| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 4, August 2024, pages 150-157
Disparities in Acute Lymphocytic Leukemia Outcomes Among Young Adults
Zoe McKinnella, b, f, Daniel Tuerffb, c, Mustafa Hammudib, c, Colleen Hamiltond, Martha Antonioe, Ramesh Subrahmanyamb, Joao Ascensaob, Maneesh Rajiv Jaina, b
aDivision of Hematology-Oncology, George Washington University Hospital, Washington, DC, USA
bDepartment of Hematology and Oncology, Washington DC VA Medical Center, Washington, DC, USA
cDivision of Internal Medicine, George Washington University Hospital, Washington, DC, USA
dDivision of Internal Medicine, Harbor UCLA Medical Center Internal Medicine, Torrance, CA, USA
eDepartment of Hematology and Oncology, Institute for Clinical Research, VA Medical Center, Washington, DC, USA
fCorresponding Author: Zoe McKinnell, Division of Hematology-Oncology, George Washington University Hospital, Washington, DC 20037, USA
Manuscript submitted June 6, 2024, accepted August 7, 2024, published online August 15, 2024
Short title: Disparities Among AYA Veterans With ALL
doi: https://doi.org/10.14740/jh1282
| Abstract | ▴Top |
Background: Age is a strong prognostic factor in acute lymphocytic leukemia (ALL), with children doing better than adults with the same disease. One hypothesis for this age-based disparity is differences in treatment regimens. Optimizing care for adolescents and young adults (AYA) with ALL has not been well defined and disparities in care exist. We conducted a retrospective study of all veterans with ALL diagnosed between the ages of 18 and 45 since the year 2000 to evaluate disparities among prognostication methods, treatment regimens, and accrual to clinical trials with regard to age and race/ethnicity and how these factors influence overall survival.
Methods: Electronic medical record data from the VA Informatics and Computing Infrastructure (VINCI) were used to identify 6,724 patients with an ICD-9 or 10 code for ALL. All patients were chart checked to confirm an ALL diagnosis between the ages of 18 and 45 and excluded if they were diagnosed before 2000, had childhood ALL, or if induction protocol was not recorded. A total of 252 patients were included in the final analysis. Multivariate analysis was performed with controls for age, ALL subtype (B, T, mixed phenotype), Ph status, cytogenetic risk (based on modified Medical Research Council-Eastern Cooperative Oncology Group (MRC-ECOG) study), obesity (body mass index (BMI) > 30), and race.
Results: Patients treated with pediatric regimens, including pediatric-inspired regimens, have statistically significant (P = 0.009) survival gains, with a hazard ratio (HR) of 0.52 after controlling for age, obesity, ALL subtype, cytogenetic risk and race. White patients had significantly improved OS compared to people of color (HR 0.57, P = 0.02) after controlling for the aforementioned covariates. Black patients were far less likely (23%) to receive a transplant than non-Black patients (46%). Only 7% of patients were treated on a clinical trial.
Conclusions: These data demonstrate that treatment with a pediatric regimen significantly improves overall survival in patients up to the age of 45 and suggests ongoing shortcomings in treatment for young adults with ALL, especially 30 to 45 years old, including persistently high use of adult induction regimens, low rates of referral to clinical trials, and significant racial disparities in bone marrow transplants for Black patients.
Keywords: Acute lymphocytic leukemia; Young adults; Health disparities; Asparaginase; Veterans health
| Introduction | ▴Top |
There is a drastic difference in survival outcomes between children up to 17 years old and adults up to age 45 with acute lymphoblastic leukemia (ALL). This drastic difference in survival outcomes has been previously described as a “survival cliff” [1]. By the numbers, the 5-year relative survival rate for people < 20 years old is 90%, whereas the 5-year survival rate for people > 20 years old is 43% [2]. Optimizing care for adolescents and young adults (AYA) with ALL who are on the edge of this “survival cliff” has not been well defined and disparities in care exist.
In addition to age, several important factors have been attributed to the “survival cliff” including treatment regimen, biologic type, and accrual to clinical trials [3]. Current NCCN guidelines say it is acceptable to treat young adult patients with either pediatric, “pediatric-inspired” or adult regimens [4]. In general, pediatric regimens are comprised of more intensive dosing of glucocorticoids, vincristine, and L-asparaginase, as well as more intensive and prolonged central nervous system prophylaxis compared to adult regimens [5]. Notably, the definition of pediatric and pediatric-inspired regimens has not been clearly defined in the literature and there are significant variations among the drugs, timing, and dosing between regimens. Both pediatric [6] and adult [7] regimens have been shown to be effective in treating ALL in young adults in prospective studies at academic centers. Consequently, differing approaches are used depending on the treatment center.
