| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 5, October 2024, pages 250-258
Allogeneic Hematopoietic Stem Cell Transplantation After Solid Organ Transplantation in Patients With Hematologic Malignancies Managed With Post-Transplant Cyclophosphamide-Based Graft-Versus-Host Disease Prophylaxis
Charley Janga, c, Jingmei Hsub
aDepartment of Medicine, NYU Grossman School of Medicine, New York, NY, USA
bDepartment of Hematology and Oncology, NYU Langone Health Perlmutter Cancer Center, NYU Grossman School of Medicine, New York, NY, USA
cCorresponding Author: Charley Jang, Department of Medicine, NYU Grossman School of Medicine, New York, NY 10016, USA
Manuscript submitted July 25, 2024, accepted September 19, 2024, published online October 21, 2024
Short title: GVHD Prophylaxis in Allogeneic HSCT After SOT
doi: https://doi.org/10.14740/jh1327
| Abstract | ▴Top |
Patients who receive solid organ transplants often require lifelong immunosuppression, which increases their risk for hematologic disorders. Allogeneic hematopoietic stem cell transplantation (HSCT) offers a potential curative treatment option for these patients. However, there is still a lack of understanding and guidance on graft-vs-host disease (GVHD) immunosuppression regimens, potential complications, and outcomes in patients with solid organ transplants who undergo HSCT. The rate of solid organ transplantation continues to increase annually, making this a common clinical scenario that hematologists encounter. In this case series, we present three patients who underwent liver, kidney and cardiac transplants and each developed hematological malignancies requiring allogeneic stem cell transplant. This is the first case report of two patients who received post-transplant cyclophosphamide with mycophenolate mofetil and tacrolimus GVHD prophylaxis. We also review recent advances in GVHD prophylaxis in allogeneic HSCT and solid organ transplantation including immune tolerance and immunosuppression-free protocols. Our case series support the use of post-transplant cyclophosphamide with mycophenolate mofetil and tacrolimus as post-transplant GVHD prophylaxis, which does not appear to compromise solid organ graft function. Our case series also provides evidence that allogeneic HSCT is a feasible and potentially life-saving treatment option in patients who develop hematologic malignancies after solid organ transplantation.
Keywords: Solid organ transplantation; Hematopoietic stem cell transplantation; Immunosuppression; Hematologic disorders; Graft-vs-host disease; Post-transplant cyclophosphamide
| Introduction | ▴Top |
Hematopoietic stem cell transplantation (HSCT) and solid organ transplantation (SOT) offer life-saving treatment options for patients with hematological diseases and end-stage organ failure and have transformed the therapeutic landscape for terminal diseases and organ dysfunctions. The rate of SOT continues to increase over the years, with over 46,000 transplants occurring in 2023 which represents a 12.7% and 8.7% increase from 2021 and 2022, respectively, in the United States alone [1]. SOT patients often require lifelong immunosuppression with a subsequent increased risk of hematologic malignancies [2-8]. Post-transplant lymphoproliferative disorders (PTLD) represent one of the major lymphoid hematological malignancies [9, 10], while acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) represent the major myeloid hematological malignancies in SOT recipients.
HSCT is one of the treatment modalities offered to patients who develop life-threatening hematologic disorders after SOT. These SOT patients who undergo HSCT face additional increased risk of complications including allograft rejection due to HSCT donor-derived immune cells, opportunistic infections due to immature post-transplant donor immune system and continued immunosuppressive therapy, and relapse due to a weakened graft-versus leukemia/lymphoma effect [11-15]. There is paucity of data and guidance on HSCT in SOT patients, leading to uncertainty in the management and outcomes in this patient population.
In this case series, we present three SOT patients who developed post-transplant hematologic malignancies and subsequently underwent allogeneic HSCT. We review recent new graft-vs-host disease (GVHD) prophylaxis in allogeneic HSCT and new advances in SOT.
| Case Reports | ▴Top |
Case 1
The patient is a 57-year-old man with a 20-year history of ulcerative colitis on mesalamine. His course was complicated by the development of primary sclerosing cholangitis which required a Roux-en-Y hepaticojejunostomy and cadaver liver transplantation in January 2016. Post-liver transplant, he was maintained on tacrolimus immunosuppressant to prevent graft rejection. He presented with pancytopenia about 2 years post-SOT. The bone marrow biopsy from April 2018 showed normal cellularity with relative decrease in the myeloid precursors and an increased hemosiderin deposition without obvious dysplasia. Myeloblast was ≤ 5%, but there were trisomy 8 cytogenetic changes concerning for low-grade myelodysplasia. However, no definitive diagnosis was made. With worsening pancytopenia, repeat bone marrow biopsy was performed in August 2018, which showed AML with 90% blasts. There were no new molecular mutations or cytogenetic changes noted. The patient underwent idarubicin and cytarabine induction, and his AML achieved complete remission. His leukemia treatment course was complicated by Enterobacter bacteremia and human herpesvirus 6 (HHV6) viremia. He received one cycle of high-dose cytarabine (HiDAC) consolidation before proceeding to double cord stem cell transplant. He received two 6/6 human leukocyte antigen (HLA) matched cord blood unit infusions on February 2019, after a fludarabine-cyclophosphamide-total body irradiation (200 cGy) (Flu-Cy-TBI) non-myeloablative conditioning regimen. He continued tacrolimus during his induction and consolidation chemotherapy and continued throughout HSCT. He also was started on mycophenolate mofetil (MMF) until day 28 post-HSCT for HSCT GVHD prophylaxis (Fig. 1).
