| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 4, August 2024, pages 142-149
Long-Term Outcome of Eltrombopag With First-Line Immunosuppressive Therapy for Newly Diagnosed Severe Aplastic Anemia
Hirofumi Yokotaa, b, p, Kotaro Miyaoa, Masashi Sawaa, Seitaro Terakurab, Shingo Kurahashic, Yoshikazu Ikomad, e, Nobuhiko Imahashif, Takanobu Morishitag, Akinao Okamotoh, Tomohiro Kajiguchii, Takaaki Onoj, Tomoko Naritak, Nobuhiro Kanemurae, Kazutaka Ozekil, Yumi Kojimam, Kensuke Naiton, Kaori Uchinoo, Akihiro Tomitah, Hiroatsu Iidaf, Naoto Imotoc, Senji Kasaharad, Yuichiro Inagakia, Tetsuya Nishidag, Makoto Muratab, on behalf of the Nagoya Blood and Marrow Transplantation Group
aDepartment of Hematology and Oncology, Anjo Kosei Hospital, Anjo, Japan
bDepartment of Hematology and Oncology, Nagoya University Graduate School of Medicine, Nagoya, Japan
cDivision of Hematology and Oncology, Toyohashi Municipal Hospital, Toyohashi, Japan
dDepartment of Hematology, Gifu Municipal Hospital, Gifu, Japan
eDepartment of Hematology and Infectious Disease, Gifu University Hospital, Gifu, Japan
fDepartment of Hematology, NHO Nagoya Medical Center, Nagoya, Japan
gDepartment of Hematology, Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Nagoya, Japan
hDepartment of Hematology, Fujita Health University School of Medicine, Toyoake, Japan
iDepartment of Hematology and Oncology, Tosei General Hospital, Seto, Japan
jDepartment of Internal Medicine, School of Medicine, Hamamatsu University, Hamamatsu, Japan
kDepartment of Hematology and Oncology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
lDepartment of Hematology and Oncology, Konan Kosei Hospital, Konan, Japan
mDepartment of Hematology, Nagoya Ekisaikai Hospital, Nagoya, Japan
nDepartment of Hematology, Hamamatsu Medical Center, Hamamatsu, Japan
oDepartment of Internal Medicine, Division of Hematology, Aichi Medical University School of Medicine, Nagakute, Japan
pCorresponding Author: Hirofumi Yokota, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, Aichi 466-8560, Japan
Manuscript submitted May 26, 2024, accepted July 23, 2024, published online August 10, 2024
Short title: Long-Term Outcome of IST for SAA
doi: https://doi.org/10.14740/jh1289
| Abstract | ▴Top |
Background: To investigate whether the addition of eltrombopag (EPAG) to rabbit anti-thymocyte globulin (ATG)-based immunosuppressive therapy (IST) for newly diagnosed severe aplastic anemia (SAA) improves outcomes and affects the cumulative incidence of clonal evolution (CE), we conducted a multicenter retrospective analysis.
Methods: Data were collected from 101 patients, aged 15 - 65 years, undergoing initial IST.
Results: No significant imbalance in age, sex, or severity was observed between the EPAG (n = 20) and non-EPAG (n = 81) groups. The median duration of EPAG administration in EPAG group was 16.1 months (range: 0.6 - 41.1 months). Six months after the initiation of IST, the complete response (CR) rate significantly improved in the EPAG group (P < 0.01). The cumulative incidence of allogeneic stem cell transplantation (allo-SCT) at 2 years and the 2-year overall survival (OS) were not significantly different between the two groups (allo-SCT, P = 0.31; OS, P = 0.64). Grade 3-4 adverse events in the EPAG group and the cumulative incidence of CE (P = 0.96) showed no increase.
Conclusion: In summary, IST showed significantly better initial efficacy in the EPAG group. Although the addition of EPAG did not reduce the need for allo-SCT, no increase was observed in the incidence of CE with long-term EPAG use.
Keywords: Aplastic anemia; Eltrombopag; Allogeneic stem cell transplantation; Immunosuppression therapy
| Introduction | ▴Top |
The first-line treatment for severe aplastic anemia (SAA) in young adults is immunosuppressive therapy (IST) using a combination of anti-thymocyte globulin (ATG) and cyclosporine (CsA), or allogeneic stem cell transplantation (allo-SCT) from a human leukocyte antigen (HLA)-matched sibling donor, if available. This is especially recommended for individuals under 40 years of age [1-4]. However, owing to concerns such as transplantation-related mortality and graft-versus-host disease (GVHD), some patients may choose to avoid allo-SCT, even when a matched sibling donor is available. In contrast, the efficacy of IST is reportedly to be 50-70%, with a recurrence rate of 30% after treatment [2]. Insufficient responses and high recurrence rates pose challenges for IST.
