| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 13, Number 5, October 2024, pages 200-206
Utility of p53 Immunohistochemical Staining for Risk Stratification of Mantle Cell Lymphoma
Ibrahim Elsharawia, b, c , Sorin Selegeana, b, Michael Cartera, b
aDepartment of Pathology and Laboratory Medicine, Division of Anatomical Pathology and Hematological Pathology, Dalhousie University, Halifax, NS, Canada
bQueen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia B3H 2Y9, Canada
cCorresponding Author: Ibrahim Elsharawi, QEII Health Sciences Centre, Halifax, NS B3H 1V8, Canada
Manuscript submitted August 6, 2024, accepted September 28, 2024, published online October 11, 2024
Short title: p53 IHC Staining for MCL
doi: https://doi.org/10.14740/jh1333
| Abstract | ▴Top |
Background: Inactivating TP53 mutations in mantle cell lymphoma (MCL) are associated with poor prognosis. While next-generation sequencing (NGS) is the gold standard for assessing TP53, p53 immunohistochemistry (IHC) is an orthogonal means of evaluating TP53 status that has not been well characterized in MCL. In this single tertiary care center laboratory study, we aimed to evaluate the concordance of p53 IHC with the TP53 status in cases of MCL in hopes of evaluating if the former could act as an accurate, timely and cost-effective way of risk stratifying these patients.
Methods: A total of 47 cases of MCL that had TP53 NGS performed were included in this study. The main objective was to correlate NGS findings with p53 IHC results. Secondary objectives included assessment of possible associations between TP53 status and other variables (demographics, unique histopathological and IHC features). The turn-around time and cost for NGS and p53 IHC were also compared.
Results: Thirteen out of 47 (28%) cases were TP53-mutated by NGS. p53 IHC showed good concordance with NGS, with moderate to high sensitivity (11/13, 85%) and excellent specificity (34/34, 100%). Secondary objectives revealed increased SOX11-negative status in TP53-mutated cases (3/13, 23% vs. 1/29, 3%, P = 0.045). The cost and turn-around time of NGS were approximately of 30- and sixfold those of p53 IHC, respectively.
Conclusion: p53 IHC shows good concordance with NGS in MCL, with high specificity and moderate sensitivity for identifying inactivating TP53 mutations. Based on our findings, p53 IHC may be an efficient and cost-effective tool in risk stratification of MCL.
Keywords: Mantle cell lymphoma; Lymphoma; Next-generation sequencing; Immunohistochemistry; TP53; p53; SOX11; Laboratory utilization
| Introduction | ▴Top |
Mantle cell lymphoma (MCL) is a type of B-cell lymphoma that generally has an aggressive course and is considered incurable [1]. It accounts for up to 10% of all B-cell lymphomas worldwide, and its incidence is increasing [1, 2]. With advances in therapeutic modalities in recent years, median survival rate has increased from about 3 years to 5 - 10 years [1]. These therapeutic advances have led to pressure being applied to laboratories to perform additional ancillary biomarker tests in a timely manner to guide treatment.
Management of MCL is based on clinical, pathological, and molecular factors. Many clinical parameters have been utilized for predicting prognosis in MCL, as determined by the MCL international prognostic index (MIPI) [1]. From an immuno- and histopathology point of view, the presence of blastoid/pleomorphic morphology, high Ki-67 index, and CD5-positive status have been variably associated with worse prognosis [1-4]. Several molecular alterations have also been associated with inferior outcomes [5]. These include TP53, NOTCH1 and CDKN2A mutations, present in up to 31%, 14%, and 34% of MCL cases, respectively [1]. Of these three genes, mutation in TP53 is associated with the worst disease outcomes [5]. TP53-mutant cases have a median survival of 1.8 years and a high (50%) relapse rate after 1 year [5].