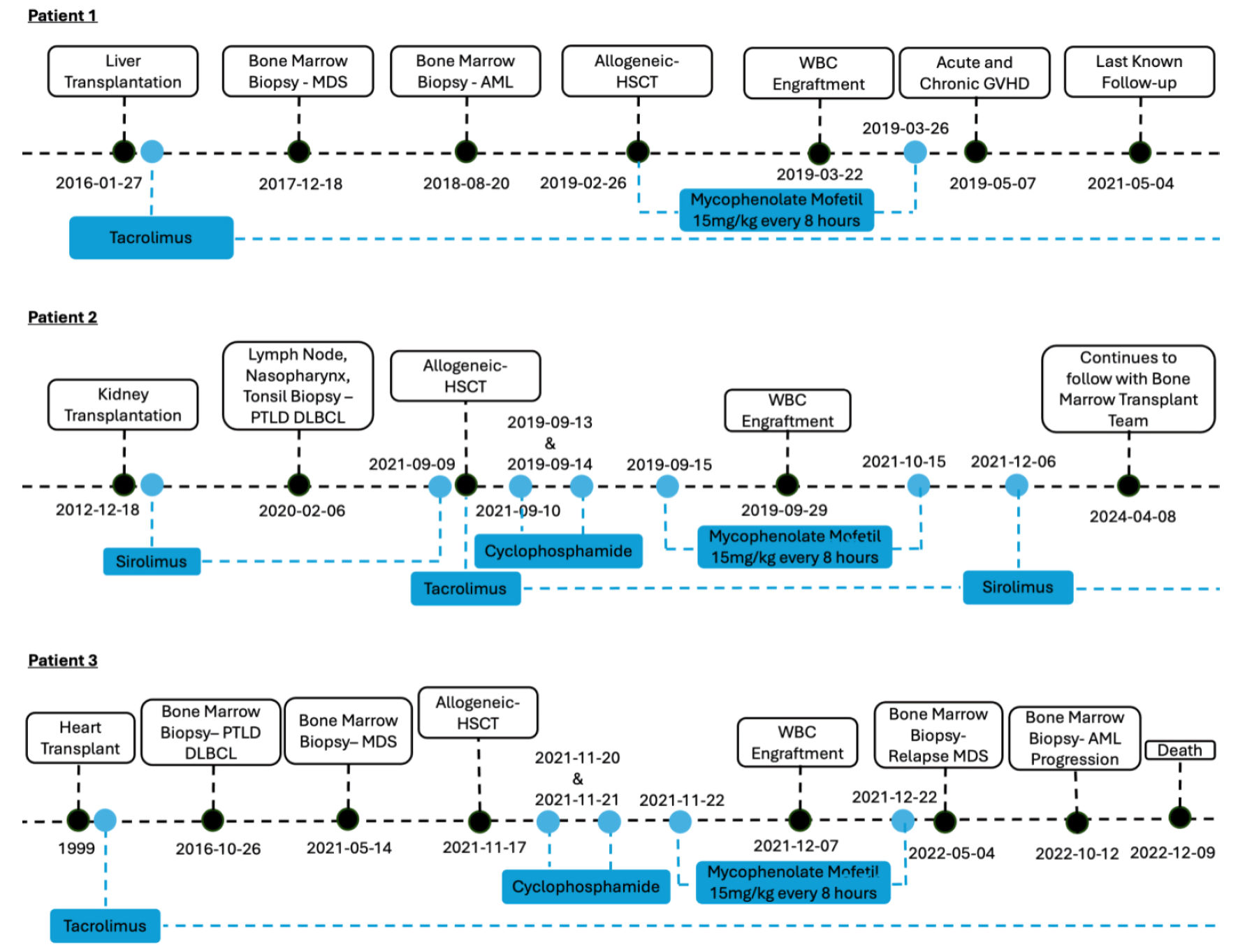 Click for large image | Figure 1. Patient timelines. MDS: myelodysplastic syndrome; AML: acute myeloid leukemia; WBC: white blood cell; HSCT: hematopoietic stem cell transplantation; GVHD: graft-vs-host disease; PTLD: post-transplant lymphoproliferative disorder; DLBCL: diffuse large B-cell lymphoma. |
The patient achieved neutrophil and platelet engraftment at 24 days and 44 days post-HSCT, respectively. He also achieved full chimerism, although with mixtures of both cords. His HSCT course was complicated by respiratory syncytial virus (RSV)/coronavirus (non-coronavirus disease 2019 (COVID-19)) upper respiratory infections, HHV6 reactivation (did not require treatment), and deep vein thrombosis and pulmonary embolism. He developed mild acute skin GVHD that only required topical hydrocortisone treatment. He developed steroid-responsive severe chronic GVHD (cGVHD) with gastrointestinal, ocular, joint, skin and pulmonary involvement. For his bronchiolitis obliterans syndrome, he developed hypoxia, which was initially treated with 1 mg/kg prednisone. His prednisone was able to be tapered to 20 mg daily without major dyspnea on exertion. The patient continued tacrolimus post-HSCT. He moved to another state at 26 months post-HSCT (Table 1). During this time, his liver remains functional without evidence of liver rejection.