Since 2017, the efficacy of thrombopoietin receptor agonists (TPO-RAs) for aplastic anemia has been demonstrated, including the additional benefit of adding eltrombopag (EPAG) to initial IST for SAA [5-8]. In response to these findings, Japanese guidelines now recommend the addition of EPAG to IST as the first-line treatment for patients under 40 years of age without an HLA-matched sibling donor [9]. Whereas the efficacy of IST is expected to improve significantly, the use of equine ATG, considered more effective than rabbit ATG, was not available in Japan until July 2023 [10-12]. Most large-scale trials demonstrating the added effect of EPAG have used equine ATG. Although reports of rabbit ATG from Japan and China have been published, they are inadequate and further accumulation is needed [13-16].
Even if IST is chosen as the first-line treatment, some patients may undergo allo-SCT as a secondary treatment if the initial response is insufficient or if recurrence occurs after the response. Currently, no reports have focused on whether adding EPAG to IST reduces the need for allo-SCT in subsequent treatments.
Furthermore, clonal evolution (CE) is a long-term issue that occurs in approximately 15% of patients who undergo IST [17]. About 40% of CE cases involve abnormalities in chromosome 7, and patients with these abnormalities have a very poor prognosis [18]. Although evidence is lacking regarding the increase in frequency of CE with the addition of EPAG, few studies have reported that high-risk events, such as abnormalities in chromosome 7, occur earlier compared to conventional treatment [19, 20]. Therefore, examining the risk of developing CE under EPAG administration in real-world data is significant.
We conducted a multicenter collaborative study to elucidate the long-term outcomes of adding EPAG to IST and to determine whether the concomitant long-term use of EPAG increases the incidence of CE.
| Materials and Methods | ▴Top |
This multicenter retrospective observational study was conducted across 15 participating Nagoya Blood and Marrow Transplantation Group facilities. Individual patient data were collected through surveys distributed to each facility. The inclusion criteria were patients aged 15 - 65 years with SAA who underwent ATG plus CsA as the initial IST between January 2009 and May 2020. The target age group was defined as the range in which allo-SCT would be actively considered if primary IST proved to be ineffective. The exclusion criteria included a history of TPO-RAs, allo-SCT, previous ATG administration, or treatment for malignant diseases.
Patients initiating EPAG within 60 days after the commencement of ATG were categorized as the EPAG group, whereas the remaining patients formed the non-EPAG group. The primary endpoint was cumulative allo-SCT rate, and the secondary endpoints were complete remission (CR), partial remission (PR), CE rate, and overall survival (OS). CR was defined as meeting all of the following: neutrophil count > 1,000/µL, Hb > 10 g/dL, platelet count > 100,000/µL; PR was defined as meeting two or more of the following: neutrophil count > 500/µL, platelet count > 20,000/µL, reticulocyte count > 60,000/µL. CE was defined as the development of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or the appearance of new chromosomal abnormalities after IST. Adverse events were evaluated using the Common Terminology Criteria for Adverse Event (CTCAE) version 5.0.
Fisher’s exact test was used to analyze nominal variables. Continuous variables were analyzed using the Kolmogorov-Smirnov test for normality, and since none of them followed a normal distribution, the Mann-Whitney U test was used. The Gray test was used to test the cumulative incidence of allo-SCT, cumulative achievement of CR/PR, and cumulative incidence of CE; competing events for allo-SCT were death, competing events for achievement of CR/PR were death, secondary treatment, CE, and competing events for CE were death and allo-SCT. Multivariate analysis was conducted using Fine-Gray proportional hazards regression. The Kaplan-Meier method was used to generate OS curves, and analysis was performed using the log-rank test. All statistical analyses were two-tailed, and a P-value < 0.05 was considered statistically significant. EZR was used for the statistical analysis [21].
The protocol for this study protocol was approved by the Institutional Review Board of Anjo Kosei Hospital (No. R20-47) and all other participating centers and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patients
Data from 101 patients were analyzed. The median follow-up period was 56.6 months (range: 4.6 - 153.1 months) for all cases and 59.9 months (range: 8.9 - 153.1 months) for survivors at the time of data collection.
All patients received rabbit ATG plus CsA, with the median ATG dose of 16.3 mg/kg (range: 5.6 - 25.0 mg/kg). Among the 101 patients analyzed, 20 who were administered EPAG within 60 days after ATG administration were categorized into the EPAG group, whereas the others formed the non-EPAG group.
The median follow-up period was significantly longer in non-EPAG group: 64.0 months (range: 7.4 - 153.1 months) compared to 29.0 months (range: 4.6 - 60.4 months) in EPAG group (P < 0.001) (Table 1). No statistically significant differences were observed between the groups in terms of age, sex, or severity, as evaluated using the Camitta criteria [22, 23].
 Click to view | Table 1. Comparison of Baseline Characteristics of Non-EPAG and EPAG Patients |
Chromosomal abnormalities at diagnosis were identified in seven patients (8.6%) in the non-EPAG group and three (15.0%) in the EPAG group (P = 0.58). Additionally, 14 patients in the non-EPAG group (17.3%) and three in the EPAG group (15.0%) had an HLA-identical sibling donor before IST (P = 1.0). The three patients in the EPAG group who had siblings were all young, under 25 years of age.
