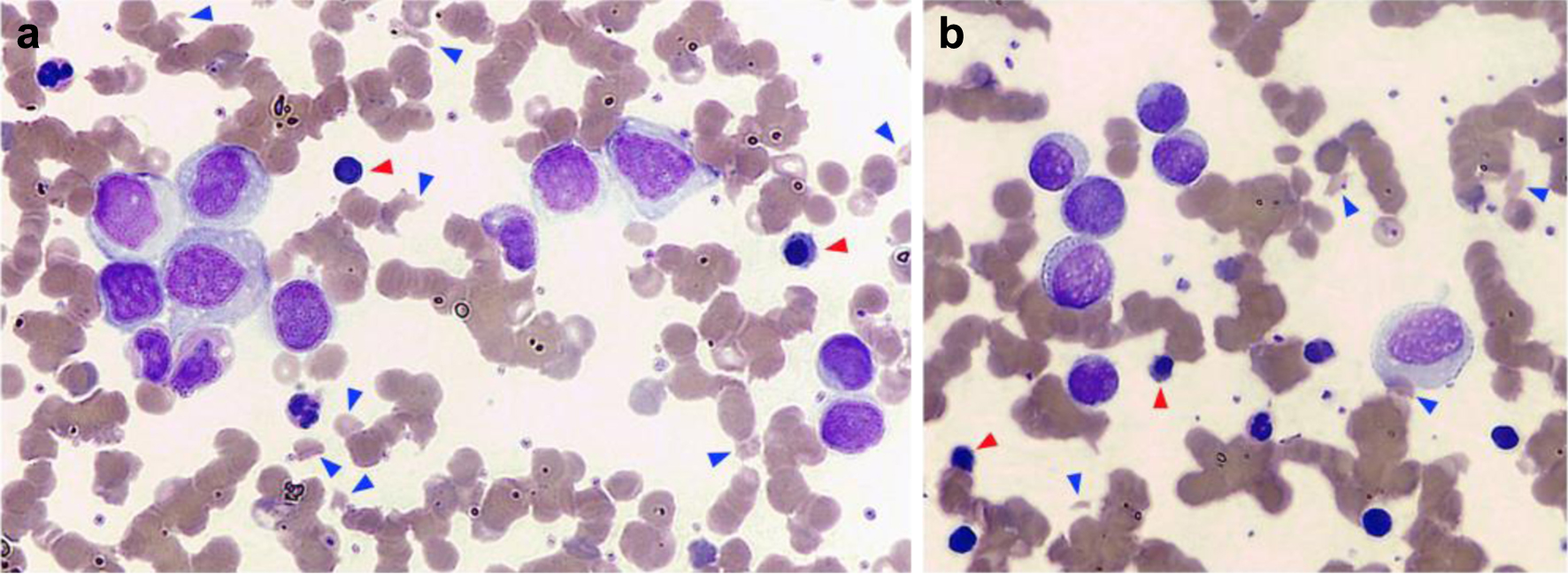 Click for large image | Figure 1. Peripheral blood smear. (a) Marked red cell anisocytosis with a remarkable proportion of schistocytes (15% of erythrocytes, blue arrowheads) and erythroblasts (3% of nucleated cells, red arrowheads) was observed. (b) A significant number of large atypical mononuclear cells with eccentric nucleus, of uncertain lineage, were also noted (15% of nucleated cells). |
Diagnosis
Therefore, in the hypothesis of a MAHA related to a de novo or relapsing cancer, total body CT scan and 18F-FDG PET/CT were ordered. The CT scan was negative, whereas only a slight 18F-FDG uptake was present in the spine, attributable to increased bone marrow activity due to MAHA. Subsequently, due to the presence of circulating abnormal cells and to exclude an acute leukemia-related MAHA, an immunophenotyping of peripheral blood cells, a bone marrow aspirate and a trephine biopsy were undertaken.