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommends next-generation sequencing (NGS) for determining tumor TP53 status, stating that p53 immunohistochemistry (IHC) is not a proven surrogate. While NGS is the gold standard, it does come with drawbacks including relatively long turn-around time, high cost, generally limited to larger centers (requires technical expertise), high specimen quality and quantity, and interpretative challenges (e.g., variants of unknown significance) [6, 7]. We set out to compare p53 IHC to TP53 NGS to assess whether the former could serve as a reliable, efficient, and cost-effective way of determining the TP53 status in MCL. The main objective was to correlate NGS findings with p53 IHC results. Secondary objectives focused on evaluating for potential associations between the TP53 status and other variables (demographics, location of involvement, and unique histopathological and IHC features). The average turn-around time and cost for both NGS for TP53 and p53 IHC for the cases were also compared.
| Materials and Methods | ▴Top |
Data collection
The study was approved by the Nova Scotia Health (NSH) research ethics board (REB). This study was conducted in accordance with our institution’s REB guidelines. Our cohort consisted of all cases of MCL received at NSH, the only center for lymph node pathology in the Canadian province of Nova Scotia, from January 1, 2020 to March 31, 2024. Case data collected included demographics (age and sex), location of involvement (subtyped into nodal/mixed nodal extranodal and exclusively extranodal), histological and IHC features (blastoid/pleomorphic morphology, CD5 status, Ki-67 index, and SOX11 status), as well as p53 IHC and TP53 NGS results. Turnaround times for p53 IHC and TP53 NGS were recorded for each case.
Diagnostic histopathology and IHC
All surgical specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned to 4 µm thickness, and stained with hematoxylin and eosin (H&E). In-house IHC studies were performed on an automated stainer (Ventana Medical Systems, Oro Valley, Arizona, USA) following the manufacturer’s instructions using the following antibodies: p53 (Clone D07, Cell Marque, OptiView DAB IHC Detection Kit), Ki-67 (MIB-1, Dako) and CD5 (SP19, Ventana). Unstained paraffin-embedded slides were sent to Mayo Clinical Laboratories (Rochester, MN) for SOX11 IHC.
NGS
To provide tumor for NGS, tissue blocks of MCL were cut at 25 µm thickness. Depending on tumor purity, estimated from H&E slides, sections were either microdissected or submitted without enrichment in tubes. DNA was extracted and sequenced using the TruSight Tumor 15 Panel on the MiSeq - Next Generation Sequencing platform (Illumina).
The following genes are included in the panel and sequenced (exons covered are indicated in brackets): AKT1 (3), BRAF (15), EGFR (12, 18-21), ERBB2 (17-21, 24, 26), FOXL2 (1), GNA11 (5), GNAQ (5), KIT (8-11, 13, 14, 17, 18), KRAS (2-4), MET (16, 18, 20), NRAS (2-4), PDGFRA (12, 14, 18), PIK3CA (9, 20), RET (16), TP53 (1-11).
At our institution, TP53 (exons 1-11, transcript NM_001276761.1) is the only gene analyzed and reported for MCL. Only variants of strong or potential clinical significance were included, corresponding to tier 1 and tier 2 variants as per Association for Molecular Pathology (AMP)/American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) classification guidelines for somatic variants (PMID: 27993330). These were defined as pathogenic or likely pathogenic TP53 variants occurring at > 5% allele frequency.
Post-sequencing bioinformatics analysis was performed using a custom NSH-developed analysis pipeline and involves alignment of amplicon sequences to a human reference genome (version GRCh37.75). Target depth of coverage for each amplicon was a minimum of × 500.
The assay cannot detect structural or copy-number variants and may not detect all gene variants. Only some intronic splice donor/acceptor sites or known clinically significant intronic variants are evaluated. The assay does not distinguish between germline and somatic variants.