In the EPAG group, patients started EPAG 5 - 42 days (median, 14 days) after ATG administration at a median dose of 75 mg (range, 25 - 75 mg). The median duration of EPAG administration was 16.1 months (range: 0.6 - 41.1 months). Ten patients (50.0%) continued to undergo EPAG at the time of data collection. Among the other 10 patients, six stopped EPAG under its best response, one due to lack of efficacy, and three due to adverse events.
Allo-SCT
The cumulative incidence of allo-SCT at 2 years after IST was 13.8% in non-EPAG group and 5.0% (95% CI: 0.3-21.1%) in EPAG group (P = 0.31) (Fig. 1). The median duration from IST to allo-SCT was 11.4 months (range: 4.1 - 70.0 months). Among the 15 patients who underwent allo-SCT, eight did so due to a lack of response to IST, followed by CE (n = 4) and relapse (n = 3) (Table 2).
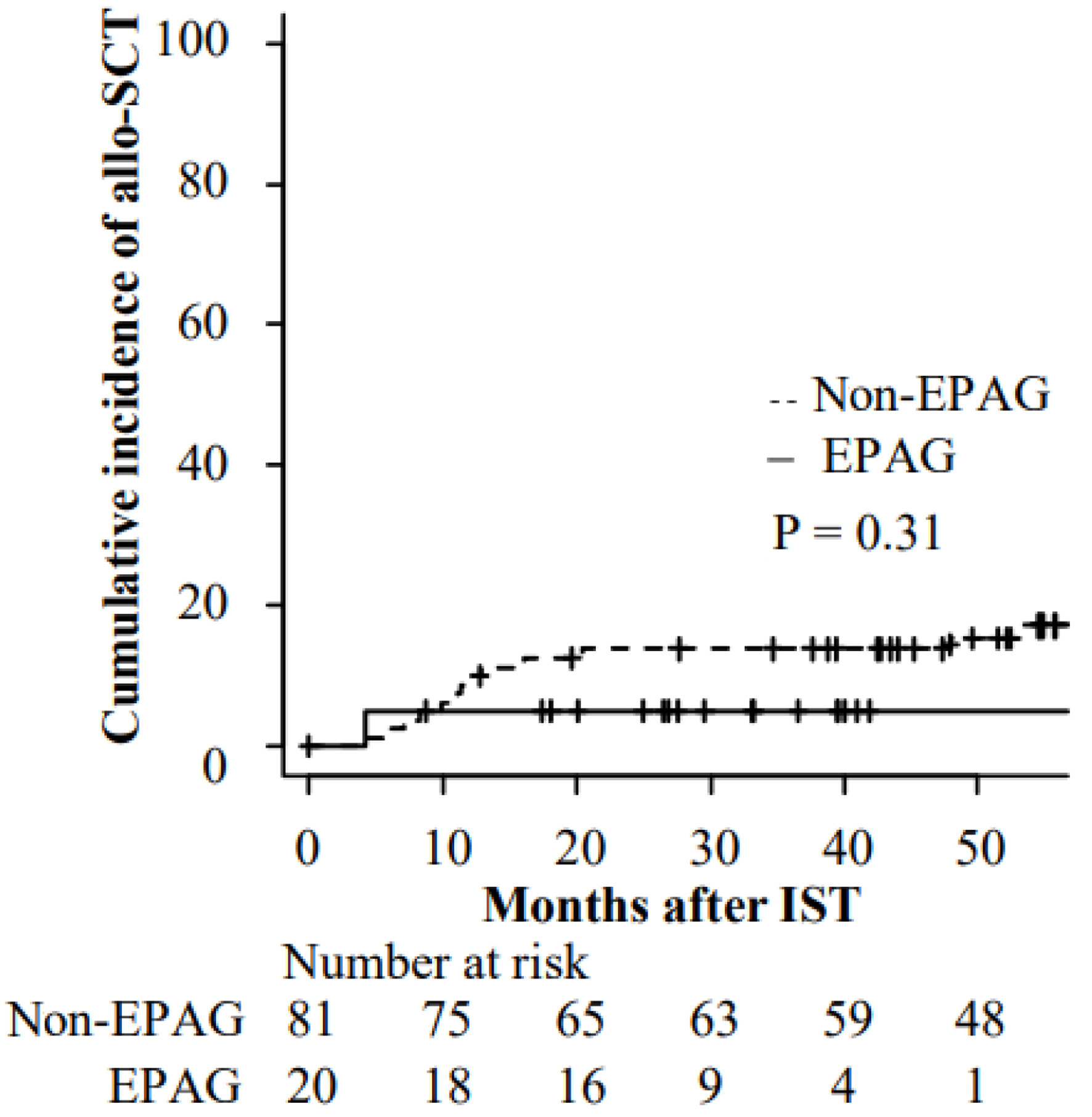 Click for large image | Figure 1. Cumulative incidence of allogenic stem cell transplantation after IST. allo-SCT: allogenic stem cell transplantation; EPAG: eltrombopag; IST: immunosuppressive therapy. |
 Click to view | Table 2. The Reason for Proceeding to allo-SCT |
According to multivariate analysis, EPAG administration did not show a statistically significant change in the cumulative incidence of allo-SCT (Table 3).
