| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 3, June 2024, pages 99-103
A Hemoglobinopathy That Produces an Array of Different Hemoglobin A1c Values
Maximo J. Marina, Bremansu Osa-Andrewsa, Patrick A. Mahera, Clive Wasserfalla, William E. Wintera, Ashraf B. Muzwagib, Neil S. Harrisa, c
aDepartment of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine Gainesville, FL, USA
bDepartment of Psychiatry, University of Florida College of Medicine Gainesville, FL, USA
cCorresponding Author: Neil S. Harris, Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL 32610, USA
Manuscript submitted March 22, 2024, accepted April 23, 2024, published online June 28, 2024
Short title: Hemoglobin Wayne and HbA1c
doi: https://doi.org/10.14740/jh1268
| Abstract | ▴Top |
Hemoglobin A1c (HbA1c) refers to non-enzymatically glycated hemoglobin and reflects the patient’s glycemic status over approximately 3 months. An elevated HbA1c over 6.5% National Glycohemoglobin Standardization Program (NGSP) (48 mmol/mol the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)) can be used to diagnose diabetes mellitus. In our laboratory, HbA1c is determined by ion-exchange chromatography which has the advantage of detecting common Hb variants such as Hb S, C, E and D without adversely affecting the HbA1c determination. Certain homozygous or compound heterozygous hemoglobinopathies such as homozygous sickle disease and Hb SC disease can significantly lower the HbA1c by reducing red cell lifespan. Occasionally however, rare and mostly benign hemoglobinopathies can interfere with this technique resulting in an apparent elevation of HbA1c in an otherwise non-diabetic patient. In this report, we describe such a hemoglobinopathy termed Hb Wayne that resulted in a significant HbA1c elevation in a normoglycemic individual. HbA1c was determined by multiple methods including immunoassay, a modified capillary electrophoresis and an alternative ion-exchange system. These techniques yielded significantly lower A1c results, more in keeping with the patient’s clinical background. The alternative ion-exchange system resulted in a low A1c that was qualified by warning flags on the chromatogram that indicated the result was not reportable. The hemoglobinopathy in question, Hb Wayne, is a frameshift mutation in the alpha globin gene that results in an extended alpha globin polypeptide that can form two variants Hb Wayne I and Wayne II. Hb Wayne is a clinically silent asymptomatic disorder with no hematologic consequences. The artifactual elevation of HbA1c is, in contrast, very significant because it may result in a misdiagnosis of diabetes mellitus leading to unnecessary treatment. In this report, we compare our findings with other descriptions of Hb Wayne in the literature and corroborate a number of previous observations and conclusions.
Keywords: Hemoglobin A1c; Globin disorder; Hb Wayne; Ion-exchange chromatography; Frameshift mutation
| Introduction | ▴Top |
Hemoglobin A1c (HbA1c) refers specifically to adult hemoglobin A (HbA) that is non-enzymatically glycated at the N-terminal valine of the β globin subunit [1, 2]. HbA1c is expressed either as a percentage of the total adult HbA (National Glycohemoglobin Standardization Program (NGSP) units) or as mmol/mol of HbA (the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) units). HbA1c gives a measure of the average blood glucose over the past 3 months [1, 2], provided the red cell lifespan is normal and is used in the diagnosis and management of diabetes mellitus. Since 2010, HbA1c has been used to diagnose diabetes; this diagnosis can be applied if the HbA1c is ≥ 6.5% (48 mmol/mol) [2]. A shortened red cell lifespan will reduce the HbA1c percentage. Homozygous hemoglobinopathies can also show glycation at the β N-terminus but such hemoglobinopathies may reduce the red cell lifespan (e.g., sickle cell anemia). HbA1c can be identified by ion-exchange chromatography, typically cation exchange high-performance liquid chromatography (HPLC) [2, 3]. This method also detects the presence of other abnormal hemoglobins if present. Indeed, HbA1c was first identified by Allen, Schroeder, and Balog using cation exchange chromatography [3]. Standard Hb electrophoresis in alkaline agarose gels or by means of the newer capillary zone electrophoresis (CZE) cannot identify A1c. CZE refers to electrophoresis performed in a fused silica capillary at a very high voltage in the order of 9 - 10 kV. However, modifications such as the incorporation of borate anions into the CZE buffers have been used to efficiently separate HbA1c [2, 4]. A commonly used technique to measure HbA1c is by immunoassay which requires an antibody directed to the 4 or 5 N-terminal amino acids of the β chain and the attached carbohydrate adduct [1, 2, 5]. This latter method can be automated and scaled down for use as a point-of-care device. The disadvantage of this technique is that it fails to recognize hemoglobinopathies such as sickle cell disease and Hb SC disease that reduce the red cell lifespan. Another technique is boronate affinity chromatography [1, 2] which measures total glycated Hb but does not separate Hb variants. Glycated Hbs contain 1,2-cis-diol groups not found in non-glycated proteins. M-aminophenylboronic acid, fixed to a solid support, reacts with these cis-diol groups. Hemoglobin can thereby be separated into two fractions: glycated and non-glycated. Empirical formulas are used to convert the total glycated Hb fraction to an HbA1c value [2]. In our laboratory, we have chosen to use cation exchange chromatography to measure HbA1c. However, rare hemoglobinopathies can interfere with this method by eluting close to the HbA1c peak despite a normal red cell lifespan. In this report, we describe such a case that could have led to the misdiagnosis of hyperglycemia and diabetes mellitus in a non-diabetic patient. In addition, different analytical methods yielded different HbA1c values because of the hemoglobinopathy.