 Click to view | Table 1. Patient Characteristics |
Case 2
The patient is a 40-year-old man with end-stage renal disease secondary to diabetic nephropathy. He underwent renal transplant in 2013 and remained on sirolimus immunosuppressant to prevent graft rejection. In 2020, 7 years after his renal transplant, the patient presented with throat and ear pain and B symptoms. He was found to have cervical and inguinal lymphadenopathy. Bilateral tonsillectomy and submental lymph node and nasopharynx biopsies in February 2020 revealed diffuse large B-cell lymphoma (DLBCL), post-transplant, non-GC type, CD5+, Pax5+, Mum-1+, BCL-2+ and negative for CD10, BCL-6, c-myc (< 5%), EMA, CD138, ALK, CD30, Epstein-Barr virus-encoded RNA (EBER) and cyclin D1. The proliferation index (Ki-67) was around 70%. Fluorescence in situ hybridization (FISH) study showed two abnormal clones: one (2.5%) had a diploid with two normal copies of BCL6 and an additional copy of 5′BCL6; the other (34.5%) had four normal fusion signals for BCL6 and two additional copies of 5′BCL6 (4F2G pattern). Staging positron emission tomography/computed tomography (PET/CT) from February 2020 showed hypermetabolic nodal masses above and below the diaphragm. Left inguinal nodal mass led to lower extremity edema. His DLBCL achieved complete remission with six cycles of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). He also received four cycles of intrathecal methotrexate and cytosine arabinoside for central nervous system (CNS) prophylaxis. Unfortunately, he had biopsy proven relapse of his DLBCL in January 2021. The Ki-67 was around 80-90%. He received two cycles of rituximab, ifosfamide, carboplatin, and etoposide (RICE) salvage chemotherapy, but unfortunately the disease progressed. He then proceeded with lenalidomide and tafasitamab treatment. He also completed 3,600 cGy (18 fractions) radiation therapy to the left groin. PET/CT in July 2021 showed complete response. His treatment was then switched to tafasitamab maintenance every 2 weeks.
The patient underwent haploidentical HSCT with Flu-Cy-TBI non-myeloablative conditioning in September 2021, 8.6 years after his renal transplant. For GVHD prophylaxis, his sirolimus was discontinued 1 day prior to HSCT. Instead, post-transplant cyclophosphamide (PTCy) on days +3 and +4, and MMF and tacrolimus started on day +5 were used. MMF was stopped on day +35 post-HSCT (Fig. 1). He achieved complete donor chimerism with neutrophil and platelet engrafted at 19 and 23 days, respectively. Two months after HSCT, a PET/CT scan showed increased uptake in his left inguinal lymphadenopathy (Deauville score 4) concerning DLBCL relapse. The tacrolimus dose was subsequently decreased to allow for potential increased graft-versus-leukemia (GVL) effect and discontinued at day +94. The patient’s immunosuppressant was transitioned back to his sirolimus and is currently maintained on 0.5 mg daily. His subsequent PET/CT scans showed continued complete remission. The patient never had acute GVHD (aGVHD) or cGVHD or infectious disease complications and is now 43 months post-HSCT (Table 1). His renal function maintained baseline function during transplant and never had signs of rejection.
Case 3
The patient is a 54-year-old woman with a history of cardiac transplant in 1999 secondary to viral myocarditis. She was on tacrolimus immunosuppressant for prevention of graft rejection. In January 2013, she developed stage IIIA human papillomavirus-associated anal cancer (cT2, cN1c, cM0), but achieved complete remission following concurrent mitomycin/fluorouracil and radiation therapy. In October 2015, she was diagnosed with DLBCL (PTLD), 16 years after her cardiac transplant. Her tacrolimus immunosuppression was decreased, and she was monitored clinically. However, PET/CT in October 2016 showed progression of PTLD. Biopsy was consistent with GC-type DLBCL. She responded to two cycles of R-EPOCH (rituximab, etoposide, prednisone, Oncovin (vincristine), and cyclophosphamide) and three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). In May 2021, she was diagnosed with MDS with bone marrow biopsy showing hypercellular marrow with erythroid and megakaryocytic dysplasia. FISH showed deletion of 5q and 7q. Cytogenetics showed monosomy 7, 15 and 19 in addition to del (5). Her MDS also carried TP53 and SH2B3 mutations. She received five cycles of azacitidine and venetoclax with reduction of myeloblasts to about 1% from 3% at diagnosis. She proceeded with a fludarabine/busulfan/cyclophosphamide/TBI (200 cGy) reduced intensity conditioning regimen followed by a haploidentical stem cell transplant in November 2021, 22 years following her cardiac transplant.
