| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 5, October 2024, pages 192-199
The Influence of Circulating Exosomes Derived From Younger and Older Donors on Hypoxia-Inducible Factor 1 Alpha Gene Expression and P21 Protein in Cord Blood Hematopoietic Stem Cells
Zahra Rastia, Reza Afrishamb, Elahe Bahrami Vahdata, Zahra Kashanikhatiba, Seyed Hadi Mousavia, Shaban Alizadeha, c
aDepartment of Hematology, School of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
bDepartment of Medical Laboratory Sciences, School of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
cCorresponding Author: Shaban Alizadeh, Department of Hematology, School of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
Manuscript submitted May 25, 2024, accepted August 26, 2024, published online October 21, 2024
Short title: The Influence of Exosomes on HIF-1α and P21 in HSCs
doi: https://doi.org/10.14740/jh1291
| Abstract | ▴Top |
Background: Exosomes are a group of extracellular vesicles that are influential in intercellular signaling and can affect aging. Hypoxia-inducible factor 1α (HIF-1α) is the principal mediator in response to hypoxia and can regulate aging. Moreover, P21 is a part of the downstream signaling pathway of hypoxia and is elevated during aging. Therefore, this research was conducted to investigate the effect of plasma exosomes of younger and older individuals on the expression of HIF-1α gene and P21 protein in hematopoietic stem cells (HSCs).
Methods: Plasma exosomes were derived from older and younger men and were characterized. Then, HSCs were isolated from cord blood samples and treated with exosomes of older and younger men. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to evaluate cell viability. Next, the expression of HIF-1α gene and P21 protein were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot, respectively.
Results: HIF-1α gene expression was considerably increased in HSCs treated with 10 µg/mL of exosomes isolated from younger men (Y10-Exo) compared to the untreated group (P = 0.002). Moreover, HIF-1α gene expression was remarkably decreased in HSCs treated with 10 µg/mL of exosomes obtained from older men (O10-Exo) in comparison with the untreated group (P < 0.001). Additionally, the expression of P21 protein was significantly increased in HSCs treated with 5 µg/mL of exosomes derived from older individuals (O5-Exo) and O10-Exo compared to the untreated group (P = 0.000 and P = 0.002, respectively).
Conclusions: Our findings showed that exosomes isolated from younger participants cause elevation in HIF-1α and may lead to delayed aging in HSCs. In addition, exosomes isolated from older participants can probably lead to aging through the reduction in HIF-1α and elevation in P21.
Keywords: Aging; Exosomes; Hematopoietic stem cells; HIF-1α; P21; Older individuals
| Introduction | ▴Top |
As the lifetime of the human population is constantly growing, a deep knowledge of underlying mechanisms of the aging process is highly needed. Not only is it crucial for understanding disease development connection with aging, but it is also important for determining long-term goals for reaching a more stable health status in later stages of life. It can be anticipated that this objective is accessible by revising or inhibiting the deterioration caused by aging in organism’s performance [1]. Studies have demonstrated that with aging, the ability to develop adaptive immune responses decreases, and hematopoietic cells shift toward the myeloid lineage, leading to a dramatic rise in myelogenous diseases such as cancer [2, 3]. Therefore, aging-related intracellular mechanisms can be important targets for therapeutic interventions to minimize the negative impacts of aging in hematopoietic stem cells (HSCs). Different intracellular and extracellular factors affect the aging process of HSCs. DNA damage is vital and irreversible. Other intracellular mechanisms are decreased telomere length, reactive oxygen species (ROS), epigenetic and chromatin structure modifications, changes in gene expression, metabolic changes, disruption of autophagy and mitochondria, increased cell polarity, and reduced homing ability [4].
One common aging feature in different tissues is DNA damage, which occurs in HSCs because of errors in DNA synthesis or endogenous factors such as raised ROS levels or environmental stressors [5]. Moreover, hypoxia-inducible factor 1α (HIF-1α) factor can affect ROS levels; this factor is the principal mediator in response to hypoxia, belongs to the helix-loop-helix family, and is regulated by oxygen pressure. HIF-1α regulates several pathways, including metabolism, ROS level, autophagy, pH regulation, angiogenesis, proteolysis, self-renewal, and maintaining quiescence [6]. In recent studies, researchers have found that HIF-1α possesses anti-aging characteristics. According to a study, the deletion of activating transcription factor 4 (ATF4) causes a reduction in HIF-1α expression, which increases ROS levels. This elevated ROS level can induce the aging phenotype, leading to dysfunction in HSCs. In addition, they found that with increased HIF-1α, age-related consequences will be eliminated in HSCs [7]. Furthermore, emerging evidence demonstrates that hypoxia is effective in regulating cell aging. HIF-1α in hypoxia accumulates in cells and regulates cell aging by modulating P53, P21, and P16 [8]. P21 is a protein that can stop the cell cycle at the G1 stage, either in a P53-dependent or P53-independent manner [9]. Altogether, P53 and P21 proteins are part of the downstream signaling pathway of aging and hypoxia and are elevated during aging and considered a crucial marker for aging evaluation [10].