 Click to view | Table 3. Multivariate Analysis on Cumulative Incidence of allo-SCT |
Donor sources included four cases of bone marrow (BM) from HLA-identical relatives, three cases of peripheral blood stem cells (PBSCs) from relatives (one of which was an HLA-one haplotype-incompatible transplant), four cases of BM from unrelated donors, and four cases of cord blood. The 3-year OS rate after stem cell infusion was 60.0% (95% CI: 31.8-79.7%). In total, six patients died after allo-SCT, and all of them died because of adverse events of allo-SCT (infection, three; bronchiolitis obliterans syndrome, one; multiple organ failure, two). The other nine patients of survival all maintained CR until the end of the observation period.
OS and CE
The 2-year OS after IST was 91.3% (95% CI: 82.6-95.8%) in non-EPAG group and 95.0% (95% CI: 69.5-99.3%) in EPAG group (P = 0.64) (Fig. 2a). The 2-year cumulative incidence of CE was 3.7% (95% CI: 1.0-9.6%) in non-EPAG group and 5.6% (95% CI: 0.3-23.2%) in EPAG group (P = 0.96) (Fig. 2b). The median duration from the start of ATG administration to the diagnosis of CE was 21.6 months (range: 3.7 - 114.1 months). All patients underwent karyotype testing at the time of CE, and three of them had chromosome 7 abnormalities (Table 4 [1, 3, 4, 7, 12, 13, 16, 17, 20]).
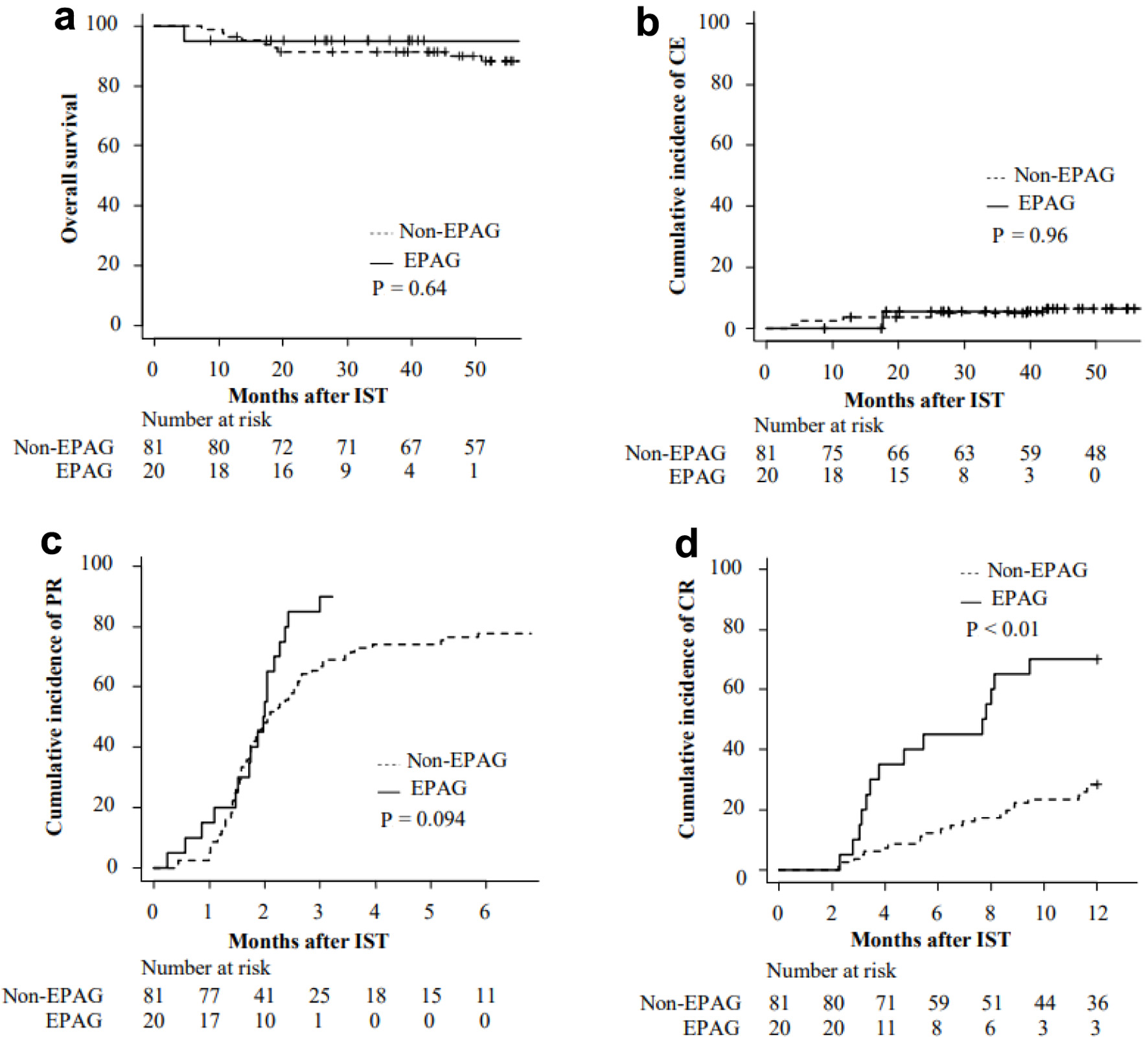 Click for large image | Figure 2. Overall survival (a) and cumulative incidence of CE (b), PR (c), and CR (d) after IST. CE: clonal evolution; CR: complete response; EPAG: eltrombopag; IST: immunosuppressive therapy; PR: partial response. |
 Click to view | Table 4. Summary of the Patient Characteristics Who Developed CE After IST |
Response of IST
