| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 5, October 2024, pages 179-185
Ferritin and Iron Levels Inversely Associated With Lymphoma Risk: A Mendelian Randomization Study
Division of Biostatistics, School of Global Public Health, New York University, New York, NY 10003, USA
Manuscript submitted August 10, 2024, accepted October 12, 2024, published online October 21, 2024
Short title: Ferritin and Iron Status and Lymphoma
doi: https://doi.org/10.14740/jh1335
| Abstract | ▴Top |
Background: Current knowledge on iron’s role in lymphoma development is very limited, with studies yielding inconsistent findings. To address this gap, we conducted a rigorous two-sample mendelian randomization study, aiming to elucidate the potential associations between iron storage and the risk of developing lymphoma.
Methods: This study leveraged extensive genetic data derived from a comprehensive genome-wide association study (GWAS) comprising 257,953 individuals. The primary objective was to pinpoint single-nucleotide polymorphisms (SNPs) that are significantly associated with iron storage. Subsequently, this genetic information was analyzed in conjunction with summary-level data pertaining to lymphoma cases and controls, sourced from the IEU open GWAS project, which included a sample size of 3,546 lymphoma cases and 487,257 controls. To evaluate the relationship between iron storage and lymphoma risk, an inverse variance-weighted method with random effects was employed, complemented by rigorous sensitivity analyses.
Results: Genetic predisposition to high ferritin and serum iron status was causally associated with lower odds of lymphoma. Ferritin exhibited an odds ratio (OR) of 0.777 (95% confidence interval (CI): 0.628 - 0.961, P = 0.020), indicating 22.3% reduced odds of lymphoma associated with a one standard deviation increase in ferritin levels. Similarly, serum iron demonstrated an OR of 0.776 (95% CI: 0.609 - 0.989, P = 0.040), corresponding to 22.4% decreased odds of lymphoma for a one standard deviation increase in serum iron.
Conclusions: This study suggests that individuals with genes linked to higher iron storage levels have a lower risk of developing lymphoma, but further research is necessary before making any clinical recommendations.
Keywords: Iron; Ferritin; Lymphoma; Mendelian randomization; Single-nucleotide polymorphism
| Introduction | ▴Top |
Lymphoma is a broad term for hematologic malignancies that originate from clonal proliferation of lymphocytes. Although they are generally classified into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), the term lymphoma includes over 50 distinct subtypes, ranging from slow-growing low-grade to aggressive high-grade neoplasms [1]. The incidence of lymphoma is approximately 6.5 cases per 100,000 individuals, and it can occur at any age, but mostly in young and middle age groups. Additionally, there are more male patients than female patients, with an incidence ratio of approximately 3:1 [2].
Classic lymphoma symptoms, referred to as B symptoms, include fever, night sweats, and unexplained weight loss. NHL typically originates in lymphoid tissues but can spread to other organs, such as the stomach, intestines, lungs, breasts, brain, and kidneys [3]. Consequently, the clinical presentation varies widely depending on the organs involved and the disease’s extent [4].
The etiology and pathogenesis of lymphoma remain unclear to date. It is widely accepted that it is related to genetic alterations, psychological stress, blood transfusion, viral infection, immune suppression caused by the treatment of other diseases such as organ transplantation, and industrial environmental pollution [2]. Lymphoma is one of the most common neoplastic causes of death in human immunodeficiency virus (HIV)-infected individuals [2].
Micronutrients are essential for maintaining immune system balance and are implicated in various diseases, including lymphoma. Iron, a crucial component of hemoglobin and myoglobin, is vital for oxygen transport, muscle storage, and cellular energy production. It also plays a pivotal role within mitochondria, facilitating electron transfer in the energy-generating electron transport chain. As a fundamental trace element involved in numerous biological processes, iron is indispensable for overall human health [5].
Maintaining a delicate balance of iron is crucial, as both iron deficiency and overload are linked to numerous diseases and adversely impact immune function and inflammation [5]. Tightly regulated iron homeostasis is essential for optimal health [6]. To the best of our knowledge, there is no direct evidence from prospective and observational studies as to the causal link between iron storage and lymphoma risk. However, some studies [5, 6] revealed that both iron deficiency and iron overload can impair immune function. Recent research has illuminated the role of iron in modulating immune cell function and its association with various human diseases [7]. For example, iron deficiency hampers B-cell proliferation and antibody responses, highlighting its potential influence on immunity [8]. Conversely, intracellular iron accumulation in neuroinflammatory diseases appears to drive the differentiation of pathogenic T helper cells by stimulating the production of the proinflammatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) [9].