Moreover, it is reported that circulating exosomes can contribute to the stem cell hemostatic disturbance during aging [11]. Nowadays, it has been proven that exosomes are important mediators of intercellular signaling and play a vital role in modulating hemostasis in the bone marrow microenvironment. Exosomes can modulate HSCs cellular processes such as proliferation, apoptosis, self-renewal, cell fate decision-making, mitochondrial quality maintenance, and DNA integrity. They can be effective in HSCs aging process [12]. Given the significance of age-related changes in various diseases and the potential role of exosomes, it is important to investigate whether exosomes can impact the aging of HSCs through HIF-1α and P21. Therefore, this study aimed to compare the influence of plasma exosomes from younger and older individuals on these factors in HSCs.
| Materials and Methods | ▴Top |
Subjects
The current research was an experimental in vitro study. Plasma samples were collected from the Iranian Blood Transfusion Organization between August to September 2022. In total, eight plasma samples were used in this research, four of which were obtained from 61 - 64 years old men, and the other half from male participants aged 32 - 42 years old. The samples were kept at -80 °C in the Allied Medical Sciences, Tehran University of Medical Sciences.
Exclusion criteria were having infection, anemia, coronary artery disease, acute and chronic diseases, inflammatory diseases, cancer, diabetes, and endocrine disorders such as hypothyroidism and hyperthyroidism. Also, suspected participants of using drugs, alcohol and smoking were excluded from the study. Moreover, various laboratory tests were done for infections such as hepatitis B and hepatitis C and syphilis. The characteristics of subjects such as age, weight, blood pressure and blood type were also obtained. This project was carried out by obtaining the consent and knowledge of the participants and maintaining confidentiality. The current investigation was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran (ethic ID number: IR.TUMS.SPH.REC.1401.169) and adhered to the ethical guidelines set by the responsible institution for research involving human participants, in accordance with the principles of the Helsinki Declaration.
Exosomes isolation
Exosomes were isolated by the ultracentrifugation method. Concisely, eight plasma samples (four samples derived from older participants and four samples isolated from younger people) were thawed at room temperature and diluted 1:3 (volume) in phosphate buffer saline (PBS). The diluted samples were centrifuged at 17,000 × g for 30 min at 4 °C to remove debris, and the supernatant containing exosomes was collected and centrifuged with ultracentrifuge (Beckman L5-L6) at 100,000 × g for 75 min at 4 °C. Next, 2 mL of PBS was added to the collected pellet, and the suspension was filtered with a 0.22-µm filter to eliminate bigger particles from exosomes. In the next step, the filtered suspension reached a volume of 13 mL, and then suspensions were centrifuged again at 100,000 × g for 75 min at 4 °C [13]. Finally, 1 mL of PBS was added to those pellets containing exosomes. For evaluation of exosomes concentration, the protein level of the suspensions was obtained by the bicinchoninic acid (BCA) method, and then the exosomes were stored at -80 °C.
Exosomes properties
To determine the characteristics of exosomes by dynamic light scattering (DLS), the hydrodynamic diameter and zeta potential of exosomes were measured with the instrument (Malvern zeta sizer, ZSP, UK). Next, transmission electron microscope (transmission electron microscopy (TEM) (Zeiss Company, EM10C-100Kv, Germany)) was used to check the shape and size of exosomes. Finally, the Western blot method (CD63 antibody: Santa Cruz Biotechnology, USA) was used to confirm the expression of the specific CD63 marker on the surface of exosomes [13].
HSCs isolation
After obtaining the consent of the participants for HSCs isolation, umbilical cord blood samples were taken from the Iranian Blood Transfusion Organization. In this study, mononuclear cells were isolated by Ficoll [14]. Firstly, the blood from the umbilical cord bags was diluted with PBS in equal proportion. Then, the samples were centrifuged for 6 min at 1,000 × g; buffy coat layer was collected. Again, the collected buffy coat was diluted with an equal ratio of PBS. Buffy coat was added at a ratio of 2:1 with Ficoll (Lymphosep-Biowest, optimized density 1.077 g/mL). Falcon tubes were then centrifuged for 35 min at 800 × g. After centrifugation, the layer related to mononuclear cells (MNCs) was collected. Then, HSCs were isolated using MACS technology. HSCs (CD34+) isolation was done based on the Milteny Biotec kit protocol and through positive selection. Then the purification of HSCs (CD34+) was confirmed by flow cytometry.
Treatment of HSCs with exosomes
Based on previous articles and literatures, two exosome concentrations (5 µg/mL and 10 µg/mL) were selected [15, 16]. The non-toxicity effect of these concentrations was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Concentrations of 5 µg/mL and 10 g/mL of exosomes were prepared using RPMI1640 medium (Gibco, USA), containing 1 × antibiotic penicillin and streptomycin. The cells were treated with concentrations of 5 µg/mL and 10 µg/mL of plasma exosomes isolated from younger and older male participants, as well as PBS for the control group (untreated cells). The plate was placed in an incubator at a temperature of 37 °C for 24 h, and after 24 h the treated cells were collected, and RNA extraction was done for real-time polymerase chain reaction (RT-PCR) test to evaluate HIF-1α mRNA expression. The remaining cells were stored at -80 °C for Western blot assay. It should be noted that the experiments were repeated three times to eliminate technical errors.