The PR rate at 3 months after IST was 66.7% (95% CI: 55.1-75.9%) in non-EPAG group and 90.0% (95% CI: 57.0-98.0%) in EPAG group (P = 0.094) (Fig. 2c). The CR rates at 6 months were 12.3% (95% CI: 6.3-20.6%) and 45.0% (95% CI: 22.3-65.4%), respectively (P < 0.01) (Fig. 2d). In multivariate analysis considering both the presence or absence of EPAG and disease severity as variables, EPAG was shown to significantly improve the CR rate (HR: 3.81, 95% CI: 1.92 - 7.56, P < 0.01).
We collected blood cell count data at baseline and 1, 3, and 6 months after the start of IST and compared the two groups (Fig. 3). The neutrophil counts at 3 months had a median of 950/µL (range: 3 - 4,139/µL) in non-EPAG group, while it was 1,416/µL (range: 3 - 3,016/µL) in EPAG group (P = 0.031). At 6 months, the respective counts were 1,265/µL (range: 10 - 3,800/µL) and 2,226/µL (range: 468 - 5,110/µL) (P < 0.01), with EPAG group showing significantly higher values. Platelet counts followed a similar trend, with 3-month medians of 3.3 × 104/µL (range: 0.1 - 22.1 × 104/µL) and 7.0 × 104/µL (range: 1.3 - 26.7 × 104/µL) for non-EPAG and EPAG groups, respectively (P = 0.025). At 6 months, the respective counts were 3.7 × 104/µL (range: 0.1 - 26.2 × 104/µL) and 11.2 × 104/µL (range: 1 - 35.2 × 104/µL) (P < 0.01), with EPAG group again demonstrating a significant increase. In contrast, reticulocytes showed no statistically significant differences at any time point.
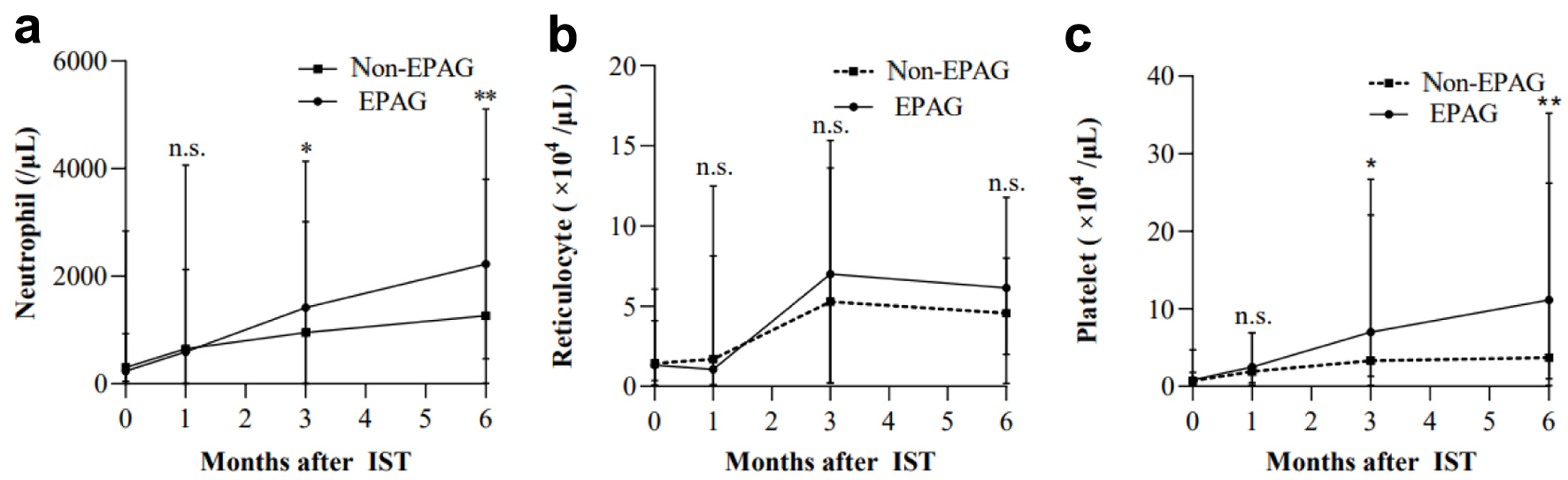 Click for large image | Figure 3. Hematologic responses over time by lineage. Data on baseline, 1, 3, and 6 months after the start of IST were collected. (a) Neutrophil. (b) Reticulocyte. (c) Platelet. *P < 0.05. **P < 0.01. EPAG: eltrombopag; IST: immunosuppressive therapy. |
Forty-two patients received second-line treatments prior to data collection. The major second-line treatments included anabolic steroids (n = 13), allo-SCT (n = 12), EPAG (n = 9), CsA resumption (n = 4), rabbit ATG re-administration (n = 4), and romiplostim (n = 1). Two patients who chose ATG re-administration and one who chose anabolic steroids underwent allo-SCT as a third-line treatment.