She was started on PTCy and MMF for GVHD prophylaxis while tacrolimus continued. Her MMF was discontinued on day +35 post-HSCT (Fig. 1). The patient achieved full chimerism with neutrophil and platelet engraftment at 20 and 31 days, respectively. She did not develop acute or cGVHD but did develop renal insufficiency post-HSCT complication.
She was noted to have worsening pancytopenia 6 months post-HSCT. Bone marrow biopsy in May 2022 showed relapsed myelodysplastic disease with 10-15% blasts. Her MDS had complex cytogenetics and two separate TP53 mutations. She was not a candidate for donor lymphocytes infusion treatment due to ongoing tacrolimus immunosuppression for her cardiac transplant rejection prevention. Her tacrolimus dose was reduced, and she received three cycles of decitabine treatment with initial response. However, 5 months later, her MDS transformed to AML with bone marrow biopsy showing 25% myeloblasts. At the time of her AML transformation, she had mixed chimerism with 43% donor (XY) and 57% host (XX). She was unable to receive induction chemotherapy for her AML due to multiple infections (pneumonia, facial cellulitis with or without sinusitis). She was enrolled in home hospice care and passed away 13 months post-HSCT (Table 1).
| Discussion | ▴Top |
Patients with solid organ transplants are at higher risk for hematologic malignancies. PTLD in the form of non-Hodgkin lymphoma is the most common hematologic malignancy after SOT and constitutes up to a 15-fold increased risk compared to the general population [2-8]. Allogeneic HSCT is a potentially curable treatment option for these patients. However, there is still little understanding and guidance in the literature of allogeneic HSCT in SOT recipients who develop hematologic malignancies, which is not an uncommon clinical scenario that hematologists face. In this case series, three of our patients underwent liver, kidney and cardiac transplant, respectively, and each developed hematological malignancies requiring allogeneic stem cell transplant. Here we review SOT patients requiring HSCT and new developments in the field.
Donor criteria for SOT varies based on the specific organ transplanted. The role of HLA matching in kidney transplantation has been well established. A higher degree of HLA matching is associated with significantly improved outcomes including graft survival and recipient survival [16-18]. However, HLA matching is not routinely performed in cardiac and liver transplantation due to organ scarcity, reducing graft cold ischemia time, and a lack of clarity of the impact of HLA matching on transplant outcomes [19-21]. In allogeneic HSCT, HLA matching is an essential component, as the degree of specific HLA mismatches has been associated with graft failure, delayed immune reconstitution, increased GVHD, and increased mortality [22, 23]. The gold standard of allogeneic HSCT is high resolution typing with either an 8/8 (in USA) or 10/10 (Europe) HLA allelic match (HLA-A, -B, -C, -DRB1, and -DQB1) [24]. In SOT patients undergoing allogeneic HSCT, there is a potential concern that HLA disparity between the transplanted solid organ and that of the HSCT donor may impact solid organ graft survival. Detailed data on HLA disparity between the solid organ and donors stem cells are often lacking in studies and case reports (Supplementary Material 1, www.thejh.org). In our case series, there were no data on the HLA disparity between the HSCT donor and solid organ transplant for either patient as solid organ transplants were performed at different institutions and years apart. Nonetheless, neither of our patients experienced solid organ transplant graft failure with the GVHD prophylaxis strategies used for HSCT. Additional studies have demonstrated successful allogeneic HSCT without SOT rejection (Supplementary Material 1, www.thejh.org) [11-13, 15]. This may partly be due to the presence and absence of permissive and nonpermissive mismatches at specific HLA loci, which may be influenced by additional clinical and immunological risk factors such as cold and warm ischemia times, donor source, and immunopeptidome overlap [25, 26]. HLA matching in solid organ transplants may also be of lesser significance due to improved immunosuppression and immunological risk assessments, which has led to successful transplantation of both well- and poorly matched solid organs.