Cell viability assessment
MTT assay was performed in order to confirm the non-toxicity of the chosen concentrations, at first, 5,000 HSCs were seeded into each well, and then exosomes from younger and older individuals were added to the wells with concentrations of 5 µg/mL and 10 µg/mL. The plate was incubated for 24 h at 37 °C and 5% CO2. After the incubation, 100 µL of MTT solution with a concentration of 0.5 mg/mL was added to each well and then incubated for 4 h at 37 °C. Afterward, 100 µL of dimethyl sulfoxide (DMSO) was added to the wells to dissolve the formazan crystals, followed by shaking and incubation in the dark for 10 min at room temperature. Finally, optical absorption was measured at a wavelength of 570 nm.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
To check the expression of the HIF-1α gene, RNA was isolated from cells using SinaClon kit, and then cDNA synthesis was done using SinaClon kit. HIF-1α gene expression was investigated by RealQ Plus SYBR Green Master Mix Green (Ampliqon, Denmark). Finally, HIF-1α mRNA expression was normalized with the β-actin gene, and delta delta cycle threshold ((ΔΔCt) was used to calculate the relative expression of genes.
Designed primers for qRT-PCR
qRT-PCR was carried out using RealQ Plus SYBR Green Master Mix Green (Ampliqon, Denmark) in order to determine HIF-1α gene expression. Total RNA extraction (SinaClon, Iran) and cDNA synthesis kit (SinClon, Iran) were used for RNA isolation and cDNA synthesis.
Primer sequences of HIF-1α and β-actin were (5'-CCGCTGGAGACACAATCATA-3' (forward), 5'-GGTGAGGGGAGCATTACATC-3' (reverse), 5'-TCCTTCCTGGGCATGGAGT-3' (forward), 5'-ACTGTGTTGGCGTACAGGTC-3' (reverse), respectively. HIF-1α mRNA expression was normalized to β-actin gene expression and ΔΔCt method was used to calculate relative expression of genes.
Western blot analysis
For confirming exosome isolation, CD63-specific marker was evaluated by Western blot analysis with anti-CD63 antibody (Santa Cruz Biotechnology, USA). Expression of P21 protein and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were measured by anti-P21 and anti-GAPDH antibodies (Santa Cruz Biotechnology USA).
At first, HSCs lysis was done by radioimmunoprecipitation assay (RIPA) buffer (PH 7.4, 1% Triton X-100, 50 mM Tris-HCL, 0.2% sodium deoxycholate, 0.2% sodium dodecyl sulfate (SDS), 1 mM Na-EDTA, and protease inhibitor cocktail). The cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and after protein band separation, they were transferred to a nitrocellulose membrane or polyvinylidene difluoride polyvinylidene fluoride (PVDF) membrane. Skimmed milk powder of 5% was used as a blocking solution. The primary antibodies were incubated for 17 h during immunoblotting, followed by incubation with secondary antibodies conjugated with horseradish peroxidase (HRP). The proteins were then detected using enhanced electrochemiluminescence (ECL) reagents. Finally, ImageJ software was utilized to quantify the protein bands.
Statistical analysis
All statistical analysis was done by SPSS (SPSS, Chicago, IL, USA), and all graphs were illustrated by GraphPad Prism 8.0 (San Diego, CA, USA); for participants’ information, descriptive statistics were used. The Shapiro-Wilk test was used to evaluate the normality of the data. The data with normal distribution have been shown as the mean ± standard error of the mean (SEM), which results from at least three independent experiments. The difference among the data was analyzed by one-way analysis of variance (ANOVA) with Tukey HSD post hoc test. The data with non-normal distribution have been indicated in the form of median ± interquartile range (IQR), and the difference among the data was analyzed by Kruskal-Wallis H analysis. A value of P < 0.05 was considered significant.
| Results | ▴Top |
Characteristics of participants in the study
In this study four younger men and four older men were involved; the mean age of younger participants was 37 ± 2.5 (standard deviation (SD)) and the mean age of older participants was 62 ± 0.75 (P = 0.0001).
Exosome characteristics and MTT assay
The characteristics of plasma exosomes are shown in Figure 1. The mean hydrodynamic size of the exosomes was 131.5 nm, and their zeta potential was -6.45 mV at 25 °C (Fig. 1a). In addition, as indicated in Figure 1b, the morphology and the size of exosomes were confirmed by the TEM. CD63 protein expression, which is a specific marker for exosomes, was approved by the Western blot test (Fig. 1c). Based on the MTT assay, there was no considerable differences in the viability percentage among five groups treated with concentrations of 5 or 10 µg/mL of exosomes (P = 0.453 among the five groups) (Fig. 2a).