Data analysis
Pathogenic and likely pathogenic variants in TP53 (variant allele frequency (VAF) > 5%), corresponding to variants of strong or potential clinical significance, designated a case as TP53-mutant. The most common variant interpretation in ClinVar database was used to establish pathogenicity. For variants not identified in ClinVar, those that predict a truncated protein product (nonsense, frameshift) and those involving a splice site (+1 and +2 positions) were considered likely pathogenic. Non-truncating TP53 variants with ambiguous ClinVar interpretation, e.g., equal number of “likely pathogenic” and “uncertain significance” classifications, were resolved by review of variant data in the TP53 Database (National Cancer Institute) [8]; features supporting pathogenicity include location within DNA binding domain, occurring within a known hotspot, number of entries in COSMIC database, and pathogenic in in vitro functional studies.
Consistent with earlier studies, a cut-off of ≥ 30% nuclear staining (intermediate/strong intensity) for p53 IHC was used to designate p53 IHC positive, and < 30% as negative [9, 10]. Additionally, a complete absence of p53 staining (“null” pattern), commonly resulting from truncating (frameshift, nonsense) mutations, was also considered a positive result, as described in earlier studies of MCL and carcinomas [11-13]. For every case, two pathologists and a senior pathology resident determined the p53 IHC score. There were no discrepant cases amongst scorers. A strong and homogenous nuclear staining pattern for SOX11 was considered positive and a lack thereof negative. We used the prognostic cut-off for Ki-67 index, with ≥ 30% classified as positive and < 30% negative, consistent with WHO fifth edition guidelines [1]. Clinical variables were used to stratify patients by age and tumor location (nodal/mixed nodal-extranodal and exclusively extranodal).