A first-level myeloid flow cytometry (FC) panel was initially performed. This panel evidenced a large sized CD45-negative cell population of about 20%, negative for CD34 and positive for CD117 (Fig. 2a). Myeloid markers, such as CD13, CD33 and CD11b were negative. Due to positivity for CD117 without expression of CD45, a more extensive panel was performed, including plasma cell markers (CD38, CD138, CD19, cytoplasmic immunoglobulin kappa and lambda light chains) and the erythroid marker CD71. Interestingly, this population resulted positive for CD138 and CD71, with negativity for CD38 (Fig. 2b). The plasma cell origin of this atypical population was excluded, therefore a second-level panel for non-hematological markers including the surface CD326 epithelial cell adhesion molecule (EpCAM) was carried out. This population resulted EpCAM positive, and also intensely expressed CD9 (Fig. 2c). Aspirate smears revealed the presence of the same cells and immunohistochemistry analysis of the bone marrow biopsy disclosed a massive infiltration of cells expressing all BC epithelial markers identified at diagnosis, such as cytokeratin, ER and GATA-binding protein 3 (GATA3) (Fig. 3). Because the immunohistochemistry results confirmed the infiltration by BC cells, G-band analysis of bone marrow and fluorescence in situ hybridization (FISH) testing were not performed.
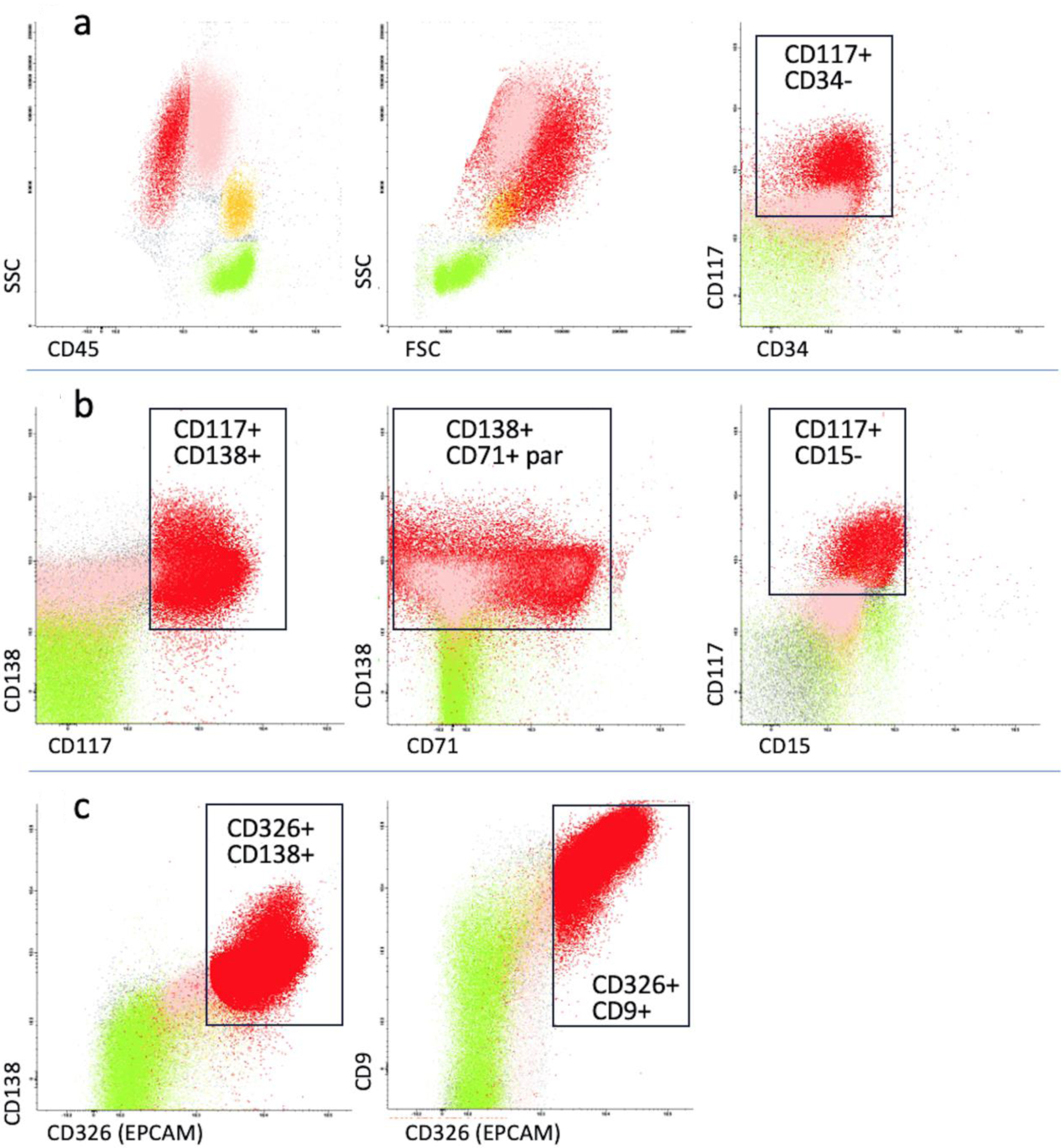 Click for large image | Figure 2. Immunophenotypic analysis of peripheral blood. Green: lymphocytes; yellow: monocytes; pink: neutrophils; red: circulating breast cancer cells. (a) The first myeloid flow cytometry panel showed an atypical population (red) of 20%, negative for CD45 and CD34, positive for CD117 with high forward and side scatter. (b) A more extensive