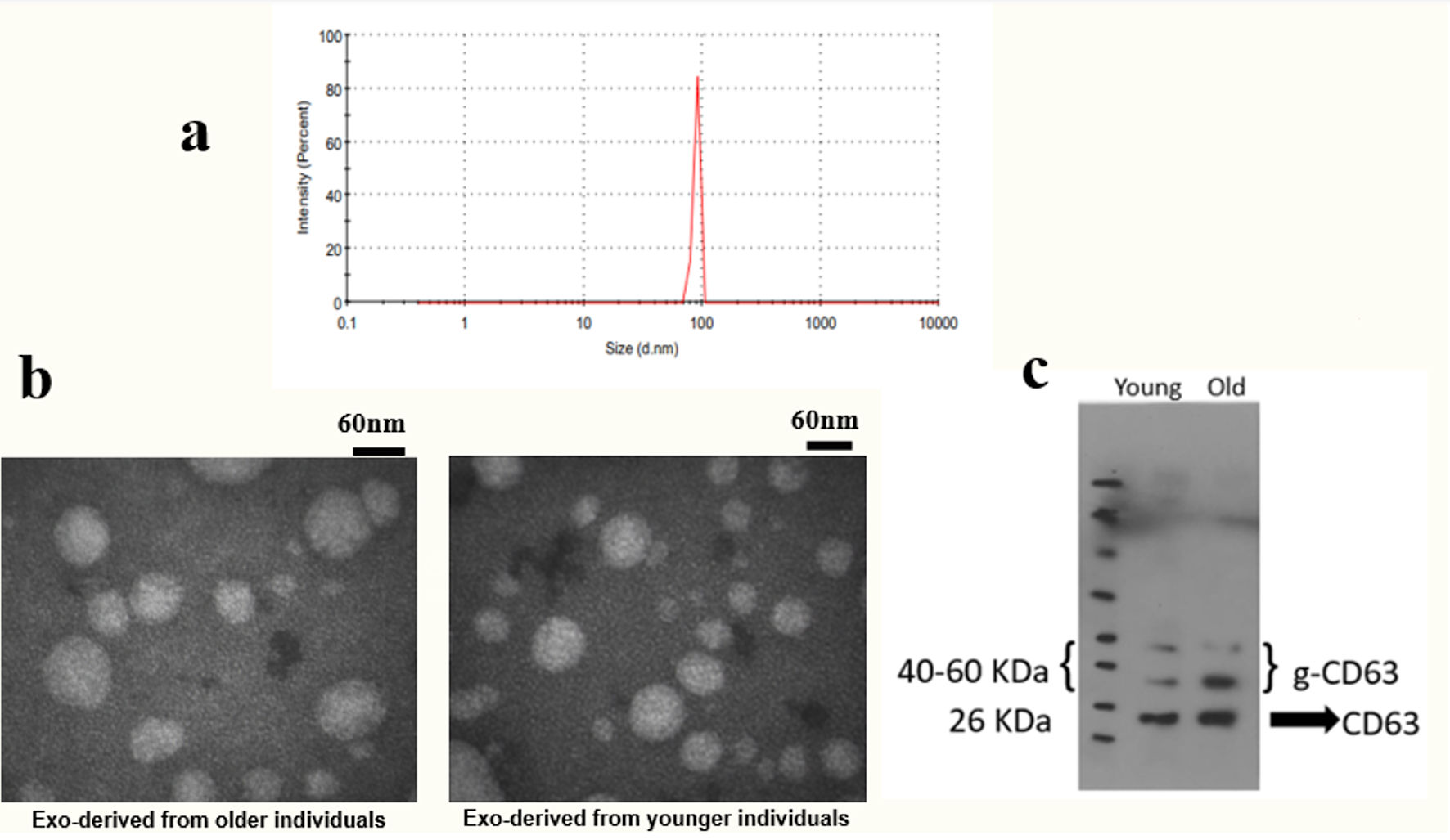 Click for large image | Figure 1. Determining the characteristics of exosomes. Isolated exosomes were evaluated in size, zeta potential, morphology, and CD63 protein expression. (a) Determination of the characteristics of exosomes by DLS. (b) Evaluation of the size and morphology of exosomes by TEM. (c) Confirmation of the expression of the specific CD63 marker by Western blot. DLS: dynamic light scattering; TEM: transmission electron microscopy. |
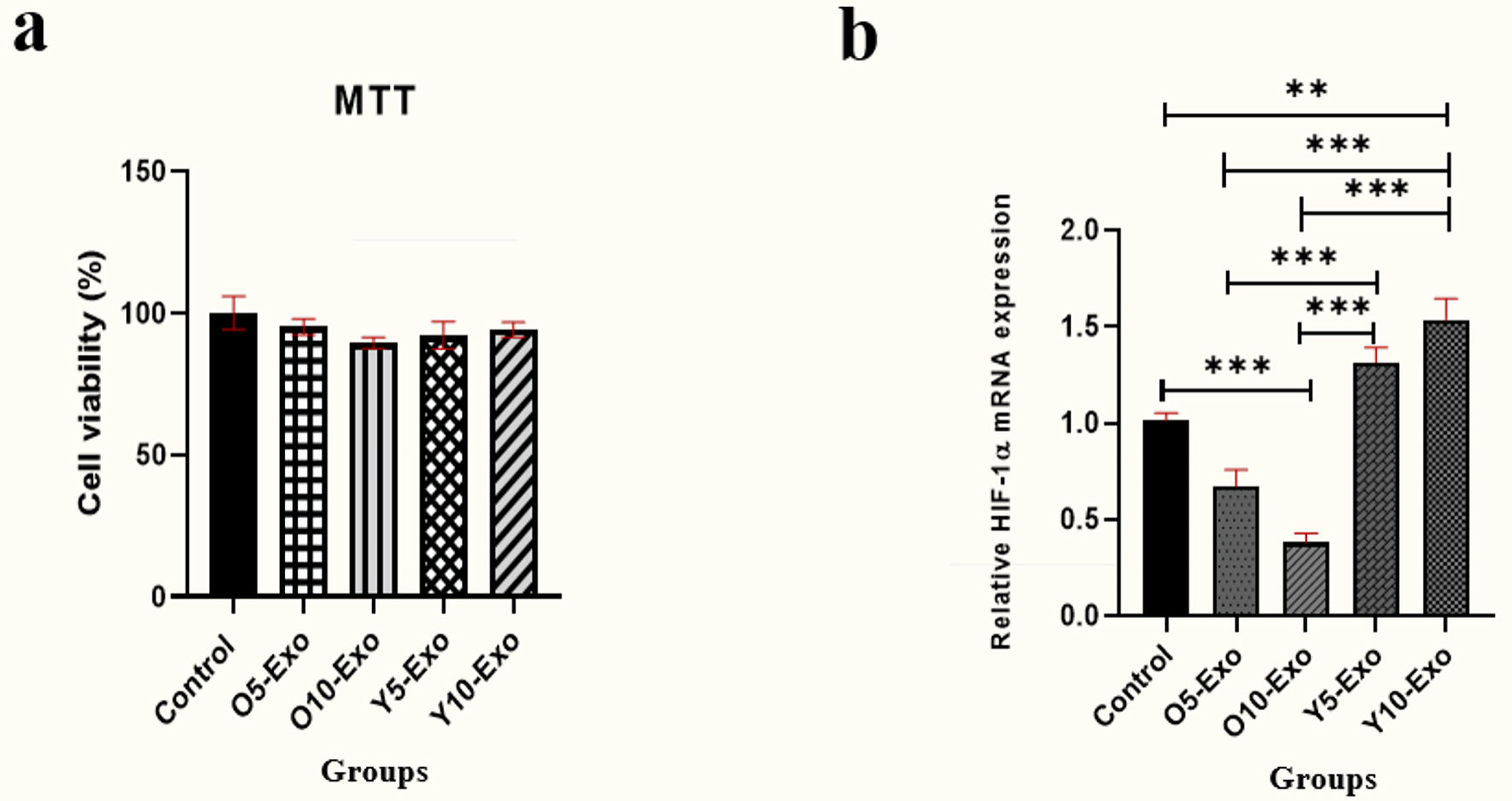 Click for large image | Figure 2. (a) MTT colorimetric assay for measuring the viability of hematopoietic stem cells (HSCs) after treatment with 5 µg/mL and 10 µg/mL concentration of exosomes isolated from young and old individuals. (b) The HIF-1α mRNA expression in the HSCs treated with the doses of 5 and 10 µg/mL Older-Exo (O5-Exo, O10-Exo) and Younger-Exo (Y5-Exo, Y10-Exo). O5-Exo: 5 µg/mL concentration of exosomes isolated from older individuals; O10-Exo: 10 µg/mL concentration of exosomes isolated from older individuals; Y5-Exo: 5 µg/mL concentration of exosomes isolated from younger individuals; Y10-Exo: 10 µg/mL concentration of exosomes isolated from younger individuals; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; HIF-1α: hypoxia-inducible factor 1α. |
The effect of older and younger exosomes on HIF-1α expression