Significance, defined as P < 0.05, was determined by Chi-square for p53 IHC, sex, tumor location, Ki-67 and SOX11. Unpaired t-test was used to compare patient age (MCL with wildtype vs. mutant TP53) and turn-around times (NGS vs. IHC).
| Results | ▴Top |
Of the 47 cases of MCL included in the study, 13 (28%) were positive for TP53 mutation by NGS (TP53-pos, Table 1). Mean and median patient age were both 67 years old, the same as that reported by the WHO [1]. Males made up most cases in both TP53-pos (77%) and TP53-neg (71%) groups. Most tumors were nodal/mixed nodal-extranodal in both the TP53-pos (13/13, 100%) and TP53-neg (29/34, 79%) groups (P = 0.14). High Ki-67 index was also common in both the TP53-pos (92%) and TP53-neg (82%) groups (P = 0.39). SOX11 status was positive more often in the TP-neg group (97%) than in the TP53-pos cohort (77%, P = 0.045). All cases were cyclin D1-positive (Fig. 1).
 Click to view | Table 1. Clinical and Disease Characteristics Stratified by TP53 Mutational Status (A), Including Variant and IHC Details of 13 TP53-Mutant Cases (B) |
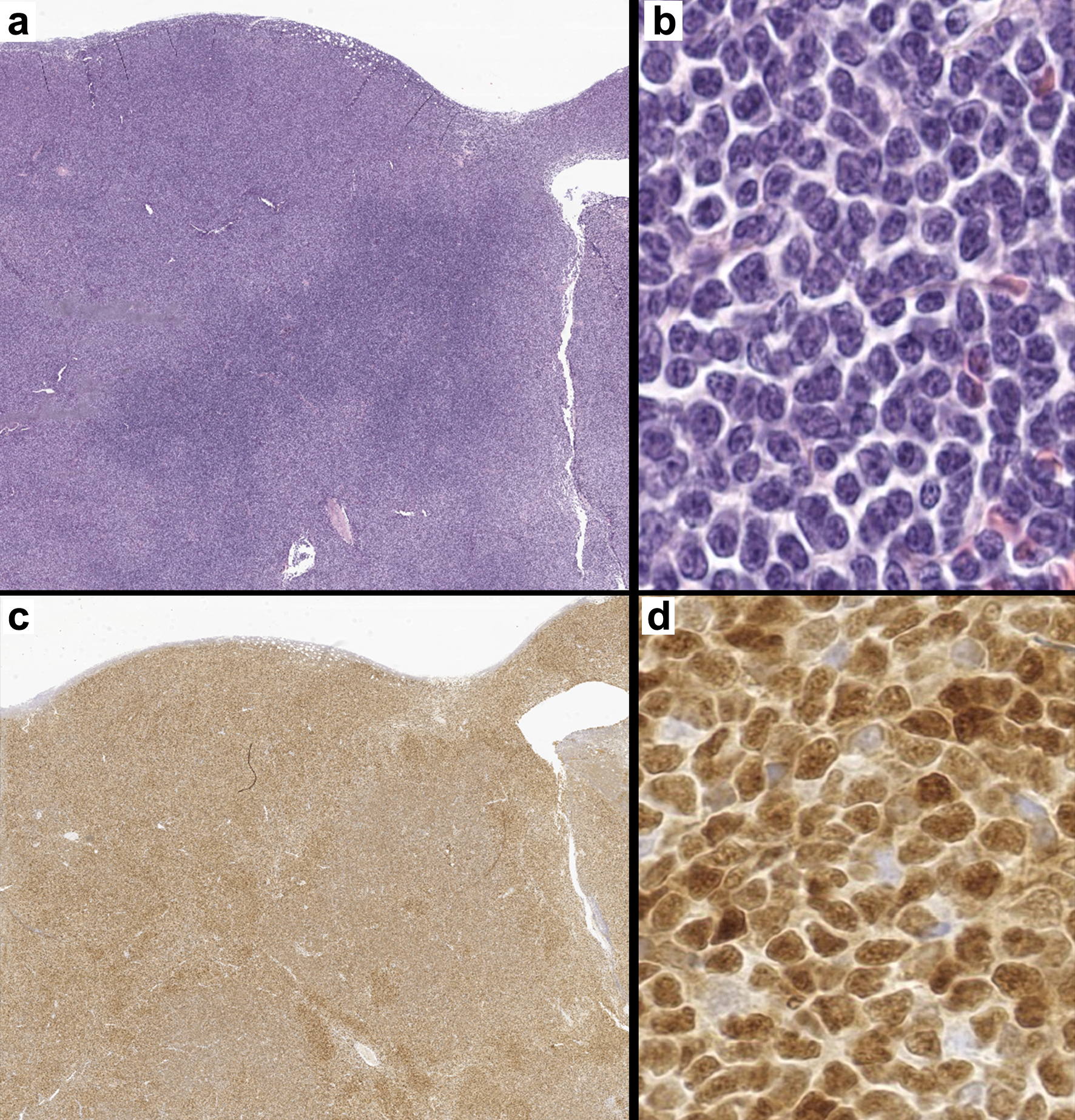 Click for large image | Figure 1. A representative case of mantle cell lymphoma. (a) Lymph node effaced by a neoplastic lymphoid process (H&E, × 7). (b) High-power image of a monotonous population of small to medium sized lymphocytes with irregular nuclear borders (H&E, × 400). (c) Cyclin D1 positivity confirming the diagnosis of mantle cell lymphoma (c: × 7, d: × 400). H&E: hematoxylin and eosin. |
Of the 13 TP53-pos cases, 11 (85%) were positive by p53 IHC and all 34 TP53-neg cases were negative by IHC (P ≤ 0.00001, Table 1). p53 IHC hence had a sensitivity of 85% and specificity of 100% in our cohort for determining TP53 status. Reviewing our institution’s costs for p53 IHC and TP53 NGS, revealed these to be $30 CAD and $842 CAD, respectively. Auditing MCL cases showed the mean turnaround time for p53 IHC was 3 days (standard deviation (SD) = 1.7) and for the TP53 NGS was 18 days (SD = 6.6) (P = 0.0001).