In addition to differences of treatment regimens administered, there is a paucity of data with regard to biologic subtype, cytogenetic classification, and prognostication for young adults with ALL. Apart from Philadelphia chromosome positivity, the cytogenetic classification and prognostication tools which have been validated in children and adults have not been specifically applied to the AYA population [8]. Lastly, clinical trial accrual differs drastically between children and young adults. Only 2% of adolescents and young adults with ALL enroll in clinical trials compared to 60% of pediatric patients [9].
The US Department of Veterans Affairs (VA) health care system serves more than 20 million US veterans and is the largest integrated health care system for cancer in the USA. The VA is considered an equal-access system compared with other large health care systems and thus provides a unique environment to investigate health disparities among young adults with ALL [10].
In this case-control study, we used the VA database to complement previous work looking at AYA patients with ALL and present a large real-world study of veterans up to the age of 45. We evaluated disparities among prognostication methods, treatment regimens, and accrual to clinical trials with regard to age and race/ethnicity and how these factors influence overall survival (OS).
| Materials and Methods | ▴Top |
Data sources and study populations
Utilizing the Veterans Affairs Informatics and Computing Infrastructure (VINCI) database, we performed a case-control study of all veterans with ALL diagnosed between the ages of 18 and 45 from January 2000 to December 2022. Retrospective data collection and review was approved by Washington DC VA IRB Committee. ICD-9 and 10 codes were used to identify patients with ALL. The age cutoff of 45 was chosen, rather than 39 which typically defines the AYA population, to specifically evaluate the “older young adult population”. Furthermore, pediatric regimens have been trialed in patients up to the age of 50 and there is a discussion that the age cutoff of 39 is arbitrarily defined [11]. VINCI contains patient demographic, clinical, and treatment data as well as outcome information. To perform a comprehensive and accurate analysis, all patients were chart checked to confirm an ALL diagnosis and excluded if they were diagnosed before the year 2000, had childhood ALL, or if an induction protocol was not recorded. The commonest reason for exclusion was an incorrectly entered ICD-9/10 code.
Study variables and end points
To evaluate the effects of treatment regimen and cytogenetic risk on OA, these variables were documented manually by chart check using the Joint Longitudinal Viewer (JLV) electronic medical record (EMR). Data from chart checkers were compared to ensure consistency and accuracy. Factors including race, ethnicity, date of birth, date of death, and body mass index (BMI) were used as controls in the analyses and were pulled directly from the VINCI database. The modified Medical Research Council-Eastern Cooperative Oncology Group (MRC-ECOG) cytogenetic risk system scoring system was used to risk stratify patients as high, intermediate, or standard risk [12]. Of the previously established cytogenetic risk classifications for Philadelphia chromosome negative (Ph-) ALL, this scoring system was the only independent prognosticator of leukemia-free survival [12]. Philadelphia chromosome positive (Ph+) patients were classified separately. Induction chemotherapy regimens were classified as pediatric, pediatric-inspired, or adult based on their description in the associated randomized clinical trial (Supplementary Material 1, www.thejh.org). There is no strict definition of a pediatric regimen in the literature and the categorizations are debatable. For this study, if the regimen described in the patient chart was not directly referred to as “pediatric”, “pediatric-inspired”, or “adult”, we classified regimens designed for children as “pediatric” and classified regimens designed for adults but inspired by pediatric regimens as “pediatric-inspired”. Characteristics of pediatric-inspired regimens we used were high doses of asparaginase, methotrexate or intrathecal methotrexate, or steroids. Given the advent of new treatment and monitoring options including blinatumomab, inotuzumab, chimeric antigen receptor T cells (CAR-T), and minimal residual disease (MRD) status which were first approved in 2014 [13-16], separate survival analyses were done for patients diagnosed before and after 2014.
Statistical analysis
Kaplan-Meier and Cox regression analyses were used to compare OS between patients treated on a pediatric-inspired regimen and patients treated on an adult regimen. Multivariate analysis was performed with controls for age, ALL subtype (B, T, mixed phenotype), Ph status, cytogenetic risk (based on modified MRC-ECOG study based on a phase III trial of 140 patients with evaluable cytogenetics, Supplementary Material 2, www.thejh.org), obesity (BMI > 30), and race. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using multivariable Cox regression to estimate associations with risk of 5-year death. To account for transplant, we analyzed OS as a function of transplant status and other aforementioned controls, where transplant status was a control variable, and found it had no effect on OS.