As displayed in Figure 2b, the level of HIF-1α expression was reduced sharply in HSCs treated with O10-Exo (10 µg/mL concentration of exosomes isolated from older individuals) in comparison with the untreated cells (P < 0.001). Also, HIF-1α expression level was considerably increased in HSCs treated with Y10-Exo (10 µg/mL concentration of exosomes isolated from younger individuals) compared to the untreated cells (P = 0.002). Moreover, HIF-1α expression level was significantly increased in HSCs treated with 5 µg/mL concentration of exosomes isolated from younger men (Y5-Exo (5 µg/mL concentration of exosomes isolated from younger individuals)) and Y10-Exo compared to the group treated with the same doses of the exosomes of older individuals (P < 0.001 for both).
The effect of older and younger exosomes on P21 protein
As shown in Figure 3, the results showed a considerable elevation in the levels of P21 protein in the groups treated with O5-Exo (5 µg/mL concentration of exosomes isolated from older individuals) and O10-Exo compared to the untreated cells (P = 0.002 and P < 0.001, respectively). Furthermore, a significant rise was observed in the group treated with O5-Exo and O10-Exo compared to the group treated with Y5-Exo and Y10-Exo. So that, the protein level showed a significant increase in the O5-Exo and O10-Exo groups compared to the Y10-Exo group (P < 0.001 for both). Also, there was a noticeable elevation in the O5-Exo and O10-Exo groups than the Y5-Exo (P = 0.001, P < 0.001, respectively).