| Discussion | ▴Top |
Our findings show a high degree of concordance between p53 IHC and NGS; all cases that were IHC-positive (aberrant) were positive by NGS, but p53 IHC failed to identify pathogenic or likely pathogenic variants in two TP53-pos cases. While we used a threshold of ≥ 30% staining to define p53 positivity, all positive cases had moderate to intense staining in > 50% of tumor cells (Fig. 2a, b), with the exception of one case that was p53 null (Fig. 2c). SOX11 was also negative in this case and Ki-67 was > 30% (Fig. 2d, e). All the p53 IHC-negative cases in our study, including the two false negatives, showed weak staining in < 30% of tumor nuclei and almost all of them were SOX11-positive (Fig. 2f-h). Of relevance to the p53 null case, Aukema et al noted poor prognostic outcomes for extremes of p53 IHC expression, i.e., in cases with “negative” (null, 0% staining) and “high” (> 50%) expression [11]. It would be interesting to further investigate the prognostic significance of p53 IHC in the future, including comparing null to high-expressors and the potential relationship of null p53 expression to specific types of TP53 mutations (single nucleotide variant, frameshift, nonsense, splice site).
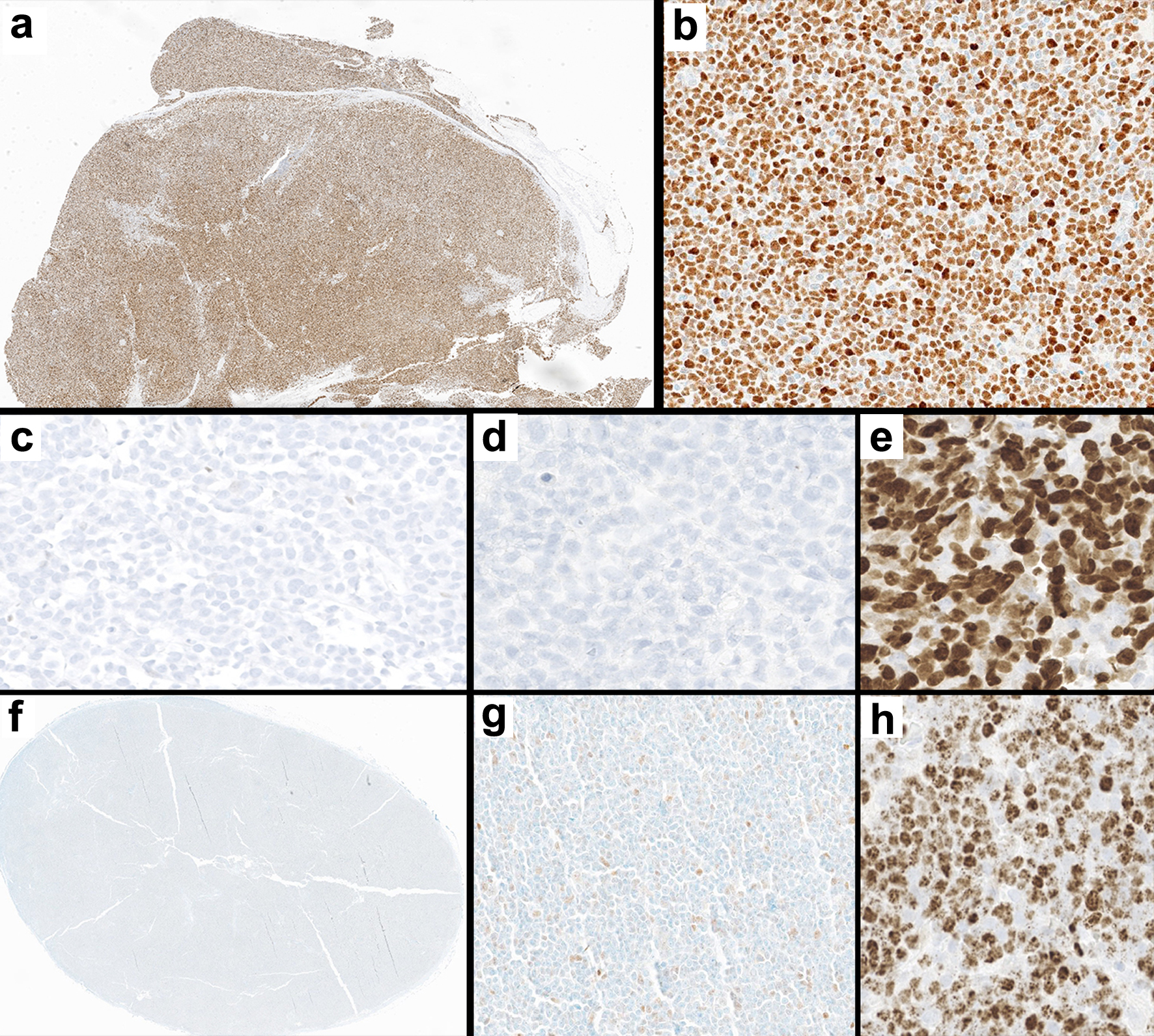 Click for large image | Figure 2. Immunohistochemical staining patterns in two TP53-pos cases (a-b and c-e) and a representative TP53-neg case (f-h). (a-b) Positive p53 IHC staining in TP53-pos MCL at low (a, × 10) and high (b, × 200) magnification. (c-e) Null p53 IHC in a TP53-pos MCL case (c, × 200), with rare non-neoplastic cells serving as internal control; this case was negative for SOX11 IHC (d, × 200) and had a high Ki-67 index (e, × 200). (f-h) Negative p53 IHC in TP53-neg MCL at low (f, × 10) and high (g, × 200) magnification; this tumor was positive for SOX11 IHC (h, × 200). IHC: immunohistochemistry; MCL: mantle cell lymphoma. |