Ethical compliance
The study protocol was thoroughly reviewed and approved by the Washington DC VA IRB. Continuous oversight was maintained to ensure ongoing compliance with ethical standards. Given the retrospective nature of the study, obtaining informed consent from subjects was not required. All data used were anonymized and de-identified to protect the privacy and confidentiality of the subjects. Measures were implemented to ensure that all patient data were handled with the highest level of confidentiality. Data were stored securely, and access was restricted to authorized personnel only. De-identification techniques were applied to ensure that individual patients could not be identified from the data.
| Results | ▴Top |
Study population and baseline characteristics
A total of 6,724 patients were identified in VINCI as having ALL by ICD-9/10 codes from January 1, 2000 to December 31, 2022. Of those, 460 patients were queried to have a diagnosis of ALL between the ages of 18 and 45 and 277 patients had a diagnosis and were available for full analysis. A total of 260 patients had an induction chemotherapy regimen documented and 252 had an induction regimen that could be classified as pediatric, pediatric-inspired or adult and were included in the analysis. Among these patients, 127 patients aged 18 - 29 years old and 125 patients aged 30 - 45 years old at the time of diagnosis with a mean age of diagnosis of 30.3 years old (Fig. 1). A total of 147 patients (58%) were non-Hispanic White, 33 (13%) were non-Hispanic Black, 33 (13%) were Hispanic, 26 (10%) were non-Hispanic of another race, and 13 (5%) did not have a race or ethnicity in the VINCI database. Of the patients, 26% were classified as obese with a BMI over 30. Black patients were the most likely to be obese (29%) and Hispanic patients were the least likely to be obese (26%), but this difference was not statistically significant. With regard to biologic subtype, 158 (65%) had B-ALL, 71 (29%) had