| Case Report | ▴Top |
Investigations
The patient is an adolescent female who was admitted to a psychiatric institution involuntarily for evaluation of worsening depression, engaging in conversations by herself, bizarre behavior and a suicide safety precaution. She has had a history of autism spectrum with intellectual developmental disorder, attention deficit hyperactivity disorder (ADHD), major depressive disorder-moderate and absence seizure. Routine laboratory studies, including an HbA1c measurement, were obtained as a baseline before considering starting an antipsychotic medication. The patient had no history of diabetes. Fasting plasma glucose on admission was 85 mg/dL (4.7 mmol/L) and 4 days later was 72 mg/dL (4.0 mmol/L) (reference interval (RI): 65 - 99 mg/dL (3.6 - 5.5 mmol/L)). An elevated HbA1c (BioRad D100 cation exchange HPLC, Hercules, CA, USA) of 11.5% NGSP (102.2 mmol/mol IFCC) (RI: > 6.5% (47.5 mmol/L) diabetic, 5.7-6.4% (38.8 - 46.4 mmol/mol) pre-diabetic, and < 5.7% (38.8 mmol/mol) non-diabetic) was recorded on the admission date (Fig. 1a). Our laboratory uses cation exchange HPLC because, unlike immunoassays, it allows identification of common hemoglobinopathies in our patient population that has a high prevalence of sickle cell disease which invalidates HbA1c determination. There was also an apparent elevation of fetal Hb (HbF) of 8.2% (RI: < 2.1%). The instrument did issue a warning flag to the operator that stated “suspected hemoglobinopathy”. In our laboratory, such warnings require review by a pathologist. The patient’s complete blood count (CBC) showed a white cell count of 5.6 × 109/L (RI: 4.5 - 13.5), a red cell count of 4.38 × 1012/L (RI: 4.1 - 5.1), Hb 10.8 g/dL (RI: 12 - 16) (108 g/L, RI: 120 - 160), hematocrit 34.2% (RI: 36 - 46), mean cell volume of 78.1 fL (RI: 78 - 100) and a red cell distribution width of 16.9% (RI: 11 - 14).