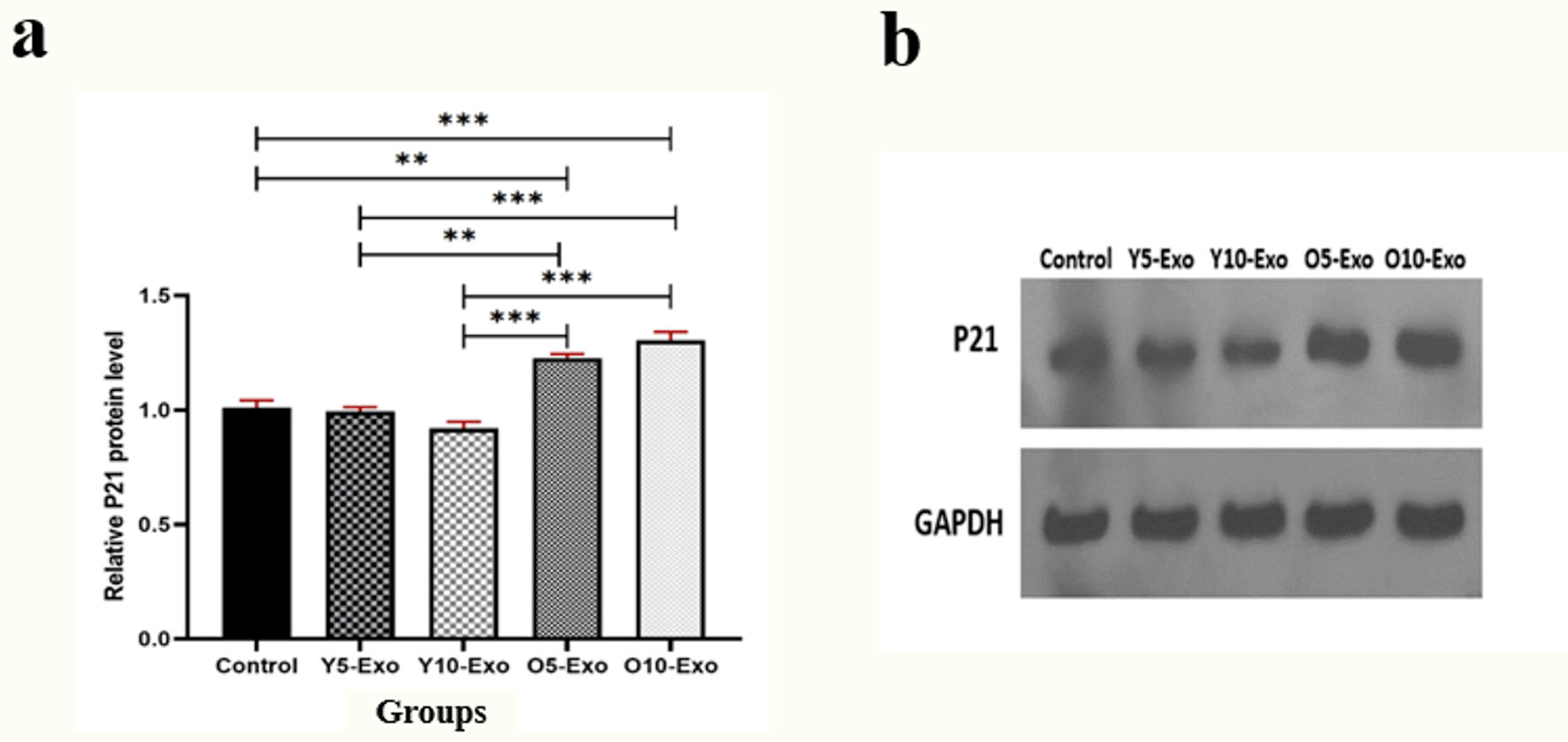 Click for large image | Figure 3. (a) The relative P21 protein level, based on P21/GAPDH ratio, in hematopoietic stem cells (HSCs) treated with the doses of 5 and 10 µg/mL Older-Exo (O5-Exo, O10-Exo) and Younger-Exo (Y5-Exo, Y10-Exo). (b) The levels of P21 protein and GAPDH evaluated by the Western blotting test. O5-Exo: 5 µg/mL concentration of exosomes isolated from older individuals; O10-Exo: 10 µg/mL concentration of exosomes isolated from older individuals; Y5-Exo: 5 µg/mL concentration of exosomes isolated from younger individuals; Y10-Exo: 10 µg/mL concentration of exosomes isolated from younger individuals; GAPDH: glyceraldehyde 3-phosphate dehydrogenase. |
| Discussion | ▴Top |
Exosomes have a vital role in cellular aging, and through exosomes, old cells can affect their microenvironment [17]. Furthermore, these exosomes are expected to impact distant organs and tissues. According to previous studies, exosomes affect different cellular processes such as aging, proliferation, regeneration, growth, migration, and immunomodulation [18]. HSCs aging and the effects of exosomes on the aging process are crucial topics, but only some studies exist. This research showed the effect of circulating exosomes of older and younger men on two important factors involved in the aging process of HSCs, HIF-1α, and P21. This study indicated that HIF-1α expression in HSCs treated with exosomes of younger participants was significantly increased compared to the untreated cells. However, HIF-1α expression in HSCs treated with exosomes of older subjects was significantly declined compared to the untreated cells. Hence, a higher level of HIF-1α expression was seen in the group treated with younger exosomes compared to older exosomes. The results of this study are in line with previous investigations and show the role of exosomes in mechanisms involved in aging. In a 2020 study conducted by Mas-Bargues et al [19], exosomes were extracted from young mesenchymal stem cells (MSCs). It was discovered that treating senescent MSCs with these exosomes reduced the aging phenotype (SA-Bgal). The study also revealed that miR-302b triggered HIF-1α, which in turn activated various pathways, including the enhanced stemness of cells, the altered metabolic pathways of cells towards glycolysis, and the delayed premature aging [19]. Furthermore, in 2022, Fang et al found that exosomes derived from MSCs rich in HIF-1α could increase the viability and autophagy in pancreatic β-cells and prevented cell aging by raising autophagy-related proteins [20]. A study conducted in 2023 by Sourki et al [21] demonstrated that plasma exosomes from different age groups may have varying effects on HSC differentiation. They found that younger donors’ exosomes improved HSC proliferation and self-renewal, while older donors’ exosomes caused senescence-associated differentiation in HSCs, especially towards the myeloid lineage [21].