Management and prevention of GVHD is crucial to the success of allogeneic HSCT and solid organ transplant. Immunosuppression regimens differ between solid organ transplants and allogeneic HSCT (Supplementary Material 1, www.thejh.org). Immunosuppression regimens in solid organ transplants generally include a calcineurin inhibitor (cyclosporin or tacrolimus), rapamycin (sirolimus, an mTOR inhibitor), antiproliferative agent (MMF or azathioprine), and glucocorticoids. In renal transplant patients, tacrolimus and MMF have been standard of care due to the landmark Symphony trial, which showed that renal transplant patients managed with tacrolimus and MMF had superior outcomes in renal function, allograft survival, and decreased acute rejection rates at 1 year compared to regimens involving cyclosporine or sirolimus [27]. Three-year follow-up results did show smaller differences among the intent-to-treat groups than those at 1 year and did not reach statistical significance, which may partly be explained by substantial transitions from one treatment to another and selection bias [28]. In liver and cardiac transplantation, the triple-drug immunosuppressive regimen with calcineurin inhibitor tacrolimus, antimetabolites mofetil mycophenolate or azathioprine, and short-term glucocorticoids remains the most commonly used accepted standard immunosuppression. Previously in allogeneic HSCT, calcineurin inhibitor and methotrexate had been the cornerstone for the prevention of GVHD [29, 30]. However, recently post-transplantation cyclophosphamide-based regimens have become standard of care for GVHD prophylaxis [31, 32]. Unlike traditional GVHD prophylaxis agents such as calcineurin inhibitors and sirolimus, cyclophosphamide can induce apoptosis of alloantigen-activated T cells, while also upregulating CD95 expression increasing the sensitivity of T cells to CD95-mediated apoptosis [33]. Post-transplant cyclophosphamide also induces alloreactive T-cell dysfunction and suppression via preferential recovery of regulatory T cells [34]. In a phase III randomized clinical trial, allogeneic HLA-matched HSCT patients, who received post-transplant GVHD prophylaxis with cyclophosphamide, MMF, and tacrolimus, were significantly more likely to have GVHD-free and relapse-free survival at 1 year, compared to those who received tacrolimus and MMF (52.7% (95% confidence interval (CI): 45.8 to 59.2) vs. 34.9% (95% CI: 28.6 to 41.3), respectively) [32]. PTCy has also been shown to be effective in HLA-matched and mismatched unrelated donor HSCT [35]. In a phase II trial of HLA-mismatched unrelated donor bone marrow transplantation using PTCy, patients who received reduced intensity conditioning had grade 2 - 4 and 3 - 4 aGVHD at day +100 of 33% and 0%, respectively, and cGVHD at 1 year of 18%. Those who received myeloablative conditioning had grade 2 - 4 and 3 - 4 aGVHD at day +100 of 43% and 18%, respectively and cGVHD at 1 year of 36% [31]. In our literature review of HSCT in SOT patients, calcineurin inhibitor-based GVHD prophylaxis was used in all patients post-HSCT. Of these 23 patients, methotrexate, MMF, and ATG was used in 12, six, and 11 patients, respectively. Twelve patients also received concurrent steroids. None of these patients received PTCy. Of these 23 patients, five patients experienced aGVHD, two patients experienced cGVHD, and one patient experienced both aGVHD and cGVHD. Two patients had unknown GVHD status (Supplementary Material 1, www.thejh.org). Similarly, in a retrospective multicenter study that evaluated allogeneic HSCT in SOT patients, patients received a range of calcineurin inhibitor-based GVHD prophylaxis with 17/31 and 7/31 patients experiencing aGVHD and cGVHD, respectively [11]. None of these patients received PTCy. In our case series, all three patients were continued on SOT immunosuppression during treatment for their hematological malignancies and throughout HSCT. Patients 2 and 3 received PTCy, MMF, and tacrolimus GVHD prophylaxis while patient 1 received tacrolimus and MMF. Patient 1 was the only patient to experience aGVHD or cGVHD. This is the first case report to our knowledge of PTCy with MMF, and tacrolimus GVHD prophylaxis in two SOT patients (Supplementary Material 1, www.thejh.org). The usage of PTCy did not affect SOT organ rejection or survival.
Novel PTCy-based regimens, such as PTCy combination with abatacept, bortezomib, vedolizumab, or PTCy with reduced-duration tacrolimus, or tacrolimus-free combinations, are being evaluated across various transplant settings, which will further refine GVHD prevention in HSCT [36-40]. As PTCy has made a substantial impact on immunosuppression in HSCT, similar transformative changes are now emerging in SOT. Solid organ transplant patients often require lifelong immunosuppression. Chronic immunosuppression predisposes patients to a myriad of potential life-threatening complications including opportunistic infections and a wide-spectrum of malignancies, as well as drug-related adverse effects, such as nephrotoxicity, neurotoxicity, cardiovascular diseases, hyperglycemia, and hypertension. Immune tolerance or immunosuppression-free protocols have the potential to reduce or eliminate the need for immunosuppressive drugs in solid organ transplants. Therapeutic cell transfer represents a potentially promising approach including regulatory T-cell infusions [41, 42]. Studies have shown that concurrent HSCT can achieve donor-specific tolerance with durable hematopoietic chimerism in preclinical models and patients [43-46]. Costimulatory blockade-based immunosuppression has also provided a promising option to improve long-term allograft function through avoidance of calcineurin inhibitor-based immunosuppression regimens. In 2011, belatacept, which binds to CD80 and CD86 on antigen-presenting cells and thus blocks CD28-mediated costimulation of T lymphocytes, was the first costimulatory blockade agent Food and Drug Administration (FDA)-approved for solid organ transplant immunosuppression. In a phase III 7-year follow-up study, patients treated with belatacept had greater sustained improvement in estimated glomerular filtration rate (eGFR) and survival compared to cyclosporine-treated patients in kidney transplant [47]. Belatacept combined with transient calcineurin inhibitor therapy was associated with superior eGFR compared to a tacrolimus-based protocol [48]. Additional promising costimulation blockade-based immunosuppression strategies include selective CD28 blockade and CD40/CD154 blockade [49-52]. Anti-CD2 depletion with siplizumab has also shown potential use in selectively expanding alloreactive regulatory T cells while depleting effector memory T cells [53, 54]. Still under early clinical trial investigation, siplizumab may be able to modulate functions to induce immune tolerance in solid organ transplant. Ongoing clinical trials are evaluating the use of siplizumab, donor-modified immune cells, and concurrent HSCT and recipient regulatory T cells in the induction of immune tolerance in solid organ transplants, which offers the potential to mitigate the deleterious effects of long-term immunosuppression [55-58]. The choice of immunosuppression regimens can have a significant impact on graft function and survival and patient morbidity and mortality. Further studies are needed to better understand the impact of post-transplant immunosuppression regimens in both SOT and HSCT patients, which may lead to more individualized approaches and improved transplant outcomes.