Adverse events
Data on grade 3-4 non-hematologic adverse events occurring up to 1 year after IST were collected (Table 5). Febrile neutropenia (FN) was recorded in 32.9% of patients in non-EPAG group and 10.0% in EPAG group (P = 0.053). No adverse events significantly increased due to EPAG administration during the follow-up period. Eight patients died within 2 years after IST, including one patient in the EPAG group. Five of the eight died due to adverse events associated with allo-SCT.
 Click to view | Table 5. Grade 3-4 Non-Hematological Adverse Events Within 1 Year After IST |
| Discussion | ▴Top |
In this retrospective, multicenter study involving collaborative examination of 101 participants, the EPAG group consisted of 20 patients. The noteworthy characteristics of the EPAG group included the presence of three patients who, despite being young and having sibling donors, opted for IST. Additionally, the median duration of EPAG administration was notably long (16.1 months). These findings are believed to reflect real-world clinical practice. As a result of the combined use of EPAG, the CR rate significantly improved after 6 months of IST in the EPAG group. However, the cumulative incidence of allo-SCT after IST did not differ significantly between the non-EPAG and EPAG groups.
Regarding the effectiveness of IST, the efficacy of equine ATG has been demonstrated in the phase III RACE trial [7]. The addition of EPAG to rabbit ATG-based IST was reported in a single-arm phase II trial involving 10 patients, with a favorable overall response rate (ORR) of 70% at 26 weeks [13]. Similar results were reported in a retrospective study involving 58 patients who received IST plus EPAG, with a 76% ORR at 6 months [16]. Furthermore, a comparing the outcomes of IST alone and IST combined with EPAG in a multicenter trial revealed a significantly better ORR in the EPAG combination group (85%) than in the non-combination group (61%) [15]. In line with these previous reports, we confirmed the improvement in rabbit ATG-based IST outcomes with the addition of EPAG.
Although the efficacy of IST has improved, the cumulative incidence of allo-SCT after IST did not decrease. In the aforementioned phase III trial (RACE trial), the cumulative incidence of allo-SCT within 1 year of randomization was 11.8% in the non-EPAG group and 11.4% in the EPAG group, showing no significant difference. A potential factor for the lack of reduction in allo-SCT could be post-IST relapse. The RACE trial demonstrated improved response rates after IST. However, the relapse rates had no significant difference. Moreover, in the long-term follow-up of the IST plus EPAG phase II trial, the cumulative relapse rate at 4 years was 43%, with many relapses occurring upon discontinuation of EPAG or CsA [6]. Based on these results, it is highly probable that post-IST relapse is a major factor preventing a reduction in allo-SCT.
The duration of EPAG administration in the aforementioned prospective trial was 3 - 6 months, which was considerably shorter than that in the current study. A single report has verified the long-term administration of EPAG [24]. According to this report, among patients who did not meet the CR criteria at 6 months after IST initiation and continued EPAG, the 2-year event-free survival (EFS) was 89%, whereas it was 49% for patients who discontinued EPAG, showing a statistically significant advantage for the continuing group. Moreover, among the 55 patients in this study who continued EPAG because they did not achieve CR, only one patient (1.8%) underwent allo-SCT during the observation period. Our study included only one case of allo-SCT in the EPAG group, and this patient underwent transplantation not due to relapse but because of an insufficient response to IST. While our study did not definitively confirm the potential for relapse suppression and avoidance of allo-SCT through extended EPAG administration, it did not rule out these possibilities.
Safety concerns arose with prolonged EPAG administration; however, in our study, no increase in grade 3-4 adverse events was noted within 1 year post-IST, and the cumulative incidence of CE during the observation period did not differ significantly between the EPAG and non-EPAG groups. Although high-risk CE involving chromosome 7 abnormalities or complex karyotypes has been reported to occur early in EPAG-combined patients, our EPAG group had no high-risk CE, and only one case of low-risk CE was observed [6, 19, 20]. Additionally, reports suggest that the 4-year incidence of CE in EPAG group (15%) is comparable to the historical control non-EPAG group, supporting our study’s findings. It seems unlikely that long-term concomitant use of EPAG leads to an increase in CE. However, as this event occurs at a certain rate, regular monitoring using fluorescence in situ hybridization (FISH) is necessary.