There was an increased frequency of SOX11-negativity in the TP53-pos group (3/13, 23%) compared to the TP53-neg group (1/29, 3%) (Table 1, Fig. 2d). This inverse relationship between p53 and SOX11 expression has been previously reported by Aukema et al, who found that 75% of p53-high tumors showed low SOX11 expression [11]. Federmann et al also noted an increased incidence of negative/low expression of SOX11 mRNA in TP53-mutant groups [14]. Whether this combination (TP53-pos, SOX11-negative) has prognostic significance beyond each biomarker on its own requires further study. SOX11 IHC is also a second diagnostic marker for MCL after cyclin D1 and, when positive, can be particularly helpful in cyclin D1-negative cases [1, 15]. While cyclin D1-negative MCL cases are quite rare, the concurrent absence of SOX11 in a TP53-mutant case might pose a major diagnostic challenge [16]. No significant differences were found between TP53-pos and TP53-neg groups for other IHC markers (CD5, Ki-67), nor with regards to histological variants or demographic features (Table 1A).
All the exclusive extranodal MCL cases were TP53-neg, consistent with their relatively favorable prognosis in comparison to MCLs with nodal involvement (Table 1A) [1]. However, the frequency of pure extranodal location was not significantly different between the TP53-pos (0/13) and TP53-neg (5/34) groups (P = 0.14). Further studies examining TP53 mutational status on a larger cohort of extranodal vs. nodal MCLs might be warranted.
Lastly, we compared the cost and turnaround times for both p53 IHC and TP53 NGS. At our institution, the cost for TP53 NGS, including reagents/consumables and technologist time, was nearly 30-fold of that of the p53 IHC ($842 CAD vs. $30 CAD). This difference could be particularly significant to large centers who process large volumes of lymphoma cases, and perhaps equally relevant to smaller centers who have p53 IHC in-house but lack access to readily available NGS. Large differences were also noted in turnaround times (3 days for IHC vs. 18 days for NGS). Additionally, while we captured the time from when NGS was ordered to when it was reported, this did not include preanalytical delays, e.g., for transportation (consult specimens). The long TAT for NGS can potentially impact care in situations where time-sensitive management is warranted, including aggressive TP53-mutant cases. Further investigation is required to identify what magnitude of impact, if any, the shorter TAT for IHC may have on patient outcomes.