T-ALL, 12 (5%) had mixed phenotype, and two patients did not have an ALL subtype recorded. Thirty-one (12%), 79 (31%), and nine (4%) patients had high-risk, intermediate- and standard-risk MRC-ECOG cytogenetic risk, respectively. Forty-four (17%) patients were Ph+. Eighty-nine (35%) patients did not have sufficient information documented to classify cytogenetic risk. With regard to treatment, 98 patients were treated with a pediatric regimen, 39 were treated with a pediatric-inspired regimen, and 115 patients were treated with an adult regimen (Table 1, Fig. 2). In total, 56 different induction chemotherapy regimens were documented (Supplementary Material 1, www.thejh.org). In addition to induction chemotherapy, 56 patients got tyrosine kinase inhibitors (TKIs) including dasatinib (n = 20), imatinib (n = 22), ponatinib (n = 4), and nilotinib (n = 2). Eight patients received multiple TKIs because they were switched from one TKI to another. Forty-nine of these 56 patients were either Ph+ or Ph+ like. One hundred eight patients received an allogenic stem cell transplant. Of these patients, 29 received salvage chemotherapy and 10 received consolidative cellular therapy prior to going to transplant. Twenty-two patients received cellular therapy as part of their overall treatment, 12 received blinatumomab, three received inotuzumab ozogamicin and four received both blinatumomab and inotuzumab ozogamicin. Three patients received CAR-T therapy.
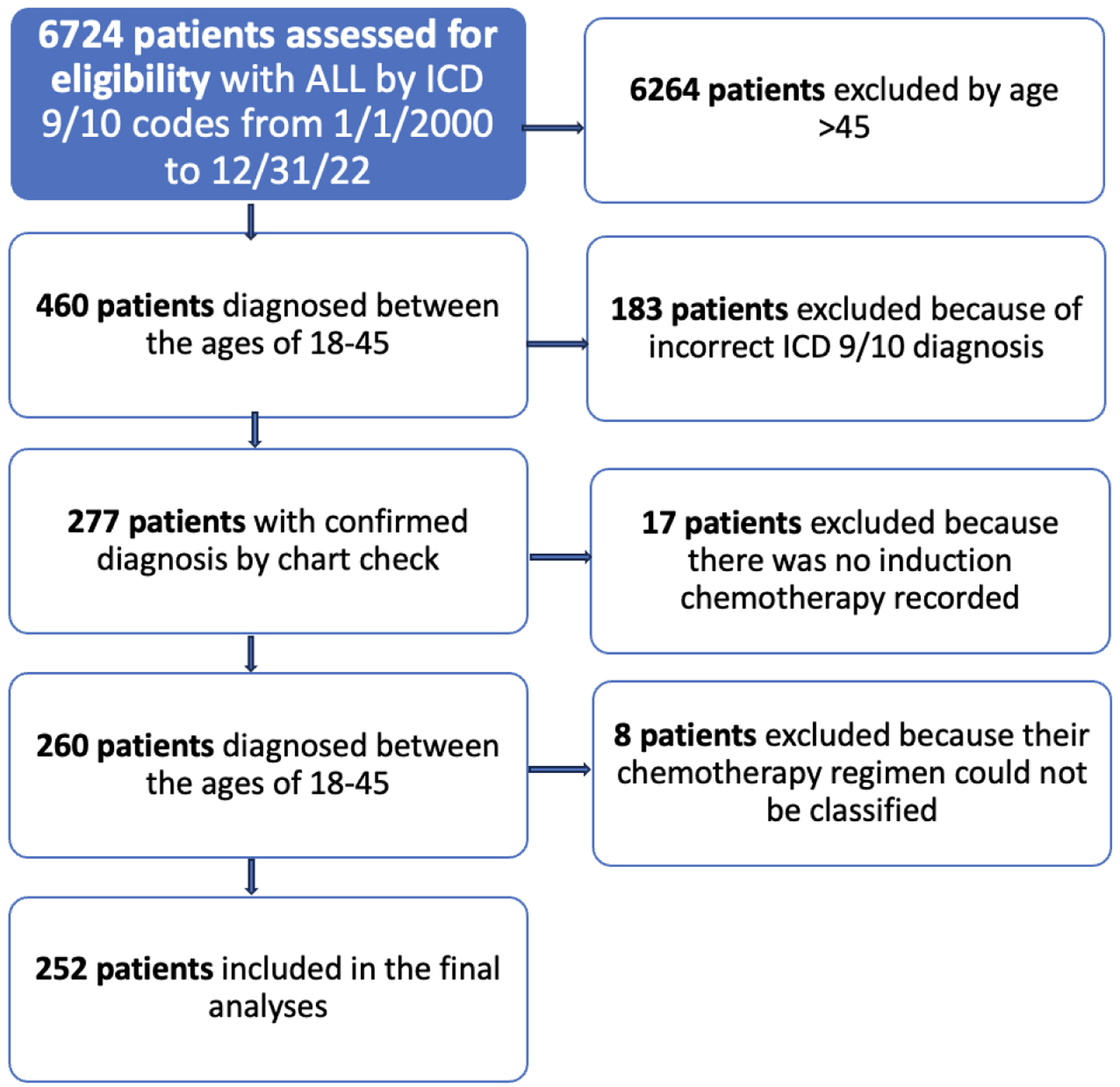 Click for large image | Figure 1. Flow diagram of patients from VINCI included in the final analysis. |
 Click to view | Table 1. Baseline Patient Characteristics |
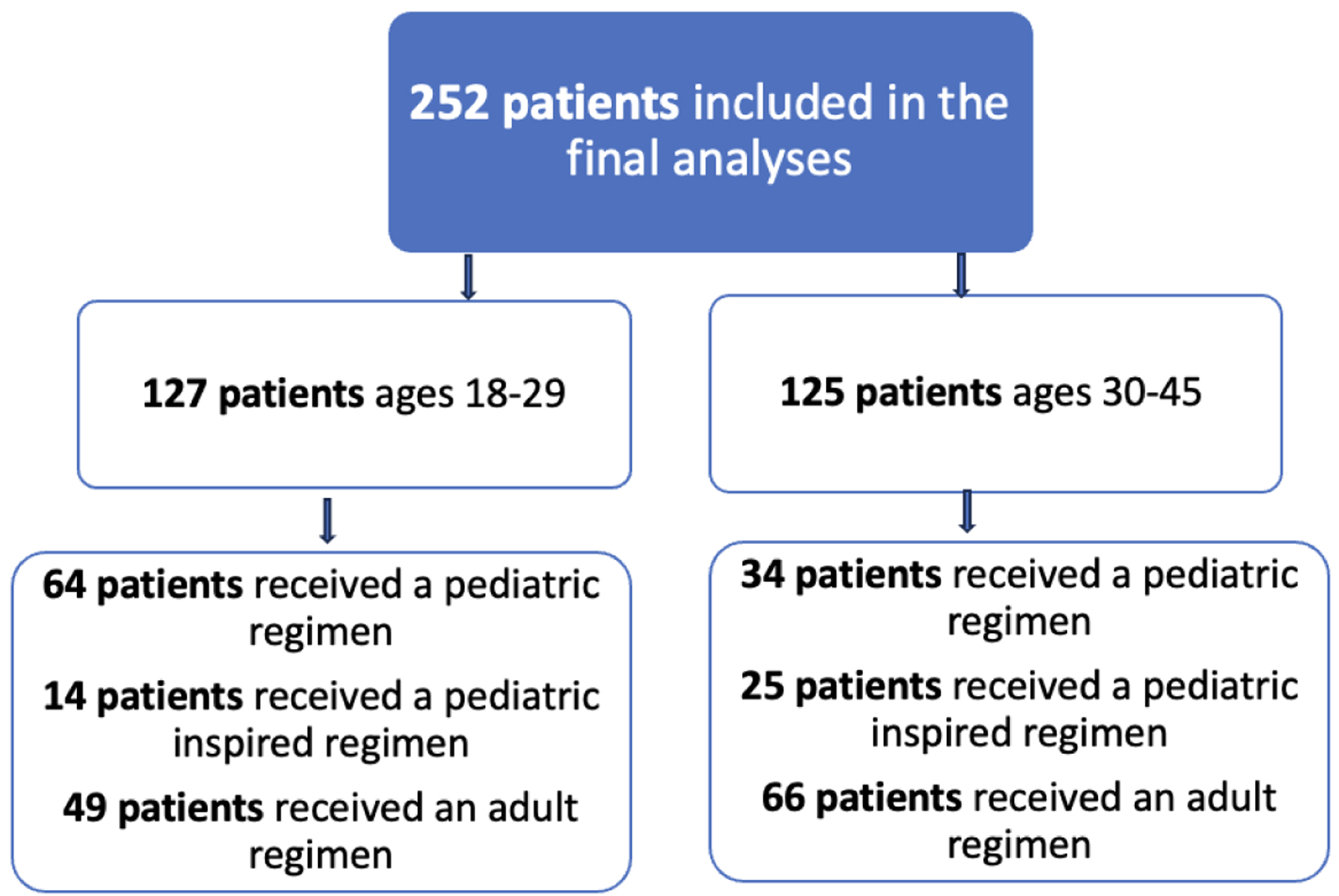 Click for large image | Figure 2. Flow diagram of treatment regimens by age category. |
Treatment regimens and OS