The 3-year OS rate after allo-SCT in this study was 60%, which is not considered favorable. However, recent reports suggest an overall improvement in allo-SCT outcomes. A large-scale study using European Cooperative Group for Bone Marrow Transplantation demonstrated that, although the incidence of GVHD was higher in unrelated donors, no significant difference in OS was observed between HLA-matched sibling donors and unrelated donors [25]. Furthermore, favorable results have been reported for salvage therapy using umbilical cord blood and HLA haploidentical transplantation in recent years [26-28]. Additionally, DeZern et al reported the remarkable success of HLA-haploidentical transplantation as an initial treatment in cases where an HLA-matched sibling donor is unavailable [29]. In this study, the 3-year OS for all patients was 92%, achieving 100% OS in 20 patients after adjusting for the total body irradiation (TBI) dose. A prospective study comparing IST plus EPAG with allo-SCT from an HLA-matched sibling donor reported equivalent 3-year OS but significantly better failure-free survival with allo-SCT [30]. Furthermore, in our current patient cohort, except for those who experienced early non-relapse mortality after allo-SCT, all patients have maintained CR status post-transplant. Even in this setting, allo-SCT has proven to be promising in terms of the sustainability of its effects. Considering these results, it can be asserted that despite the confirmed favorable treatment outcomes of IST plus EPAG at present, allo-SCT remains a viable option for SAA.
A limitation of this study was its retrospective and multicenter nature. Prospective studies are generally preferred to confirm the findings. The decision to choose between IST, allo-SCT, and the addition of EPAG was left to the discretion of the individual facility/physician, leading to inevitable selection bias and variability in treatment approaches. Additionally, the EPAG group had a limited number of cases, which may limit the generalizability of the findings and the statistical power to detect differences between groups. Furthermore, the significant difference in observation periods between the two groups requires caution in interpreting outcomes, such as allo-SCT or CE. Insufficient data on the presence of glycosylphosphatidylinositol (GPI)-deficient cells limit the ability to predict the effectiveness of IST and understand the underlying mechanisms of treatment response [31, 32].
In summary, the initial effectiveness of IST was significantly better in the EPAG group, but OS was comparable, and there was no observed reduction in the rate of allo-SCT implementation. The long-term benefit of adding EPAG could not be demonstrated. On the other hand, there was no increase in CE with long-term addition of EPAG, and the safety of adding EPAG in real-world clinical practice was confirmed.
Acknowledgments
We would like to thank all the physicians and staff at the cooperating centers for contributing patient data for this study.
Financial Disclosure
This study was not supported by any sponsor or funder.
Conflict of Interest
The authors declare no direct competing financial interests.
Informed Consent
Written informed consent was not required for this study because of its retrospective nature, which was approved by the Institutional Review Board of Anjo Kosei Hospital.
Author Contributions
HY and KM designed the study, performed the statistical analysis, and analyzed the data. MS and Y. Inagaki contributed to the study design. T. Nishida and MM organized the project. S. Kurahashi, Y. Ikoma, N. Imahashi, TM, AO, TK, TO, T. Narita, NK, KO, YK, KN, KU, AT, HI, N. Imoto, S. Kasahara, and T. Nishida contributed to data collection. HY wrote the first draft of the paper, and KM, ST, N. Imahashi, and T. Nishida contributed to the final version.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy—The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000;37(1):69-80.
doi pubmed - Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428-1436.
doi pubmed - Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187-207.
doi pubmed - Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, Kudo K, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99(12):1784-1791.
doi pubmed pmc - Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540-1550.
doi pubmed pmc - Patel BA, Groarke EM, Lotter J, Shalhoub R, Gutierrez-Rodrigues F, Rios O, Quinones Raffo D, et al. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: a phase 2 study. Blood. 2022;139(1):34-43.
doi pubmed pmc - Peffault de Latour R, Kulasekararaj A, Iacobelli S, Terwel SR, Cook R, Griffin M, Halkes CJM, et al. Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. N Engl J Med. 2022;386(1):11-23.
doi pubmed - Shinn LT, Benitez LL, Perissinotti AJ, Reid JH, Buhlinger KM, van Deventer H, Barth D, et al. Multicenter evaluation of the addition of eltrombopag to immunosuppressive therapy for adults with severe aplastic anemia. Int J Hematol. 2023;118(6):682-689.
doi pubmed - Yamazaki H. [Reference guide for the treatment of aplastic anemia: key points of the 2022 revision]. Rinsho Ketsueki. 2023;64(9):892-899.
doi pubmed - Hayakawa J, Kanda J, Akahoshi Y, Harada N, Kameda K, Ugai T, Wada H, et al. Meta-analysis of treatment with rabbit and horse antithymocyte globulin for aplastic anemia. Int J Hematol. 2017;105(5):578-586.