panel included plasma cell (CD138, CD38, CD19) and erythroid markers (CD71). The atypical population resulted positive for CD138 and CD71 with negativity for CD38. (c) A panel for non-hematological markers including CD326 epithelial cell adhesion molecule (EpCAM) was carried out. The abnormal population expressed EpCAM and CD9. The black rectangles indicate the areas of positive expression of cell markers, where applicable. FSC: forward scatter; SSC: side scatter; par: partial expression. |
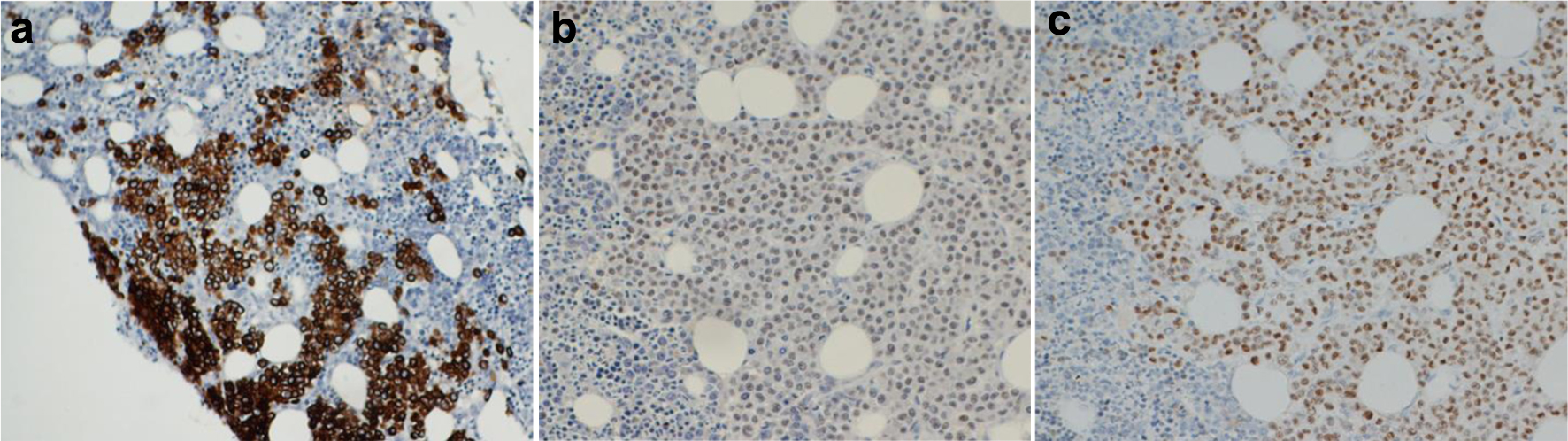 Click for large image | Figure 3. Immunohistochemistry analysis of bone marrow biopsy. A massive infiltration of cancer cells expressing all epithelial markers identified at diagnosis, such as cytokeratin (a), GATA-binding protein 3 (GATA3) (b) and estrogen receptor (c) was demonstrated. |
Follow-up and outcomes
After a few days, the patient presented with high fever and 24 h later died for suspected septic shock. However, no microbiological data are available. No specific chemotherapy was undertaken. In absence of renal failure and/or neurological symptoms, no plasma-exchange was undertaken.
| Discussion | ▴Top |
Here we describe an unusual case of a patient with previous BC, who developed a clinical picture resembling CR-MAHA, due to an increased number of schistocytes, increased value of LDH and indirect bilirubin, low haptoglobin and mild thrombocytopenia, however without renal failure and/or neurological symptoms or coagulation abnormalities. The level of anemia remained stable during the hospitalization. These clinical features may be also associated to sepsis [6, 7], and the patient died shortly after being evaluated, with a clinical picture of likely septic shock. In any case, the diagnosis of CR-MAHA was excluded.