Iron restriction inhibits the proliferation of lymphocytic cell lines in vitro and upregulates transferrin receptor 1 (TfR1) expression, indicating increased cellular iron demand. Correspondingly, blocking TfR1 function suppresses lymphocyte proliferation. Lymphocytes from iron-deficient elderly individuals exhibit reduced proliferative capacity compared to iron-replete controls [10]. The critical role of iron in adaptive immunity is underscored by a rare inherited combined immunodeficiency. Jabara et al described patients with a hypomorphic TfR1 mutation that impaired iron uptake, leading to severely compromised T- and B-cell function, including proliferation and antibody class switching. These individuals suffered from recurrent, often fatal, infections. Remarkably, providing non-transferrin-bound iron restored lymphocyte proliferation ex vivo, confirming the indispensable role of iron in adaptive immunity [11].
Given the strong association between lymphoma susceptibility and immunodeficiency [1, 2, 4], we hypothesize that abnormal iron status may be a causal risk factor for the disease. However, current understanding of iron storage in lymphoma development remains very limited.
Mendelian randomization (MR) is a powerful method for estimating the causal impact of an exposure on disease development. It utilizes genetic variants as instrumental variables (IVs) to isolate the causal effect, effectively mitigating confounding biases inherent in observational studies. To ensure valid results, MR relies on several key assumptions: 1) a strong association between the IV and the exposure; 2) no independent association between the IV and confounders of the exposure-outcome relationship; and 3) the IV’s influence on the outcome is exclusively mediated through the exposure. Additionally, the monotonicity assumption is often invoked to strengthen causal inference.
The potential bidirectional relationship between iron storage and lymphoma necessitates a rigorous approach to disentangle their association. MR is well suited to this challenge. This study will employ MR to investigate the causal influence of iron storage on lymphoma risk.
| Materials and Methods | ▴Top |
MR study design
We conducted a two-sample MR analysis to explore the potential causal relationship between iron storage, as indicated by ferritin and serum iron levels, and lymphoma risks. Our study was predicated on three core assumptions: 1) the genetic IVs are robustly associated with iron storage; 2) these genetic variants are independent of potential confounders; and 3) the effect of the genetic variants on lymphoma risk is exclusively mediated through iron storage (Fig. 1).
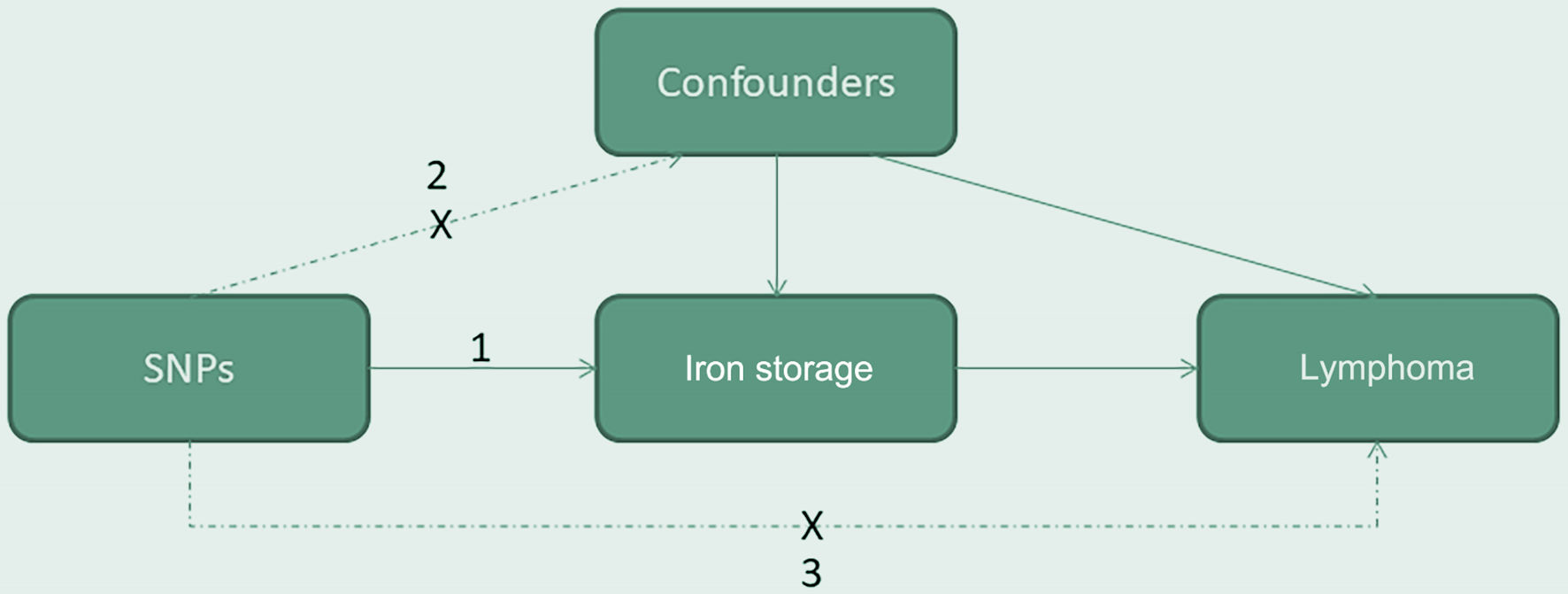 Click for large image | Figure 1. The causal directed acyclic graph of the two-sample mendelian randomization study. Our study was predicated on three core assumptions: 1) the genetic instrumental variables are robustly associated with iron storage; 2) these genetic variants are independent of potential confounders; and 3) the effect of the genetic variants on lymphoma risk is exclusively mediated through iron storage. SNP: single nucleotide polymorphism. |
Data source and software
The work presented was conducted using publicly available summary-level data from published genome-wide association studies (GWAS). The iron storage data were sourced from the largest available GWAS on iron traits [12, 13], which is a meta-analysis of studies conducted in six European populations (DeCODE, INTERNAL, SardiNIA, DBDS, HUNT, and MGI). We analyzed two sets of biomarkers related to iron storage: serum iron (n = 236,612) and ferritin (n = 257,953). The GWAS datasets are publicly available for download through the NTNU open research data.