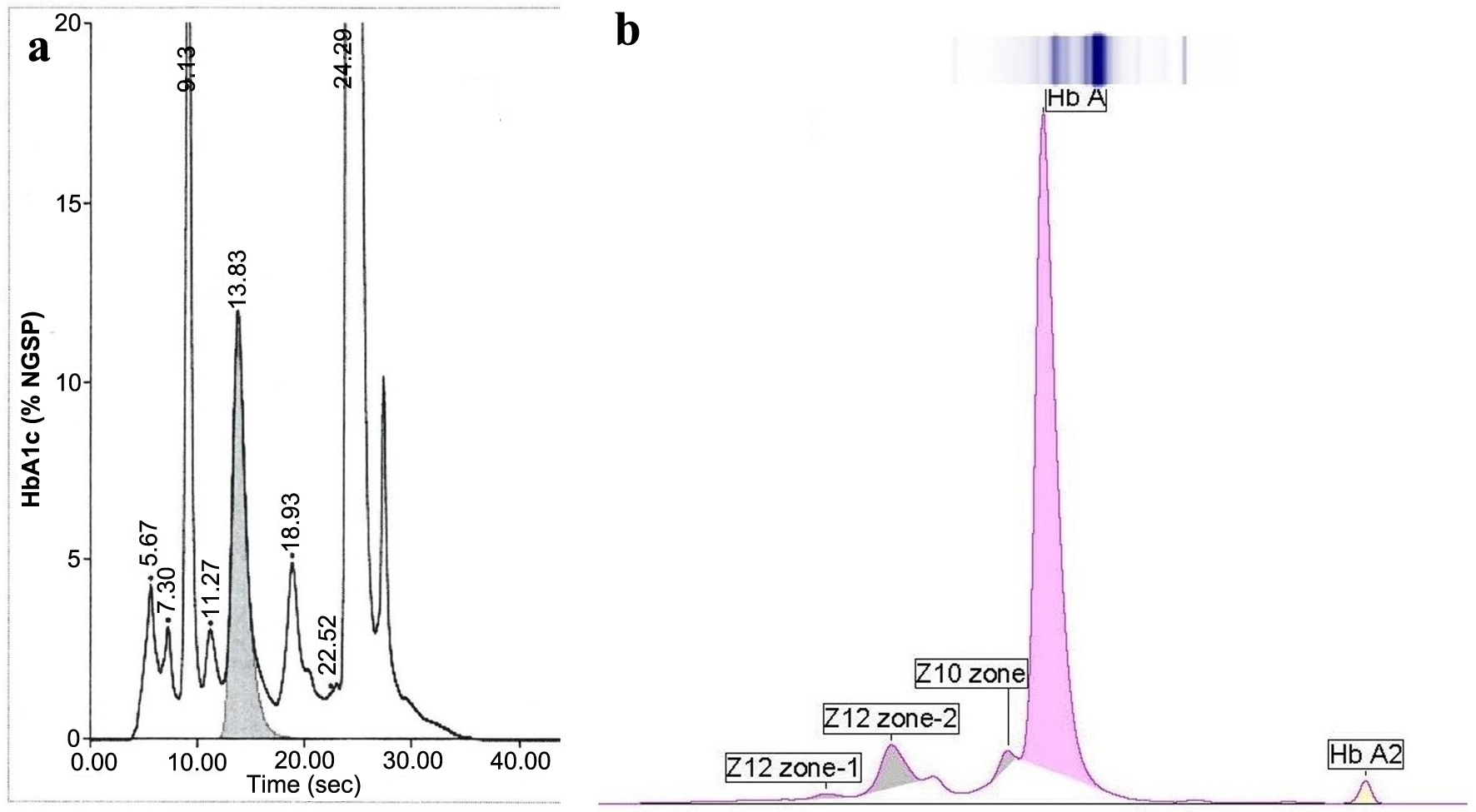 Click for large image | Figure 1. (a) Hemoglobin A1c analysis using BioRad D100 HPLC (A1c 11.5% NGSP) (102 mmol/mol IFCC). (b) Hemoglobin electrophoresis by CZE (Sebia Capillarys). This method does not show the HbA1c. CZE: capillary zone electrophoresis; IFCC: The International Federation of Clinical Chemistry and Laboratory Medicine; NGSP: National Glycohemoglobin Standardization Program. |
Diagnosis
Because of the discrepancy between the HbA1c and blood glucose, an Hb analysis was performed by CZE (Sebia Capillarys, Norcross, GA, USA) (Fig. 1b). Three small variant bands were identified, anodal to Hb A, the largest of which was 6.4% with a mobility position of 106 in the Z12 zone. For reference, Hb A migrates in zone Z9 with a mobility of 150. Typically, this type of electrophoretic Hb analysis is designed to detect Hb variants but is unable to quantitate the HbA1c.
HbA1c was then analyzed by additional platforms/methods including an alternative cation exchange HPLC (Tosoh G8 Tosoh Bioscience, Inc., South San Francisco, CA, USA) (Fig. 2a). This demonstrated several unknown peaks including P-HV3 which is the elution position of Hb E [6]. Other methods used for analysis included immunoassay (Beckman Coulter AU680, Beckman Coulter, Inc., Brea, CA, USA) as well as CZE in the A1c mode (Sebia Capillarys) (Fig. 2b). This latter electrophoretic method uses a unique proprietary buffer and program that allows separation of and quantitation of HbA1c unlike standard Hb electrophoresis. In this report, this latter method is referred to as “Sebia Capillarys HbA1c mode”. HbA1c results are displayed in Figures 1 and 2 and in Table 1. The relevant chromatographic and electrophoretic profiles are shown in Figures 1 and 2.