Learning points
Although case report studies are subject to publication bias, they can provide guidance in developing individualized treatment approaches in the face of limited data. Our case series provides evidence that allogeneic HSCT is a feasible and potentially life-saving treatment option in patients who develop hematologic malignancies after SOT. Our patients achieved rapid engraftment and durable relapse-free survival with sustained solid organ transplant function despite unknown HLA disparity between the HSCT and SOT donors. Post-transplant GVHD prophylaxis with cyclophosphamide, MMF, and tacrolimus also appears to be feasible and effective after allogeneic HSCT in SOT patients without compromising solid organ graft function. Further advancements in post-immunosuppression therapies including costimulatory blockade agents may improve transplant outcomes, while cellular therapies may remove the need for chronic immunosuppression in SOT patients, mitigating the increased risk of complications that these patients face. However, more studies, including comparisons of post-transplant GVHD prophylaxis, conditioning regimens, and donor availability and donor-recipient histocompatibility, are needed to better understand the potential complications and outcomes of HSCT in this not so uncommon patient population. This will allow for a more individualized approach with improved guidance on HSCT donor selection, GVHD/GVL management, surveillance, and immunosuppression regimens.
| Supplementary Material | ▴Top |
Suppl 1. Allogeneic-HSCT in SOT patients.
Acknowledgments
The authors would like to express their gratitude to the clinical pharmacists, nurses, physicians, and staff who contributed to the care of these patients.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
CJ and JH conceptualized and performed background research. CJ and JH wrote, edited, and finalized the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Organ Procurement and Transplantation Network. Continued increase in organ donation drives new records in 2023. New milestones exceeded. Health Resources and Services Administration.
- Park B, Yoon J, Choi D, Kim HJ, Jung YK, Kwon OJ, Lee KG. De novo cancer incidence after kidney and liver transplantation: Results from a nationwide population based data. Sci Rep. 2019;9(1):17202.
doi pubmed pmc - Quinlan SC, Morton LM, Pfeiffer RM, Anderson LA, Landgren O, Warren JL, Engels EA. Increased risk for lymphoid and myeloid neoplasms in elderly solid-organ transplant recipients. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1229-1237.
doi pubmed pmc - Engels EA, Pfeiffer RM, Fraumeni JF, Jr., Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891-1901.
doi pubmed pmc - Huo Z, Li C, Xu X, Ge F, Wang R, Wen Y, Peng H, et al. Cancer Risks in Solid Organ Transplant Recipients: Results from a Comprehensive Analysis of 72 Cohort Studies. Oncoimmunology. 2020;9(1):1848068.
doi pubmed pmc - Morton LM, Gibson TM, Clarke CA, Lynch CF, Anderson LA, Pfeiffer R, Landgren O, et al. Risk of myeloid neoplasms after solid organ transplantation. Leukemia. 2014;28(12):2317-2323.
doi pubmed pmc - Clarke CA, Morton LM, Lynch C, Pfeiffer RM, Hall EC, Gibson TM, Weisenburger DD, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109(1):280-288.
doi pubmed pmc - Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. Am J Transplant. 2018;18(3):537-549.
doi pubmed - Asleh R, Alnsasra H, Habermann TM, Briasoulis A, Kushwaha SS. Post-transplant lymphoproliferative disorder following cardiac transplantation. Front Cardiovasc Med. 2022;9:787975.
doi pubmed pmc - Cheung CY, Ma MKM, Chau KF, Chak WL, Tang SCW. Posttransplant lymphoproliferative disorders in kidney transplant recipients: a retrospective cohort analysis over two decades in Hong Kong. Oncotarget. 2017;8(57):96903-96912.
doi pubmed pmc - Basak GW, Wiktor-Jedrzejczak W, Labopin M, Schoemans H, Ljungman P, Kobbe G, Beguin Y, et al. Allogeneic hematopoietic stem cell transplantation in solid organ transplant recipients: a retrospective, multicenter study of the EBMT. Am J Transplant. 2015;15(3):705-714.