Patients treated with pediatric regimens, including pediatric-inspired regimens, have statistically significant (P = 0.009) survival gains, with an HR of 0.52 after controlling for age, obesity, ALL subtype, cytogenetic risk and race in a Cox proportional hazards regression model. The 5-year OS gains are similar for 18 - 29 years old and 30 - 45 years old populations when treated with pediatric regimen, with an HR of 0.5 and 0.53 respectively; however, the gains for each group are not statistically significant due to smaller sample when evaluated separately (P = 0.067, P = 0.092). The 5-year OS for patients who received a pediatric regimen vs. an adult regimen was 73% (CI 66-81%) vs. 61% (53-71%) respectively.
When the treatment regimens are further subclassified into pediatric, pediatric-inspired, and adult, the OS benefit of pediatric regimens becomes more pronounced. While there is no formal definition of a pediatric regimen, the pediatric regimens designed for children fared significantly better among adults than those designed for adults but inspired by pediatric regiments (Table 2). There was no evidence that pediatric-inspired regimens outperformed adult regimens. If we only count the designed-to-be pediatric regimens, then the advantage over the other regimens is even larger than the more broadly defined group of pediatric-inspired regimens (Fig. 3).
 Click to view | Table 2. Overall 5-Year Survival Probability by Age Category Based on Treatment Regimen and MRC-ECOG Cytogenetic Risk Stratification |
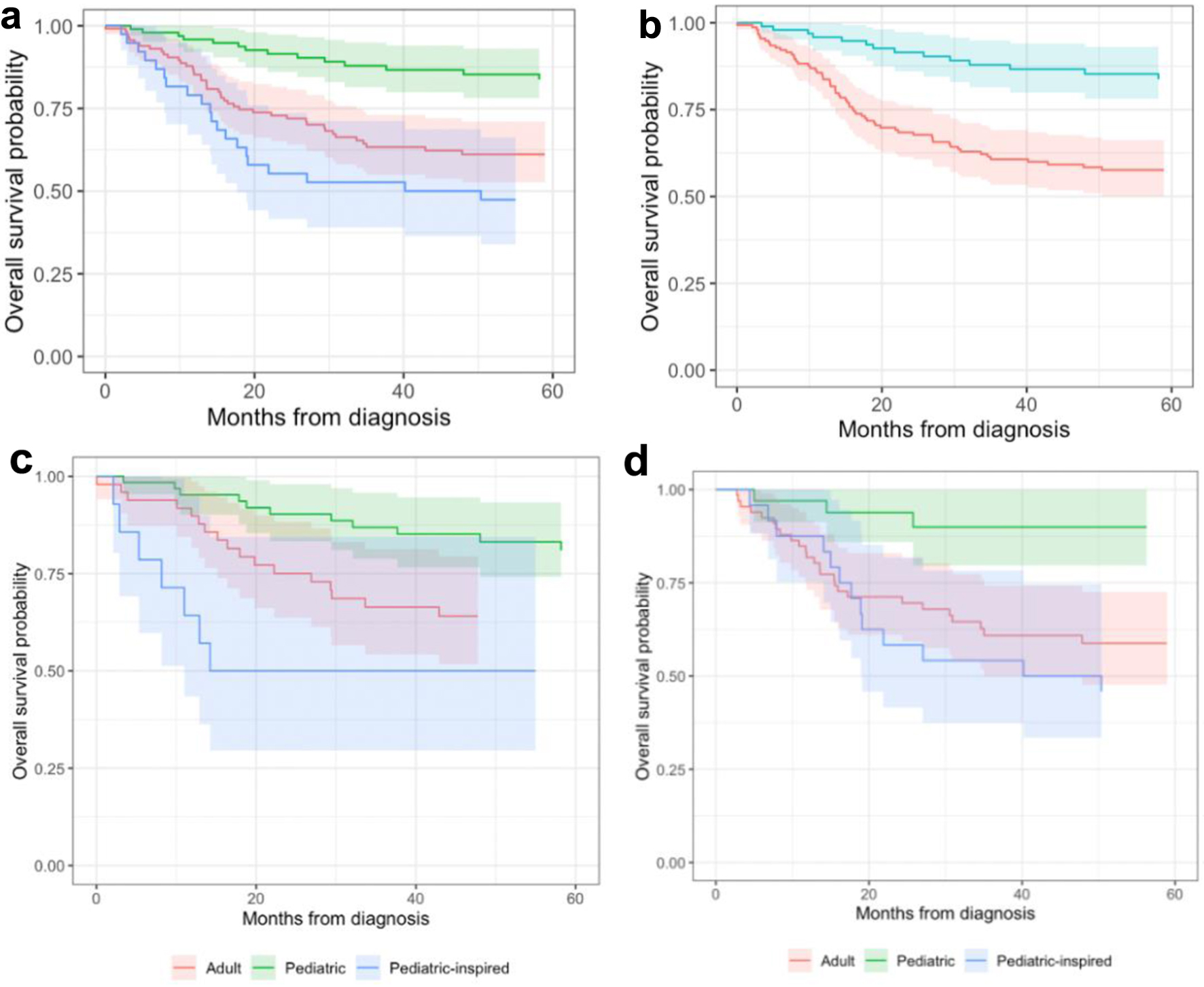 Click for large image | Figure 3. Kaplan-Meier plot of overall survival probability by treatment regimen and age category. (a, b) Patients of all ages 18 - 45 years old. (c) Overall survival of patients 18 - 29 years old by treatment regimen. (d) Overall survival of patients 30 - 45 years old by treatment regimen. |
A separate survival analysis was done on patients diagnosed between 2014 and 2018 and the same findings hold true in the setting of new treatments. Patients who received pediatric vs. adult vs. pediatric-inspired regimens had a 5-year OS of 88%, 59%, and 38% respectively.