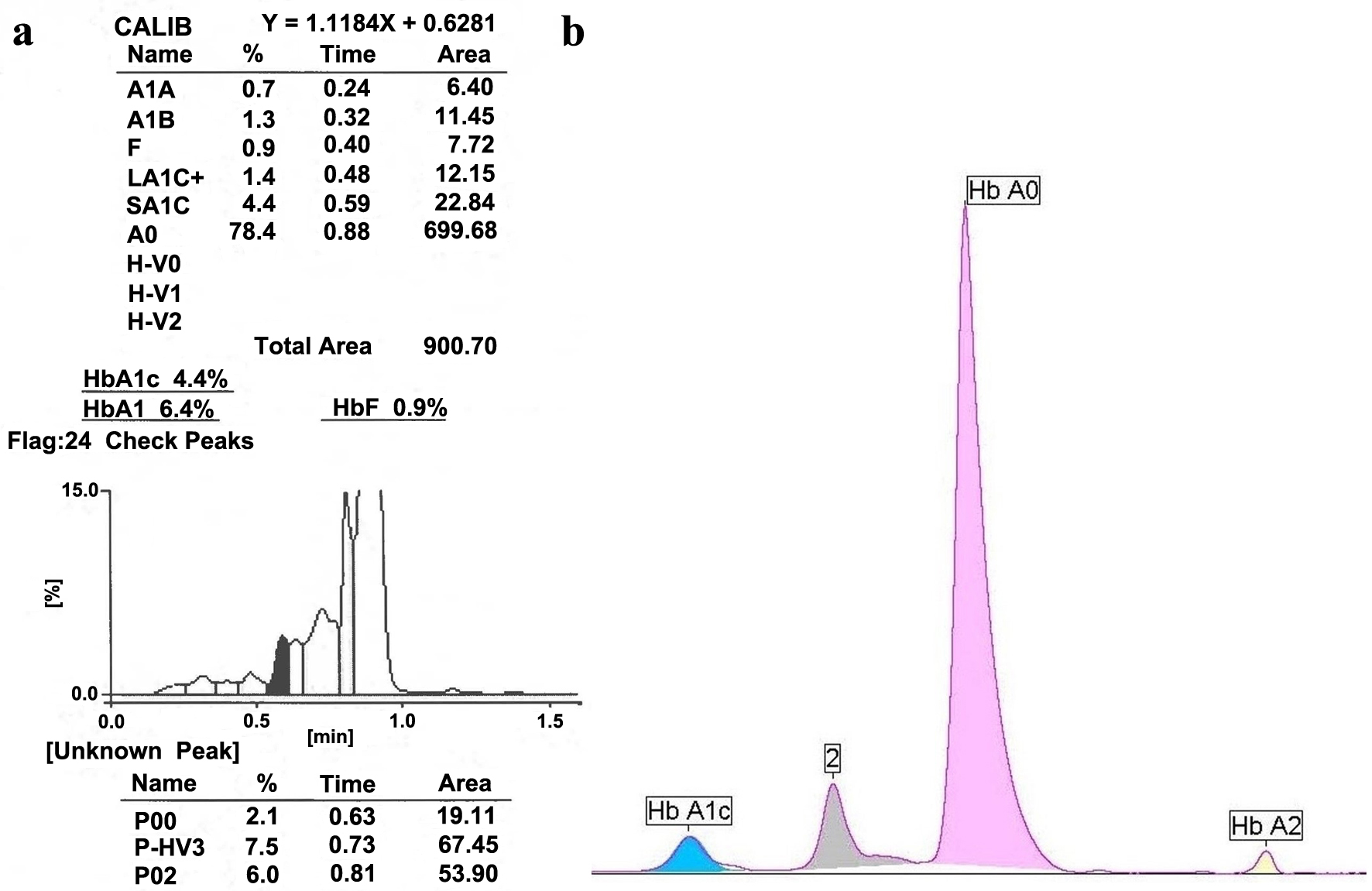 Click for large image | Figure 2. The specimen was analyzed by two additional separation modalities. (a) Tosoh G8 HPLC (A1c 4.4% NGSP (25 mmol/mol IFCC)). The P00, P-HV3 and P02 are variant peaks that would have prevented release of the A1c result. (b) Sebia Capillarys HbA1c Mode (A1c 5.7% NGSP (39 mmol/mol IFCC)). Refer to Table 1. HPLC: high-performance liquid chromatography; IFCC: The International Federation of Clinical Chemistry and Laboratory Medicine; NGSP: National Glycohemoglobin Standardization Program. |
 Click to view | Table 1. Hemoglobin A1c Determination by Four Different Methods |
Treatment
During her hospitalization, home psychotropic medications; Intuniv 4 mg and ethosuximide 1,000 mg were continued for ADHD and absence seizure consequently. Focalin 10 mg, and Focalin XR 25 mg were held off, pending workup for seizure and medical cause for altered mental status. Once medical issues and seizure worsening were excluded, her Intuniv was decreased to 3 mg for ADHD given side effects of low blood pressure and being drowsy. Prozac was started and titrated to 20 mg for mood and psychostimulant restarted for ADHD. She was discharged stable at baseline. She continued stability with a monthly follow-up with a child psychiatrist. No treatment for diabetes was ever instituted because of the communication with the laboratory.