The current study also showed that the expression level of P21 protein was remarkably increased in HSCs treated with plasma exosomes from older men compared to the untreated group and the group treated with plasma exosomes from younger men. Based on a study by Zhang et al in 2020, MSCs of aged individuals were rejuvenated by exosomes derived from umbilical cord MSCs; these exosomes increased proliferation, migration, differentiation, and anti-apoptotic effect and reduced factors associated with aging, including P21, P16, and P53 in aged MSC cells [22]. In 2023, Jin et al observed that young exosomes isolated from stem cells caused a reduction in the aging phenotype (SA-Bgal) and a significant decline in the expression of P21 and P53 proteins related to aging in mouse tendons [23]. In another study in 2022, Guo et al found that exosomes isolated from adipose-derived stem cells improved the aging process in skin fibroblasts by reducing aging phenotypes, such as decreasing ROS and reducing expression of SA-Bgal, P21, and P53 proteins [24]. Also, in another study in 2021, Chen et al reported that exosomes isolated from human MSCs could reduce aging phenotype, P21, and P16 expression in aged mouse cholangioids and had a protective effect against aging induced by oxidative stress [25].
The studies mentioned above demonstrate that HIF-1α can be transferred via exosomes. Also, exosomes can contain miRNAs such as miR-302b, miR-126, and miR31 and these miRNAs lead to an increase in HIF-1α expression [19, 26, 27]. Based on studies conducted about aging, it is revealed that increased HIF-1α prevents aging through different mechanisms; for instance, HIF-1α by decreasing ROS production, leads to an overall reduction in cell aging [7]. Besides, according to another study, anti-aging impact of HIF-1α is due to increased expression of MIF. MIF reduces P53 and P21, which prevents cell aging [28]. These findings strengthen the idea that HIF-1α by using some factors has anti-aging effects which is its main role. The results of the present study are consistent with previous studies and show that circulating plasma exosomes isolated from younger men increase HIF-1α in HSCs. In contrast, circulating plasma exosomes isolated from aged men have led to the reduction of HIF-1α in HSCs. Also, the present study found that by treating HSCs with exosomes from older male participants, the expression of P21 protein was increased, which is a sign of the effect of exosomes on the aging process of HSCs. In the current study, there are some limitations, for example, the contents of exosomes, their uptake into HSCs and their impact on other aging-related pathways like ROS were not investigated in this study. Additionally, we could not analyze the effect of exosomes from the intermediate-age group on HSCs. Also, it would be great to analyze the effect of exosomes from different age groups on adult HSCs. So, more complementary studies are needed to confirm our findings.
Conclusions
Our results showed that circulating exosomes of older individuals lead to a reduction in the expression of the HIF-1α gene and an increase in the expression of P21 protein in HSCs. However, circulating exosomes of younger individuals have led to the increasing expression of the HIF-1α gene in HSCs. The findings of this study and previous research point out the distinct content of circulating exosomes of people of different age groups, which can affect the underlying mechanisms of cellular aging. Due to the increasing importance of the topic of aging and related diseases and the significance of the aging of HSCs in bone marrow transplantation, we need further investigations in this field so that we can move toward preventing the aging process of HSCs in the future.
Acknowledgments
The current study is derived from master thesis with grant number 1402-2-102-62916, we would like to acknowledge the Tehran University of Medical Sciences for their financial support.
Financial Disclosure
The current investigation was supported by the Tehran University of Medical Sciences (grant number: 1402-2-102-62916).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from all participants.
Author Contributions
SA, and RA designed the experiments. ZR, EBV and ZK conducted the experiments. ZR and SHM wrote the manuscript. RA and ZR analyzed data and edited the manuscript. All authors discussed the results and contributed to the final manuscript.
Data Availability
The datasets used and/or analyzed during the study available from the corresponding author on reasonable request.
Abbreviations
ATF4: activating transcription factor 4; BCA: bicinchoninic acid; DLS: dynamic light scattering; HSCs: hematopoietic stem cells; HIF-1α: hypoxia-inducible factor 1α; IPSC: induced pluripotent stem cell; MACS: magnetic-activated cell sorting; MIF: macrophage migration inhibitory factor; MSC: mesenchymal stem cell; MNCs: mononuclear cells; O5-Exo: 5 µg/mL concentration of exosomes isolated from older individuals; O10-Exo: 10 µg/mL concentration of exosomes isolated from older individuals; PBS: phosphate buffered saline; ROS: reactive oxygen species; SASP: senescence-associated secretory phenotype; SA-Bgal, senescence-associated-beta galactosidase; SEM: standard error of the mean; TEM: transmission electron microscopy; Y5-Exo: 5 µg/mL concentration of exosomes isolated from younger individuals; Y10-Exo: 10 µg/mL concentration of exosomes isolated from younger individuals
| References | ▴Top |
- Wahlestedt M, Pronk CJ, Bryder D. Concise review: hematopoietic stem cell aging and the prospects for rejuvenation. Stem Cells Transl Med. 2015;4(2):186-194.
doi pubmed - Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol. 2016;7:502.
doi pubmed - Yang D, de Haan G. Inflammation and aging of hematopoietic stem cells in their niche. Cells. 2021;10(8):1849.
doi pubmed - Mejia-Ramirez E, Florian MC. Understanding intrinsic hematopoietic stem cell aging. Haematologica. 2020;105(1):22-37.
doi pubmed - Montazersaheb S, Ehsani A, Fathi E, Farahzadi R. Cellular and molecular mechanisms involved in hematopoietic stem cell aging as a clinical prospect. Oxid Med Cell Longev. 2022;2022:2713483.
doi pubmed - Rouault-Pierre K, Hamilton A, Bonnet D. Effect of hypoxia-inducible factors in normal and leukemic stem cell regulation and their potential therapeutic impact. Expert Opin Biol Ther. 2016;16(4):463-476.