doi pubmed - Shinohara A, Oshima K, Fuji S, Umeda K, Kako S, Kurokawa M, Tsukada N, et al. Hematopoietic stem cell transplantation in solid organ recipients with emphasis on transplant complications: a nationwide retrospective survey on behalf of the Japan Society for Hematopoietic Stem Cell Transplantation Transplant Complications Working Group. Biol Blood Marrow Transplant. 2020;26(1):66-75.
doi pubmed - Doney KC, Mielcarek M, Stewart FM, Appelbaum FR. Hematopoietic cell transplantation after solid organ transplantation. Biol Blood Marrow Transplant. 2015;21(12):2123-2128.
doi pubmed - Schechter T, Gassas A, Weitzman S, Grant D, Pollock-BarZiv S, Dipchand A, Alexander S, et al. Hematopoietic stem-cell transplantation following solid-organ transplantation in children. Bone Marrow Transplant. 2011;46(10):1321-1325.
doi pubmed - El Jurdi N, DeFor T, Adamusiak AM, Brunstein CG, Pruett T, Weisdorf DJ. Hematopoietic cell and solid organ transplantation in the same patient: long-term experience at the University of Minnesota. Transplant Cell Ther. 2021;27(1):87.e1-87.e6.
doi - Hafeez MS, Awais SB, Razvi M, Bangash MH, Hsiou DA, Malik TH, Haq MU, et al. HLA mismatch is important for 20-year graft survival in kidney transplant patients. Transpl Immunol. 2023;80:101861.
doi pubmed - Takemoto S, Terasaki PI, Cecka JM, Cho YW, Gjertson DW. Survival of nationally shared, HLA-matched kidney transplants from cadaveric donors. The UNOS Scientific Renal Transplant Registry. N Engl J Med. 1992;327(12):834-839.
doi pubmed - Vu LT, Baxter-Lowe LA, Garcia J, McEnhill M, Summers P, Hirose R, Lee M, et al. HLA-DR matching in organ allocation: balance between waiting time and rejection in pediatric kidney transplantation. Arch Surg. 2011;146(7):824-829.
doi pubmed - Balan V, Ruppert K, Demetris AJ, Ledneva T, Duquesnoy RJ, Detre KM, Wei YL, et al. Long-term outcome of human leukocyte antigen mismatching in liver transplantation: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Hepatology. 2008;48(3):878-888.
doi pubmed - Navarro V, Herrine S, Katopes C, Colombe B, Spain CV. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transpl. 2006;12(4):652-658.
doi pubmed - Firoz A, Geier S, Yanagida R, Hamad E, Rakita V, Zhao H, Kashem M, et al. Heart transplant human leukocyte antigen matching in the modern era. J Card Fail. 2024;30(2):362-372.
doi pubmed - Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, Yoshida T, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339(17):1177-1185.
doi pubmed - Furst D, Muller C, Vucinic V, Bunjes D, Herr W, Gramatzki M, Schwerdtfeger R, et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis. Blood. 2013;122(18):3220-3229.
doi pubmed - Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348.
doi pubmed pmc - Leeaphorn N, Pena JRA, Thamcharoen N, Khankin EV, Pavlakis M, Cardarelli F. HLA-DQ mismatching and kidney transplant outcomes. Clin J Am Soc Nephrol. 2018;13(5):763-771.
doi pubmed pmc - Meurer T, Crivello P, Metzing M, Kester M, Megger DA, Chen W, van Veelen PA, et al. Permissive HLA-DPB1 mismatches in HCT depend on immunopeptidome divergence and editing by HLA-DM. Blood. 2021;137(7):923-928.
doi pubmed - Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, Margreiter R, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562-2575.
doi pubmed - Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, Acevedo RR, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9(8):1876-1885.
doi pubmed - Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729-735.
doi pubmed - Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73(6):1729-1734.
pubmed - Shaw BE, Jimenez-Jimenez AM, Burns LJ, Logan BR, Khimani F, Shaffer BC, Shah NN, et al. National marrow donor program-sponsored multicenter, phase II trial of HLA-mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2021;39(18):1971-1982.
doi pubmed pmc - Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, Elmariah H, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388(25):2338-2348.
doi pubmed pmc - Strauss G, Osen W, Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. 2002;128(2):255-266.
doi pubmed pmc - Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129(6):2357-2373.
doi pubmed pmc - Gooptu M, Bolanos-Meade J, Koreth J. Expanding post-transplant cyclophosphamide to matched unrelated donor transplants and beyond. Blood Rev. 2023;62:101053.
doi pubmed - Al-Homsi AS, Cirrone F, Wo S, Cole K, Suarez-Londono JA, Gardner SL, Hsu J, et al. PTCy, abatacept, and a short course of tacrolimus for GVHD prevention after haploidentical transplantation. Blood Adv. 2023;7(14):3604-3611.
doi pubmed pmc - Bruno B, Cirrone F, Cole K, et al. Post-transplant high dose cyclophosphamide and bortezomib as graft-versus-host disease prophylaxis following allogeneic hematopoietic stem cell transplantation. Blood. 2021;138:3892.