While there is the possibility of missing a small number of TP53-pos MCL cases by p53 IHC (i.e., false negatives), the lack of false positive cases in our study coupled with the quick turnaround time of IHC suggests it may be a cost-effective option as an initial test. p53 IHC might also be of particular importance in specific scenarios, e.g., nearly exhausted tissue blocks and cases with low tumor purity, where NGS may be unreliable. Lastly, IHC may be helpful in potentially resolving variants of unknown significance identified by NGS. One of the two false negative cases in our study, for instance, had a TP53 variant characterized by our methods as likely pathogenic; however, there is conflicting evidence regarding its pathogenicity (Table 1B) [17]. This raises the possibility that the variant (T284P) is not in fact pathogenic, or has only a minor impact on p53 function, and that the p53 IHC result may hence represent a true negative rather than a false negative. In fact, this patient remains alive 4 years post diagnosis and has not shown signs of progression while being actively treated with ibrutinib and venetoclax. We see a potential role for p53 IHC in helping resolve cases harboring TP53 variants of unknown significance. Comparing outcomes in a larger study of MCL patients could help indicate whether p53 IHC is non-inferior to TP53 NGS in prognosticating survival.
Further studies may suggest integrating p53 and SOX11 IHC as part of a larger IHC panel to risk stratify MCL, especially when considering the major impact that IHC profiling has had for categorizing diffuse large B-cell lymphomas and the emerging evidence of its utility in determining prognosis for T-cell lymphomas [1, 18].
As we continue to learn more about the optimal treatment of TP53-mutant MCL, it is apparent that many of these patients have an aggressive course with a high tendency for early progression and initial treatment failures [5, 19]. The impacts of relapse seem to be higher when it occurs within 6 months of initiating therapy, necessitating a timely approach in managing high risk patients [20]. Recent studies have highlighted the potential benefits of novel therapies like the combination of ibrutinib and venetoclax and the early application of CAR T-cell therapy or allo-hematopoietic stem cell transplant in treating MCL. Some of these therapies have shown particularly promising results in TP53-pos cases [21-23].
Based on our findings, p53 IHC should be further investigated as a potential surrogate for TP53 status and prognostic marker in MCL. It could prove to be a fast and cost-effective tool for identifying MCLs that are TP53-mutant (i.e., p53 IHC-positive), eliminating the need for NGS assessment in these cases. With further study, p53 IHC may even be shown to be as effective as NGS at risk stratifying MCL.
Acknowledgments
There are no acknowledgments to make.
Financial Disclosure
The project did not receive any external funding.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Informed Consent
Written informed consent was not required per exemption from the Institutional REB. An REB letter is available upon request.
Author Contributions
I. Elsharawi: conception and design, literature review, acquisition of data, drafting of article and article guarantor. S. Selegean: analysis and interpretation of data, supervision, reviewing and editing of manuscript. M. Carter: analysis and interpretation of data, design, supervision, reviewing and editing of manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748.
doi pubmed pmc - Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725.
doi pubmed - Soleimani A, Navarro A, Liu D, Herman SEM, Chuang SS, Slavutsky I, Narbaitz M, et al. CD5-negative mantle cell lymphoma: clinicopathologic features of an indolent variant that confers a survival advantage. Leuk Lymphoma. 2022;63(4):911-917.
doi pubmed pmc - Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, Barth TF, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394.
doi pubmed - Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, Montano-Almendras CP, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910.
doi pubmed - Bacher U, Shumilov E, Flach J, Porret N, Joncourt R, Wiedemann G, Fiedler M, et al. Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018;8(11):113.
doi pubmed pmc - Zhong Y, Xu F, Wu J, Schubert J, Li MM. Application of Next Generation Sequencing in Laboratory Medicine. Ann Lab Med. 2021;41(1):25-43.
doi pubmed pmc - https://dceg.cancer.gov/tools/public-data/tp53-database.