The most impressive feature of this case was the massive circulation of abnormal cells (15-20% of white cells) resembling an acute leukemia, that were demonstrated by FC to be of epithelial origin.
Rare tumor cells can be sometimes detected in the peripheral blood and bone marrow of patients with epithelial cancers, usually at very low concentration [8]. Many studies have shown that the number of circulating tumor cells (CTCs), as detected by a spectrum of techniques, can be correlated with disease progression and poor prognosis in various carcinomas [9-13]. It was reported that in about 58% of patients with BC, CTCs could be detected in peripheral blood, using immunomagnetic enrichment method based on fluorescent immunocytochemistry, with a sensitivity of one tumor cell in 1 × 107 peripheral blood mononuclear cells [14]. In another study, the CTCs were found in 71.4% of metastatic BC patients and the highest number of CTCs detected was 1,491 per 7.5 mL blood [15]. The direct detection of evident amounts of malignant tumor cells in peripheral blood by conventional FC is however reported in very few real-life studies [16, 17], with a maximum percentage of abnormal circulating cells between 5% and 9% of leukocytes.
In the present case, the FC analysis allowed the characterization of the non-hematological nature of an abnormal cell population, circulating at unusually high level in the peripheral blood (about 15-20% of white cells), mimicking AML. The resulting phenotyping of this population was CD45- CD34- CD13- CD33- CD38- CD9+ CD71+ CD117+ CD138+ CD326+. The initial AML hypothesis was supported by the positivity for CD117 and CD71. These markers are typically expressed in erythroid leukemia, in absence of CD45 and CD34 (FAB: M6), nevertheless the co-expression of CD138 and CD326 in erythroid leukemia has never been reported.
It is known that CD117 is expressed on KIT-dependent cell types, including mast cells, some hematopoietic stem cells, germ cells, melanocytes and erythroid precursors [18]. CD117-KIT is also expressed in pulmonary and other small cell carcinomas, adenoid cystic carcinoma, renal chromophobe carcinoma, thymoma, some ovarian cancers and in about 29% of BCs [19]. The lack of CD117 expression in invasive BC was reported to be associated with features of more aggressive tumor behavior with lymph node metastasis and negativity of ER and PgR [20]. Conversely, in the present report, a case of invasive CD117 positive BC is described. Furthermore, CD117 can be detected among 30% of malignant plasma cells, as identified by the CD138 lineage marker [21]. In our case, circulating BC cells co-expressed both markers. As reported by several studies, the expression of CD138 is also typically observed on the surface of mature epithelial cells (squamous and transition types) and in a variety of soft tissue tumors [22, 23]. In BC, the CD138 expression is controversial. Some studies reported cases of strong expression of CD138 on cancer cells in metastatic lobular carcinoma associated to poor prognosis [24, 25]. Other authors reported the shift from membranous and stromal CD138 expression to cytoplasmic CD138 expression as associated with poor prognosis in BC [26].
In this case report, FC allowed to quickly identify the epithelial nature of the abnormal circulating cells by the use of CD326 (EpCAM) marker. EpCAM is a transmembrane cell surface glycoprotein that is highly expressed in epithelial cancers and at lower levels in normal epithelia. Due to its frequent overexpression in carcinomas, EpCAM has been used as a diagnostic marker [27]. Several studies reported the usefulness of this marker for the detection of cancer cells by FC in various biological samples [28, 29]. Some groups have previously proposed different panels to identify non-hematopoietic neoplasms, including EpCAM in association with other markers such as CD45, CD56, CD71 or CD99 [30, 31]. In this study, we used a panel including CD326, CD138, CD45, CD117, CD71 and CD9. As reported by other authors, CD9 is widely expressed in various normal and cancer tissues [32, 33]. Non-neoplastic mammary epithelial cells were observed to be negative or weakly positive for CD9 expression, while the stromal inflammatory cells exhibited moderate-to-strong immunoreactivity for CD9 [34]. Baek and colleagues demonstrated that CD9 expression in patients with invasive lobular breast carcinoma is associated with poor prognosis [35]. Other authors reported an association between the overexpression of CD9 in human BC cells and the development of bone metastases [36]. Here we have described a case of metastatic BC, with peripheral blood and bone marrow invasion, strongly expressing CD9 and CD326, as evaluated by FC.