Follow-up and outcomes
Globin Gene analysis was performed at ARUP Laboratories (Salt Lake City, UT, USA). A heterozygous frameshift mutation known as Hb Wayne was identified in an alpha-2 globin gene [7].
| Discussion | ▴Top |
Szuberski et al have identified 62 cases of Hb Wayne in the USA over a 16-year period [8]. Hb Wayne is a clinically silent alpha globin chain variant. It is silent because only one of the four alpha globin genes is affected. However, isolated Hb Wayne has high oxygen affinity and a markedly reduced Bohr effect mainly due to the switch in residue 139 [9]. The Hb Wayne mutation eliminates a stop codon that results in replacement of the last three amino acids (Lys-Tyr-Arg) with eight novel amino acids (Asn-Thr-Val-Lys-Leu-Glu-Pro-Arg) at the C-terminus [7]. The ninth codon after the frameshift is the new stop codon [7]. The Human Genome Variation Society (HGVS) nomenclature for Hb Wayne is p.Lys140AsnfsTer9. An alternative name is Lys139fs when numbered from the mature protein.
The Hb Wayne frameshift mutation leads to two Hb Wayne isoforms. The asparagine at position 139 (when numbered from the mature protein) can undergo spontaneous deamidation to aspartate resulting in the formation of Hb Wayne I and Hb Wayne II, respectively [7].
Hb Wayne is known to interfere with HbA1c measurement by ion-exchange chromatography as was observed in this case. Specifically, it leads to an elevation of HbA1c resulting in the misdiagnosis of diabetes in an otherwise normal patient [10-12]. In this particular case, the HPLC profile demonstrated another abnormality due to the apparent elevation of HbF to 8.23%. The analyzer also issued a “suspected hemoglobinopathy” warning flag. In 2017 Rodriguez-Capote et al published a case series where HbA1c was determined by five different methods in 12 cases of Hb Wayne [10]. Our data corroborate these findings, namely: 1) The HbA1c determined by cation exchange chromatography on a BioRad D100 system shows an elevated HbA1c as well as an elevated HbF. 2) An immunoassay resulted in an A1c that was significantly lower than the above result and within the normal/non-diabetic RI. 3) The A1c measured by the Sebia Capillarys CZE (in the A1c mode) was also within the normal RI but was slightly higher than the immunoassay A1c. 4) The algorithm proposed by Rodriguez-Capote et al [10] assisted in the identification of Hb Wayne. The algorithm is initiated by finding an HbF > 7% and an HbA1c > 9% (75 mmol/mol) with a discordant blood glucose and a discordant HbA1c by immunoassay. 5) The Tosoh G8 instrument indicated the possible presence of Hb E at 7.5% as indicated by a peak labelled as P-HV3 [6]. This was also noted in the report by Rodriguez-Capote et al. The Tosoh G8 chromatogram did yield an apparent A1c result albeit with warning flags to check the result. Because of the unidentified peaks on the HPLC profile, the Tosoh G8, HbA1c was deemed unreportable. The latter measurement of 4.4% (25 mmol/mol IFCC) was much lower than the other methods used in our analysis.