Effects of cytogenetic risk stratification on OS
These data showed that patients with B-ALL had better OS compared to patients with T-ALL and MPAL. By contrast, these data offer no support for the modified MRC-ECOG risk stratification. In fact, the high-risk cytogenetic group was the most likely to survive after controlling for age, obesity, ALL subtype, race and use of the pediatric regimen (Table 2). As expected, the high-risk cytogenetic patients were more likely to get a transplant than those who were intermediate- or standard-risk cytogenetics with 52% (n = 31) of the high-risk patients getting a transplant compared to 35% (n = 79) of the intermediate-risk and 22% (n = 9) of the standard-risk patients. Additionally, since Ph+ status is not included in the MRC-ECOG cytogenetic risk stratification, a separate survival analysis was done controlling for Ph status and it had no significant impact with an HR for death (HR 0.92, P > 0.9).
Effects of race and age on clinical trial accrual, transplant referral, and OS
Significant racial disparities exist with regard to OS. White patients fare better (P = 0.02) than people of color (HR = 0.57) after controlling for age, obesity, ALL subtype, cytogenetic risk, whether the patient received a pediatric regimen, and including patients whose regimen was unknown or could not be classified (Table 3). Black patients were less likely (41%) to receive a pediatric regimen than non-Black patients (51%) and more likely to receive an unknown or unclassified regimen (15% vs. 8%). Black patients were far less likely (23%) to receive a transplant than non-Black patients (46%). Of note, White patients were not more likely (45%) to receive transplants than other non-Black patients (Hispanic, other people of color, unknown race). Only 7% (n = 18) of patients were referred to a clinical trial, including just 2% (n = 3) of the 30 - 45 years old group. Twenty percent (n = 16) of 18 - 29 years old were referred to a clinical trial.