In conclusion, although FC is widely used in the diagnosis of hematological neoplasms and it is not routinely used in the diagnosis or follow-up of non-hematopoietic neoplasms, we demonstrated its usefulness, in a rare case with high-grade invasion of peripheral blood. The major limiting factor of this approach resides in the need of highlighting clusters of large sized CD45-negative cells, that may be overlooked in ordinary immunophenotypic analyses of peripheral blood, bone marrow or lymph node aspirates, when admixed with cell debris. It is reasonable to think that CD45-negative epithelial cell contaminants may become self-evident during routine FC analyses of hematopoietic cell suspensions only when greater than 4-5%.
FC may play the role of a useful ancillary test to conventional cytology and immunohistochemistry during the analysis of peripheral blood, bone marrow and lymph node aspirates showing unexpected clusters of CD45-negative cells.
Acknowledgments
None to declare.
Financial Disclosure
There is no external funding for this article.
Conflict of Interest
The authors declare no potential conflict of interest.
Informed Consent
According to our Ethical Committee, informed consent is not requested in retrospective clinical case studies after the death of the patient.
Author Contributions
AG and BB prepared the manuscript; IC, NV, LB, MS, SM, DM and LC managed the clinical case, collected the data and reviewed the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Valentini CG, Fianchi L, Voso MT, Caira M, Leone G, Pagano L. Incidence of acute myeloid leukemia after breast cancer. Mediterr J Hematol Infect Dis. 2011;3(1):e2011069.
doi pubmed pmc - Martin MG, Welch JS, Luo J, Ellis MJ, Graubert TA, Walter MJ. Therapy related acute myeloid leukemia in breast cancer survivors, a population-based study. Breast Cancer Res Treat. 2009;118(3):593-598.
doi pubmed pmc - Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91(4):195-205.
doi pubmed - Thomas MR, Scully M. How I treat microangiopathic hemolytic anemia in patients with cancer. Blood. 2021;137(10):1310-1317.
doi pubmed pmc - Takabatake D, Oishi K. Microangiopathic hemolytic anemia associated with metastatic breast cancer: case report and literature review. Springerplus. 2016;5(1):684.
doi pubmed pmc - Zini G, d'Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, McFadden S, et al. ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol. 2012;34(2):107-116.
doi pubmed - Patel JJ, Taneja A, Niccum D, Kumar G, Jacobs E, Nanchal R. The association of serum bilirubin levels on the outcomes of severe sepsis. J Intensive Care Med. 2015;30(1):23-29.
doi pubmed - Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, Zhu F, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6(1):404.
doi pubmed pmc - Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781-791.
doi pubmed - Potdar PD, Lotey NK. Role of circulating tumor cells in future diagnosis and therapy of cancer. J Cancer Metastasis Treat. 2015;1:44-56.
- Tieng FYF, Abu N, Nasir SN, Lee LH, Ab Mutalib NS. Liquid biopsy-based colorectal cancer screening via surface markers of circulating tumor cells. Diagnostics (Basel). 2021;11(11):2136.
doi pubmed pmc - Garrido Castillo LN, Mejean A, Vielh P, Anract J, Decina A, Nalpas B, Benali-Furet N, et al. Predictive Value of Circulating Tumor Cells Detected by ISET((R)) in Patients with Non-Metastatic Prostate Cancer Undergoing Radical Prostatectomy. Life (Basel). 2022;12(2):165.
doi pubmed pmc - Gruijs M, Zeelen C, Hellingman T, Smit J, Borm FJ, Kazemier G, Dickhoff C, et al. Detection of Circulating Tumor Cells Using the Attune NxT. Int J Mol Sci. 2022;24(1):165-179.
doi pubmed pmc - Cai QQ, Huang HQ, Lin TX, Jiang WQ. [Detection and clinical significance of circulating tumor cells in peripheral blood of breast cancer patients]. Ai Zheng. 2005;24(7):837-841.