doi pubmed - Sun Y, Lin X, Liu B, Zhang Y, Li W, Zhang S, He F, et al. Loss of ATF4 leads to functional aging-like attrition of adult hematopoietic stem cells. Sci Adv. 2021;7(52):eabj6877.
doi pubmed - Gao H, Nepovimova E, Heger Z, Valko M, Wu Q, Kuca K, Adam V. Role of hypoxia in cellular senescence. Pharmacol Res. 2023;194:106841.
doi pubmed - Papismadov N, Gal H, Krizhanovsky V. The anti-aging promise of p21. Cell Cycle. 2017;16(21):1997-1998.
doi pubmed - Zhu LY, Yu LM, Zhang WH, Deng JJ, Liu SF, Huang W, Zhang MH, et al. Aging induced p53/p21 in genioglossus muscle stem cells and enhanced upper airway injury. Stem Cells Int. 2020;2020:8412598.
doi pubmed - Grenier-Pleau I, Tyryshkin K, Le TD, Rudan J, Bonneil E, Thibault P, Zeng K, et al. Blood extracellular vesicles from healthy individuals regulate hematopoietic stem cells as humans age. Aging Cell. 2020;19(11):e13245.
doi pubmed - Goldberg LR. Extracellular vesicles and hematopoietic stem cell aging. Arterioscler Thromb Vasc Biol. 2021;41(8):e399-e416.
doi pubmed - Sadegh-Nejadi S, Afrisham R, Emamgholipour S, Izadi P, Eivazi N, Tahbazlahafi B, Paknejad M. Influence of plasma circulating exosomes obtained from obese women on tumorigenesis and tamoxifen resistance in MCF-7 cells. IUBMB Life. 2020;72(9):1930-1940.
doi pubmed - Jia Y, Xu H, Li Y, Wei C, Guo R, Wang F, Wu Y, et al. A modified ficoll-paque gradient method for isolating mononuclear cells from the peripheral and umbilical cord blood of humans for biobanks and clinical laboratories. Biopreserv Biobank. 2018;16(2):82-91.
doi pubmed - Afrisham R, Sadegh-Nejadi S, Meshkani R, Emamgholipour S, Paknejad M. Effect of circulating exosomes derived from normal-weight and obese women on gluconeogenesis, glycogenesis, lipogenesis and secretion of FGF21 and fetuin A in HepG2 cells. Diabetol Metab Syndr. 2020;12:32.
doi pubmed - Afrisham R, Sadegh-Nejadi S, Meshkani R, Emamgholipour S, Bagherieh M, Paknejad M. Anti-inflammatory effects of plasma circulating exosomes obtained from normal-weight and obese subjects on hepatocytes. Endocr Metab Immune Disord Drug Targets. 2021;21(3):478-484.
doi pubmed - Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17(2):e12734.
doi pubmed - Hamdan Y, Mazini L, Malka G. Exosomes and micro-RNAs in aging process. Biomedicines. 2021;9(8):968.
doi pubmed - Mas-Bargues C, Sanz-Ros J, Roman-Dominguez A, Gimeno-Mallench L, Ingles M, Vina J, Borras C. Extracellular vesicles from healthy cells improves cell function and stemness in premature senescent stem cells by miR-302b and HIF-1alpha activation. Biomolecules. 2020;10(6):957.
doi pubmed - Fang J, Chen Z, Lai X, Yin W, Guo Y, Zhang W, Ma J, et al. Mesenchymal stem cells-derived HIF-1alpha-overexpressed extracellular vesicles ameliorate hypoxia-induced pancreatic beta cell apoptosis and senescence through activating YTHDF1-mediated protective autophagy. Bioorg Chem. 2022;129:106194.
doi pubmed - Abbasi Sourki P, Pourfathollah AA, Kaviani S, Soufi Zomorrod M, Ajami M, Wollenberg B, Multhoff G, et al. The profile of circulating extracellular vesicles depending on the age of the donor potentially drives the rejuvenation or senescence fate of hematopoietic stem cells. Exp Gerontol. 2023;175:112142.
doi pubmed - Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, et al. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther. 2020;11(1):273.
doi pubmed - Jin S, Wang Y, Wu X, Li Z, Zhu L, Niu Y, Zhou Y, et al. Young exosome bio-nanoparticles restore aging-impaired tendon stem/progenitor cell function and reparative capacity. Adv Mater. 2023;35(18):e2211602.
doi pubmed - Guo JA, Yu PJ, Yang DQ, Chen W. The antisenescence effect of exosomes from human adipose-derived stem cells on skin fibroblasts. Biomed Res Int. 2022;2022:1034316.
doi pubmed - Chen W, Zhu J, Lin F, Xu Y, Feng B, Feng X, Sheng X, et al. Human placenta mesenchymal stem cell-derived exosomes delay H(2)O(2)-induced aging in mouse cholangioids. Stem Cell Res Ther. 2021;12(1):201.
doi pubmed - Alique M, Bodega G, Giannarelli C, Carracedo J, Ramirez R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1alpha which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci Rep. 2019;9(1):7381.
doi pubmed - Zhu D, Wang Y, Thomas M, McLaughlin K, Oguljahan B, Henderson J, Yang Q, et al. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1alpha pathway. J Mol Cell Cardiol. 2022;162:10-19.
doi pubmed - Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006;20(24):3366-3371.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