 Click to view | Table 3. Overall 5-Year Survival by Race |
| Discussion | ▴Top |
In this case-control study, we present further evidence that a pediatric chemotherapy induction regimen improves OS in veterans < 45 years old. Unlike prior studies done at large academic centers, the patients in this study were treated at veteran health centers, community, and academic centers across the USA. Our data suggest that in patients < 45 years old, who are not treated on a clinical trial and who are Philadelphia chromosome negative, a pediatric regimen (designed for children) improves survival. Despite these findings, we found that only 39% of patients were treated with a pediatric regimen.
Furthermore, we identified 56 different induction chemotherapy regimens given to 260 patients. This large array of treatment regimens reflects conflicting treatment approaches among providers. Our results specifically show that the purely pediatric regimens (designed for children) fare significantly better than the pediatric-inspired regimens (designed for adults) suggesting that pediatric and pediatric-inspired should be clearly defined. The NCCN guidelines currently recommend AYA patients be treated with pediatric or pediatric-inspired regimens. Taken together, our data suggest that for patients who are not treated in clinical trials, a more standardized approach to treating young adults up to the age of 45 may be beneficial. Our findings are consistent with other large database studies which found that only 33% of AYA with ALL treated at adult cancer centers receive pediatric regimen yet there are significant survival advances for these patients when treated in a pediatric or NCI-designated cancer center [17].
While we hypothesized that young adults would benefit from a pediatric regimen, we were surprised to find that patients with high-risk MRC-ECOG cytogenetic risk scores fared significantly better than patients with low risk and patients who were Ph+. This discrepancy was especially prominent among 30 - 45 years old with high-risk cytogenetics where the 5-year OS was 100%. We chose to analyze Ph+ patients in a separate risk category since there is evidence that Philadelphia chromosome positivity confers favorable prognosis and allows for de-escalation of chemotherapy and chemotherapy-free treatments such as blinatumomab and ponatinib [18]. All of the Ph+ patients in this dataset whose treatment included a TKI had a 5-year OS of 69% - lower than the patients classified as high risk. As expected, a significantly higher percentage of patients with high-risk cytogenetics were referred for transplant. This discrepancy may be explained by MRD status. MRD refers to chemo- and radiotherapy surviving leukemia cells. Stratification based on MRD status has helped to maximize treatment effectiveness of ALL in children and adults while minimizing side effects [19, 20]. Because only a small percentage of the patients in our data had an MRD status documented, it was not included in our analyses. In sum, our findings suggest that traditional cytogenetic prognostication methods such as the MRC-ECOG are not valid among young adults and while MRD testing may be accurate, it had not been widely adopted amongst the patients in this study.
Lastly, we noted persistent disparities among survival outcomes and referral to transplant even in a relatively equal access system such as the VA system. Black patients have statistically significant worse OS compared to non-Black patients and were significantly less likely to get referred for an allogeneic hematopoietic stem cell transplantation (allo-HSCT). Several patient-, health-, and policy-level factors have been shown to contribute to these disparities. First, race/ethnicity has been associated with delayed or discontinued cancer treatment. In a cohort study, non-Hispanic Black and Hispanic individuals were more likely to have cancer treatment delays of at least 14 days or treatment discontinuation relative to non-Hispanic White individuals [21]. While not specifically examined, this 2-week delay in care may result in worse outcomes in a patient with ALL. Second, Black patients continue to be less likely to receive an allo-HSCT than White patients and continue to be underrepresented in national marrow donor registries. By the numbers, a Black patient has a 23% chance of finding a matched donor compared to a White person who has a 77% chance [22]. Lack of referral by providers and a lack of availability may also have contributed to this disparity in bone marrow transplant (BMT) referral. Notably, BMT was previously restricted to three VA centers: Nashville, TN, San Antonio, TX, and Seattle, WA. Lastly, while co-morbid conditions often preclude patients from getting referred to BMT, we suspect this is a less significant factor in this younger population. In sum, our data suggest that further efforts need to be made to improve care equality.
Limitations
There were several limitations of this study including: 1) our inability to evaluate toxicities given the lack of standardization among different medical centers; 2) some survival data were not available such as relapse-free survival and cause of death of transplanted patients; 3) the disease status at the time of transplant was not available; 4) the VINCI interface is a complex integration of data from different sources; therefore, missing data and data quality may have affected the analysis.
Conclusion
Overall, these data demonstrate that in veterans up to the age of 45, a pediatric regimen significantly improves OS and identifies disparities in care with regard to race, age, and referral to clinic trial, and transplant. We hope that our work will stimulate recommendations for ALL as part of the VA’s national oncology program which currently has guidelines for many hematology malignancies and has a goal of standardized evidence-based practices for all veterans. Above all, we hope that more veterans be referred for clinical trial as the field shifts towards cellular therapies to both eliminate the “survival cliff” and drive for cure in this young patient population.
| Supplementary Material | ▴Top |
Suppl 1. ALL chemotherapy regimen classification.