pubmed - Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920-928.
doi pubmed - Rowan DJ, Logunova V, van Tuinen P, Olteanu H, Peterson JF. Circulating breast carcinoma cells mimicking therapy-related acute myeloid leukemia. Int J Surg Pathol. 2017;25(1):87-93.
doi pubmed - De Miguel Sanchez C, Robles de Castro D, Cordoba Alonso AI, Guinea de Castro JM. Diffuse bone marrow metastases of small cell carcinoma with associated erythrophagocytosis mimicking acute leukaemia. Hematol Transfus Cell Ther. 2022;44(3):459-461.
doi pubmed pmc - Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13(3):205-220.
doi pubmed - Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol. 2004;202(3):375-381.
doi pubmed - Amin MM, El-Hawary AK, Farouk O. Relation of CD117 immunoreactivity and microvascular density in invasive breast carcinoma. Indian J Pathol Microbiol. 2012;55(4):456-460.
doi pubmed - Chen F, Hu Y, Wang X, Fu S, Liu Z, Zhang J. Expression of CD81 and CD117 in plasma cell myeloma and the relationship to prognosis. Cancer Med. 2018;7(12):5920-5927.
doi pubmed pmc - O'Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121(2):254-263.
doi pubmed - Orosz Z, Kopper L. Syndecan-1 expression in different soft tissue tumours. Anticancer Res. 2001;21(1B):733-737.
pubmed - Nguyen TL, Grizzle WE, Zhang K, Hameed O, Siegal GP, Wei S. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am J Clin Pathol. 2013;140(4):468-474.
doi pubmed - Canevari M, Wells J, Pryor E. Metastatic CD138+ lobular breast carcinoma mimicking a plasma cell neoplasm. American Journal of Clinical Pathology. 2019;152(Supplement 1):S107.
- Kind S, Jaretzke A, Buscheck F, Moller K, Dum D, Hoflmayer D, Hinsch A, et al. A shift from membranous and stromal syndecan-1 (CD138) expression to cytoplasmic CD138 expression is associated with poor prognosis in breast cancer. Mol Carcinog. 2019;58(12):2306-2315.
doi pubmed - Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122-128.
doi pubmed - Wong-Arteta J, Rey M, Aragon L, Gil-Rodriguez E, Bujanda L. The utility of flow cytometry in the diagnostic work up of malignant effusions due to nonhematopoietic neoplasms. Cytometry B Clin Cytom. 2020;98(6):504-515.
doi pubmed - Annunziata J, Miller ML, Park DC, Vlad G, Bhagat G, Alobeid B. Detection of nonhematologic neoplasms by routine flow cytometry analysis. Am J Clin Pathol. 2020;153(1):99-104.
doi pubmed - Kentrou NA, Tsagarakis NJ, Tzanetou K, Damala M, Papadimitriou KA, Skoumi D, Stratigaki A, et al. An improved flow cytometric assay for detection and discrimination between malignant cells and atypical mesothelial cells, in serous cavity effusions. Cytometry B Clin Cytom. 2011;80(5):324-334.
doi pubmed - Chang A, Benda PM, Wood BL, Kussick SJ. Lineage-specific identification of nonhematopoietic neoplasms by flow cytometry. Am J Clin Pathol. 2003;119(5):643-655.
doi pubmed - Lazo PA. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci. 2007;98(11):1666-1677.
doi pubmed pmc - Huan J, Gao Y, Xu J, Sheng W, Zhu W, Zhang S, Cao J, et al. Overexpression of CD9 correlates with tumor stage and lymph node metastasis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(3):3054-3061.
pubmed pmc - Kwon HJ, Choi JE, Kang SH, Son Y, Bae YK. Prognostic significance of CD9 expression differs between tumour cells and stromal immune cells, and depends on the molecular subtype of the invasive breast carcinoma. Histopathology. 2017;70(7):1155-1165.
doi pubmed - Baek J, Jang N, Choi JE, Kim JR, Bae YK. CD9 expression in tumor cells is associated with poor prognosis in patients with invasive lobular carcinoma. J Breast Cancer. 2019;22(1):77-85.
doi pubmed pmc - Kischel P, Bellahcene A, Deux B, Lamour V, Dobson R, E DEP, Clezardin P, et al. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer Res. 2012;32(12):5211-5220.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