- Rodrigues JM, Hassan M, Freiburghaus C, Eskelund CW, Geisler C, Raty R, Kolstad A, et al. p53 is associated with high-risk and pinpoints TP53 missense mutations in mantle cell lymphoma. Br J Haematol. 2020;191(5):796-805.
doi pubmed pmc - Nolan J, Murphy C, Dinneen K, Lee G, Higgins E, Bacon L, O'Brien D, et al. p53 immunohistochemistry must be confirmed by TP53 next generation sequencing for accurate risk stratification of patients with mantle cell lymphoma. Leuk Lymphoma. 2022;63(14):3504-3507.
doi pubmed - Aukema SM, Hoster E, Rosenwald A, Canoni D, Delfau-Larue MH, Rymkiewicz G, Thorns C, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood. 2018;131(4):417-420.
doi pubmed - Vermij L, Leon-Castillo A, Singh N, Powell ME, Edmondson RJ, Genestie C, Khaw P, et al. p53 immunohistochemistry in endometrial cancer: clinical and molecular correlates in the PORTEC-3 trial. Mod Pathol. 2022;35(10):1475-1483.
doi pubmed pmc - Kobel M, Kang EY. The Many Uses of p53 immunohistochemistry in gynecological pathology: proceedings of the ISGyP companion society session at the 2020 USCAP Annual9 Meeting. Int J Gynecol Pathol. 2021;40(1):32-40.
doi pubmed - Federmann B, Frauenfeld L, Pertsch H, Borgmann V, Steinhilber J, Bonzheim I, Fend F, et al. Highly sensitive and specific in situ hybridization assay for quantification of SOX11 mRNA in mantle cell lymphoma reveals association of TP53 mutations with negative and low SOX11 expression. Haematologica. 2020;105(3):754-764.
doi pubmed pmc - Lee W, Shin E, Kim BH, Kim H. Diagnostic accuracy of SOX11 immunohistochemistry in mantle cell lymphoma: A meta-analysis. PLoS One. 2019;14(11):e0225096.
doi pubmed pmc - Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, Chiorazzi M, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106(13):4315-4321.
doi pubmed pmc - National Center for Biotechnology Information. ClinVar. [VCV001763670.3] https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV001763670.3.
- Oishi N, Satou A, Miyaoka M, Kawashima I, Segawa T, Miyake K, Mochizuki K, et al. Genetic and immunohistochemical profiling of NK/T-cell lymphomas reveals prognostically relevant BCOR-MYC association. Blood Adv. 2023;7(1):178-189.
doi pubmed pmc - Lew TE, Minson A, Dickinson M, Handunnetti SM, Blombery P, Khot A, Anderson MA, et al. Treatment approaches for patients with TP53-mutated mantle cell lymphoma. Lancet Haematol. 2023;10(2):e142-e154.
doi pubmed - Kumar A, Eyre TA, Lewis KL, Thompson MC, Cheah CY. New directions for mantle cell lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1-15.
doi pubmed - Hammons L, Fenske TS. Treatment of mantle cell lymphoma in the frontline setting: are we ready for a risk-adapted approach? J Pers Med. 2022;12(7):1134.
doi pubmed pmc - Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, Burbury K, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211-1223.
doi pubmed - Lin RJ, Ho C, Hilden PD, Barker JN, Giralt SA, Hamlin PA, Jakubowski AA, et al. Allogeneic haematopoietic cell transplantation impacts on outcomes of mantle cell lymphoma with TP53 alterations. Br J Haematol. 2019;184(6):1006-1010.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