- Shelikhova L, Perminova M, Molostova O, et al. Post-transplant cyclophosphamide, abatacept, and vedolizumab to prevent graft-versus-host disease after hematopoietic stem cells transplantation from haploidentical donors in children with acute leukemia: results of a prospective trial. Blood. 2023;142:3555.
- Kasamon YL, Fuchs EJ, Zahurak M, Rosner GL, Symons HJ, Gladstone DE, Huff CA, et al. Shortened-duration tacrolimus after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2018;24(5):1022-1028.
doi pubmed pmc - Koura D, Mirocha J, Dykes K, et al. A prospective randomized clinical trial comparing a calcineurin-free gvhd prophylaxis regimen with post-transplant cyclophosphamide and abatacept to methotrexate and tacrolimus. Blood. 2023;142:3551.
- Proics E, David M, Mojibian M, Speck M, Lounnas-Mourey N, Govehovitch A, Baghdadi W, et al. Preclinical assessment of antigen-specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation. Gene Ther. 2023;30(3-4):309-322.
doi pubmed pmc - Mathew JM, J HV, LeFever A, Konieczna I, Stratton C, He J, Huang X, et al. A phase i clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8(1):7428.
doi pubmed pmc - Strober S. Use of hematopoietic cell transplants to achieve tolerance in patients with solid organ transplants. Blood. 2016;127(12):1539-1543.
doi pubmed pmc - Scandling JD, Busque S, Shizuru JA, Lowsky R, Hoppe R, Dejbakhsh-Jones S, Jensen K, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant. 2015;15(3):695-704.
doi pubmed - Leventhal JR, Elliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, Mathew JM, Konieczna I, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99(2):288-298.
doi pubmed - Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, Tolkoff-Rubin N, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599-1611.
doi pubmed pmc - Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333-343.
doi pubmed - Adams AB, Goldstein J, Garrett C, Zhang R, Patzer RE, Newell KA, Turgeon NA, et al. Belatacept Combined With Transient Calcineurin Inhibitor Therapy Prevents Rejection and Promotes Improved Long-Term Renal Allograft Function. Am J Transplant. 2017;17(11):2922-2936.
doi pubmed pmc - Adams AB, Ford ML, Larsen CP. Costimulation Blockade in Autoimmunity and Transplantation: The CD28 Pathway. J Immunol. 2016;197(6):2045-2050.
doi pubmed pmc - Parsons RF, Larsen CP, Pearson TC, Badell IR. Belatacept and CD28 Costimulation Blockade: Preventing and Reducing Alloantibodies over the Long Term. Curr Transplant Rep. 2019;6(4):277-284.
doi pubmed pmc - Zhang T, Pierson RN, 3rd, Azimzadeh AM. Update on CD40 and CD154 blockade in transplant models. Immunotherapy. 2015;7(8):899-911.
doi pubmed pmc - Xu H, Yan J, Huang Y, Chilton PM, Ding C, Schanie CL, Wang L, et al. Costimulatory blockade of CD154-CD40 in combination with T-cell lymphodepletion results in prevention of allogeneic sensitization. Blood. 2008;111(6):3266-3275.
doi pubmed pmc - Podesta MA, Binder C, Sellberg F, DeWolf S, Shonts B, Ho SH, Obradovic A, et al. Siplizumab selectively depletes effector memory T cells and promotes a relative expansion of alloreactive regulatory T cells in vitro. Am J Transplant. 2020;20(1):88-100.
doi pubmed pmc - Cvetkovski F, Razavi R, Sellberg F, Berglund E, Berglund D. Siplizumab combination therapy with belatacept or abatacept broadly inhibits human T cell alloreactivity in vitro. Am J Transplant. 2023;23(10):1603-1611.
doi pubmed - A study of TCD601 in the induction of tolerance in de novo renal transplantation (PANORAMA). ClinicalTrials.gov identifier: NCT04803006. Updated July 05, 2024. Accessed July 25, 2024. https://www.clinicaltrials.gov/study/NCT04803006.
- A study of TCD601 in the induction of tolerance in renal transplantation (PERSPECTIVE). ClinicalTrials.gov identifier: NCT04803058. Updated July 05, 2024. Accessed July 25, 2024. https://www.clinicaltrials.gov/study/NCT04803058.
- Delayed blood stem transplantation in HLA matched kidney transplant recipients to eliminate immunosuppressive drugs. ClinicalTrials.gov identifier: NCT03591302. Updated May 10, 2022. Accessed July 25, 2024. https://www.clinicaltrials.gov/study/NCT03591302.
- Clinical trial with donor modified immune cells in living donor kidney transplantation. ClinicalTrials.gov identifier: NCT05365672. Updated December 04, 2023. Accessed July 25, 2024. https://clinicaltrials.gov/study/NCT05365672.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