Which is the “true” HbA1c? Inaccurate results could be reported even if the manufacturer’s instructions are followed depending on the level of scrutiny by the laboratory personnel and the attending pathologist. In our laboratory, we follow a policy that all HbA1c values above 15% NGSP (140 mmol/mol IFCC) be reviewed prior to release. In this particular case, the elevated HbA1c was not high enough to meet this particular criterion. Based on the data of Rodriguez-Capote et al, the immunoassay result and the Sebia Capillarys CZE (A1c mode) are most likely reflective of the true glycemic status of the patient. Immunoassays typically recognize epitopes at the N-terminus of the beta globin chain [5] and are unlikely to be affected by a frameshift mutation at the C-terminus of the alpha chain. It is uncertain if the immunoassay result is significantly different (from a clinical perspective) from the CZE HbA1c mode data (5.2 vs. 5.7% NGSP (33.3 mmol/mol vs. 38.8 mmol/mol)), a difference of 0.5% NGSP units or a percentage difference of 9%. It is worth noting however that the College of American Pathologists proficiency testing (external quality assurance (EQA)) for glycated Hb only allows for a percentage difference of 6% or less when measured in NGSP percentage units.
In summary, we describe a case of heterozygous Hb Wayne that presented as an artificially high HbA1c and HbF as measured by the BioRad D100 HPLC analyzer. The sample was analyzed by different modalities leading to results within the normal RI but still somewhat discrepant from one another. Hb Wayne is a benign hemoglobinopathy in that it has no hematologic consequences but can alter the determination of HbA1c with significant clinical effects and lead to the misdiagnosis of diabetes mellitus and unnecessary treatment. It is important both for clinicians and laboratory workers alike to be cognizant of the HbA1c methods used by the diagnostic laboratory.
Acknowledgments
A special thanks to the staff of the Shands Core Laboratory, in particular Elisha Roberts and to Pinal Patel at UF Health Pathology Laboratories.
Financial Disclosure
Authors have no financial disclosure to report.
Conflict of Interest
The authors declare that they have no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
NSH documented the case together with ABM and PAM, and MJM. NSH, BOA, PAM, CW and WEW collected and analyzed data. NSH, ABM and MJM wrote and approved the final paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CZE: capillary zone electrophoresis; EQA: external quality assurance; HbA1c: hemoglobin A1c; HGVS: Human Genome Variation Society; HPLC: high-performance liquid chromatography; IFCC: The International Federation of Clinical Chemistry and Laboratory Medicine; NGSP: National Glycohemoglobin Standardization Program
| References | ▴Top |
- Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33(6):393-400.
doi pubmed pmc - Harris NS, Weaver KD, Beal SG, Winter WE. The interaction between Hb A1C and selected genetic factors in the African American population in the USA. J Appl Lab Med. 2021;6(1):167-179.
doi pubmed - Allen D, Schroeder W, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin - a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Am Chem Soc. 1958;80(7):1628-1634.
- Koval D, Kasicka V, Cottet H. Analysis of glycated hemoglobin A1c by capillary electrophoresis and capillary isoelectric focusing. Anal Biochem. 2011;413(1):8-15.
doi pubmed - Chang J, Hoke C, Ettinger B, Penerian G. Evaluation and interference study of hemoglobin A1c measured by turbidimetric inhibition immunoassay. Am J Clin Pathol. 1998;109(3):274-278.
doi pubmed - G8 variant analysis mode training manual V2.0. South San Francisco, CA: Tosoh Bioscience, Inc; 2021. p. 26-31.
- Seid-Akhavan M, Winter WP, Abramson RK, Rucknagel DL. Hemoglobin Wayne: a frameshift mutation detected in human hemoglobin alpha chains. Proc Natl Acad Sci U S A. 1976;73(3):882-886.
doi pubmed pmc - Szuberski J, Oliveira JL, Hoyer JD. A comprehensive analysis of hemoglobin variants by high-performance liquid chromatography (HPLC). Int J Lab Hematol. 2012;34(6):594-604.
doi pubmed - Moo-Penn WF, Jue DL, Johnson MH, McDonald MJ, Turci SM, Shih TB, Jones RT, et al. Structural and functional studies of hemoglobin Wayne: an elongated alpha-chain variant. J Mol Biol. 1984;180(4):1119-1140.
doi pubmed - Rodriguez-Capote K, Estey MP, Barakauskas VE, Burton T, Holmes D, Krause R, Higgins TN. Identification of Hb Wayne and its effects on HbA1c measurement by 5 methods. Clin Biochem. 2015;48(16-17):1144-1150.
doi pubmed - Mulpuri N, Bryant A, Shahin D, Soe K. The hemoglobin Wayne variant and association with falsely elevated HbA(1c). JCEM Case Rep. 2023;1(3):luad043.
doi pubmed pmc - Ao X, Ganta N, Choe S, Patel P, Turro J, Cheriyath P. Hemoglobin Wayne: a rare variant that can cause falsely elevated hemoglobin A1c. Cureus. 2022;14(7):e26559.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