doi pubmed - Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430-438.
doi pubmed pmc - Yang N, Chen J, Zhang H, Dai Z, Yao H, Ma X, Bai J, et al. Horse versus rabbit antithymocyte globulin in immunosuppressive therapy of treatment-naive aplastic anemia: a systematic review and meta-analysis. Ann Hematol. 2017;96(12):2031-2043.
doi pubmed - Imada K, Obara N, Iida H, Imajo K, Maeda T, Usuki K, Fanghong Z, et al. Eltrombopag in combination with rabbit anti-thymocyte globulin/cyclosporine a in immunosuppressive therapy-naive patients with aplastic anemia in Japan. Intern Med. 2021;60(8):1159-1168.
doi pubmed pmc - Jie M, Fu L, Li S, He Y, Yao J, Cheng X, Zhang L, et al. Efficacy and safety of eltrombopag in the first-line therapy of severe aplastic anemia in children. Pediatr Hematol Oncol. 2021;38(7):647-657.
doi pubmed - Jin Y, Li R, Lin S, Jia J, Yang Y, Zhang D, He G, et al. A real-word experience of eltrombopag plus rabbit antithymocyte immunoglobulin-based IST in Chinese patients with severe aplastic anemia. Ann Hematol. 2022;101(11):2413-2419.
doi pubmed - Li R, Zhou J, Liu Z, Chen X, Long Q, Yang Y, Lin S, et al. Predicting response of severe aplastic anemia to rabbit-antithymocyte immunoglobulin based immunosuppressive therapy combined with eltrombopag. Front Immunol. 2022;13:884312.
doi pubmed pmc - de Planque MM, Bacigalupo A, Wursch A, Hows JM, Devergie A, Frickhofen N, Brand A, et al. Long-term follow-up of severe aplastic anaemia patients treated with antithymocyte globulin. Severe Aplastic Anaemia Working Party of the European Cooperative Group for Bone Marrow Transplantation (EBMT). Br J Haematol. 1989;73(1):121-126.
doi pubmed - Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99(9):3129-3135.
doi pubmed - Winkler T, Fan X, Cooper J, Desmond R, Young DJ, Townsley DM, Scheinberg P, et al. Treatment optimization and genomic outcomes in refractory severe aplastic anemia treated with eltrombopag. Blood. 2019;133(24):2575-2585.
doi pubmed pmc - Groarke EM, Patel BA, Shalhoub R, Gutierrez-Rodrigues F, Desai P, Leuva H, Zaimoku Y, et al. Predictors of clonal evolution and myeloid neoplasia following immunosuppressive therapy in severe aplastic anemia. Leukemia. 2022;36(9):2328-2337.
doi pubmed pmc - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed pmc - Camitta BM, Storb R, Thomas ED. Aplastic anemia (first of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306(11):645-652.
doi pubmed - Camitta BM, Storb R, Thomas ED. Aplastic anemia (second of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306(12):712-718.
doi pubmed - Li R, Wang N, Chai X, Yang L, Liu K, He H, Lin S, et al. Prolonged use of eltrombopag in patients with severe aplastic anemia in the real world. Clin Exp Med. 2023;23(6):2619-2627.
doi pubmed - Bacigalupo A, Socie G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, Cybicka A, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100(5):696-702.
doi pubmed pmc - DeZern AE, Eapen M, Wu J, Talano JA, Solh M, Davila Saldana BJ, Karanes C, et al. Haploidentical bone marrow transplantation in patients with relapsed or refractory severe aplastic anaemia in the USA (BMT CTN 1502): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2022;9(9):e660-e669.
doi pubmed pmc - Prata PH, Eikema DJ, Afansyev B, Bosman P, Smiers F, Diez-Martin JL, Arrais-Rodrigues C, et al. Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: a report from the EBMT Severe Aplastic Anemia Working Party. Bone Marrow Transplant. 2020;55(6):1050-1058.
doi pubmed - Kuwatsuka Y, Kanda J, Yamazaki H, Mori T, Miyamura K, Kako S, Uchida N, et al. A comparison of outcomes for cord blood transplantation and unrelated bone marrow transplantation in adult aplastic anemia. Biol Blood Marrow Transplant. 2016;22(10):1836-1843.
doi pubmed - DeZern AE, Zahurak M, Symons HJ, Cooke KR, Huff CA, Jain T, Swinnen LJ, et al. Alternative donor BMT with posttransplant cyclophosphamide as initial therapy for acquired severe aplastic anemia. Blood. 2023;141(25):3031-3038.
doi pubmed - Liu L, Lei M, Fu R, Han B, Zhao X, Liu R, Zhang Y, et al. Matched related transplantation versus immunosuppressive therapy plus eltrombopag for first-line treatment of severe aplastic anemia: a multicenter, prospective study. J Hematol Oncol. 2022;15(1):105.
doi pubmed pmc - Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107(4):1308-1314.
doi pubmed - Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, Vavilov V, et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol. 2014;164(4):546-554.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