Suppl 2. Modified MRC-ECOG cytogenetic risk stratification.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was not deemed to be necessary by the IRB and the requirement was waived.
Author Contributions
ZM had the idea for the article, conducted the required tests, and drafted the work. DT, MH, ZM, and CH manually chart checked each patient and created the data set. MA, RS, JA, and MJ critically revised the work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Siegel SE, Stock W, Johnson RH, Advani A, Muffly L, Douer D, Reed D, et al. Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol. 2018;4(5):725-734.
doi pubmed pmc - Leukemia - acute lymphocytic - ALL - statistics. Cancer.Net. Published June 25, 2012. Accessed August 6, 2023. https://www.cancer.net/cancer-types/leukemia-acute-lymphocytic-all/statistics.
- Bleyer A. Important factors improving outcome of young adults with acute lymphoblastic leukemia (ALL). Best Pract Res Clin Haematol. 2021;34(4):101322.
doi pubmed - National Comprehensive Cancer Network. Acute lymphoblastic leukemia (Version 2.2023). https://www.nccn.org/professionals/physician_gls/pdf/all.pdf. Accessed August 9, 2023.
- Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, Mattano LA, Jr., et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with High-Risk B-Acute lymphoblastic leukemia: a report from children's oncology group study AALL0232. J Clin Oncol. 2016;34(20):2380-2388.
doi pubmed pmc - Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, Willman CL, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548-1559.
doi pubmed pmc - Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM, Pemmaraju N, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819-823.
doi pubmed pmc - Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577.
doi pubmed pmc - Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12(7):816-824.
doi pubmed - Velarde KE, Romesser JM, Johnson MR, Clegg DO, Efimova O, Oostema SJ, Scehnet JS, et al. An initiative using informatics to facilitate clinical research planning and recruitment in the VA health care system. Contemp Clin Trials Commun. 2018;11:107-112.
doi pubmed pmc - Haiat S, Marjanovic Z, Lapusan S, Vekhoff A, Rio B, Corre E, Dimicoli S, et al. Outcome of 40 adults aged from 18 to 55 years with acute lymphoblastic leukemia treated with double-delayed intensification pediatric protocol. Leuk Res. 2011;35(1):66-72.
doi pubmed - Lazaryan A, Dolan M, Zhang MJ, Wang HL, Kharfan-Dabaja MA, Marks DI, Bejanyan N, et al. Impact of cytogenetic abnormalities on outcomes of adult Philadelphia-negative acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation: a study by the Acute Leukemia Working Committee of the Center for International Blood and Marrow Transplant Research. Haematologica. 2020;105(5):1329-1338.
doi pubmed pmc - Blincyto (blinatumomab) FDA approval history. Drugs.com. (n.d.). https://www.drugs.com/history/blincyto.html.
- Besponsa (inotuzumab ozogamicin) FDA approval history. Drugs.com. (n.d.-a). https://www.drugs.com/history/besponsa.html.
- Dekker SE, Rea D, Cayuela JM, Arnhardt I, Leonard J, Heuser M. Using measurable residual disease to optimize management of AML, ALL, and chronic myeloid leukemia. Am Soc Clin Oncol Educ Book. 2023;43:e390010.
doi pubmed - Kymriah (tisagenlecleucel) FDA approval history. Drugs.com. (n.d.-c). https://www.drugs.com/history/kymriah.html.
- Muffly L, Alvarez E, Lichtensztajn D, Abrahao R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
doi pubmed pmc - Jabbour E, Haddad FG, Short NJ, Kantarjian H. Treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia-from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 2022;8(9):1340-1348.
doi pubmed - Pieters R, de Groot-Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, Egeler RM, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch childhood oncology group. J Clin Oncol. 2016;34(22):2591-2601.
doi pubmed - Sala Torra O, Othus M, Williamson DW, Wood B, Kirsch I, Robins H, Beppu L, et al. Next-generation sequencing in adult B cell acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 2017;23(4):691-696.
doi pubmed pmc - Llanos AAM, Ashrafi A, Ghosh N, Tsui J, Lin Y, Fong AJ, Ganesan S, et al. Evaluation of inequities in cancer treatment delay or discontinuation following SARS-CoV-2 infection. JAMA Netw Open. 2023;6(1):e2251165.
doi pubmed pmc - BeTheMatch.org. How does a patient’s ethnic background affect matching? Published March 10, 2023. Accessed August 24, 2023. https://bethematch.org/transplant-basics/how-blood-stem-cell-transplants-work/how-does-a-patients-ethnic-background-affect-matching/.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