Lymphoma outcome data were retrieved from the IEU open GWAS project, specifically from GWAS ID “ebi-a-GCST90018878”. This dataset includes 3,546 lymphoma cases and 487,257 controls from the European population, mostly from the UK. Mean age of participants at recruitment was 56.8 years old, and 53.8% were female. The number of single-nucleotide polymorphisms (SNPs) is 24,193,511.
All analyses were conducted using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). The MR analysis code is accessible upon reasonable request from the corresponding author. As this study exclusively utilized publicly available summary-level data and involved no patient interaction, the Institutional Review Board approval was deemed unnecessary. Ethical compliance in this MR study is ensured through the exclusive use of publicly available summary-level data and the absence of patient interaction.
IV selection
To ensure the robustness of our MR analysis, we implemented a stringent quality control protocol for IV selection. This protocol involved several steps: 1) Strong associations with iron traits: SNPs significantly associated with ferritin and serum iron levels at a genome-wide level (P < 5 × 10-8) were extracted from the iron GWAS meta-analysis. 2) Minimizing linkage disequilibrium (LD). We excluded SNPs in high LD (defined by r2 < 0.001 and clump distance > 10,000 kb) to ensure the selected IVs have independent effects. 3) Mitigating pleiotropic effects. SNPs directly associated with lymphoma risk (P < 5 × 10-6) were excluded to minimize potential confounding influences beyond iron storage. 4) Controlling for established confounders. Known confounders, such as smoking, high-fat diet and obesity, Epstein-Barr (EB) virus infection, HIV infection, systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome and Hashimoto’s thyroiditis, were retrieved from the PhenoScanner database and removed from the analysis. 5) Palindromic SNP exclusion: SNPs with ambiguous allele frequencies due to their palindromic nature were also excluded. 6) This multi-step approach ensured the selected IVs have robust associations with iron storage, minimal LD, limited pleiotropic effects, and are not confounded by established factors like smoking and obesity. In addition, the F-statistic for each instrument variable was calculated, so as to assess the bias of all the IVs. If the F-statistic is bigger than 10, the possibility of weak instrument variable bias is very small.
Statistical analysis
A two-sample MR analysis was conducted using the “TwoSampleMR” package to assess the causal relationship between iron storage and lymphoma development. The primary analysis employed the inverse variance-weighted (IVW) method, which is optimal under standard IV assumptions. To enhance robustness, several complementary methods were applied: MR-Egger to detect and account for potential pleiotropic effects, weighted median for estimation when at least half of the IVs are valid, and simple/weighted mode for alternative estimates. Given the lower power of these complementary methods compared to IVW, primary interpretation focused on the IVW results.
To assess potential heterogeneity, which can bias MR results, we employed the MR-heterogeneity assay and visually inspected funnel plots. Outlier identification and removal were conducted using MR-PRESSO and leave-one-out analyses. Additionally, the MR-Egger intercept test (P < 0.05) was used to detect pleiotropic effects, where genetic variants influence both the exposure and other traits unrelated to the outcome. A flowchart detailing the study methodology is presented in Figure 2.
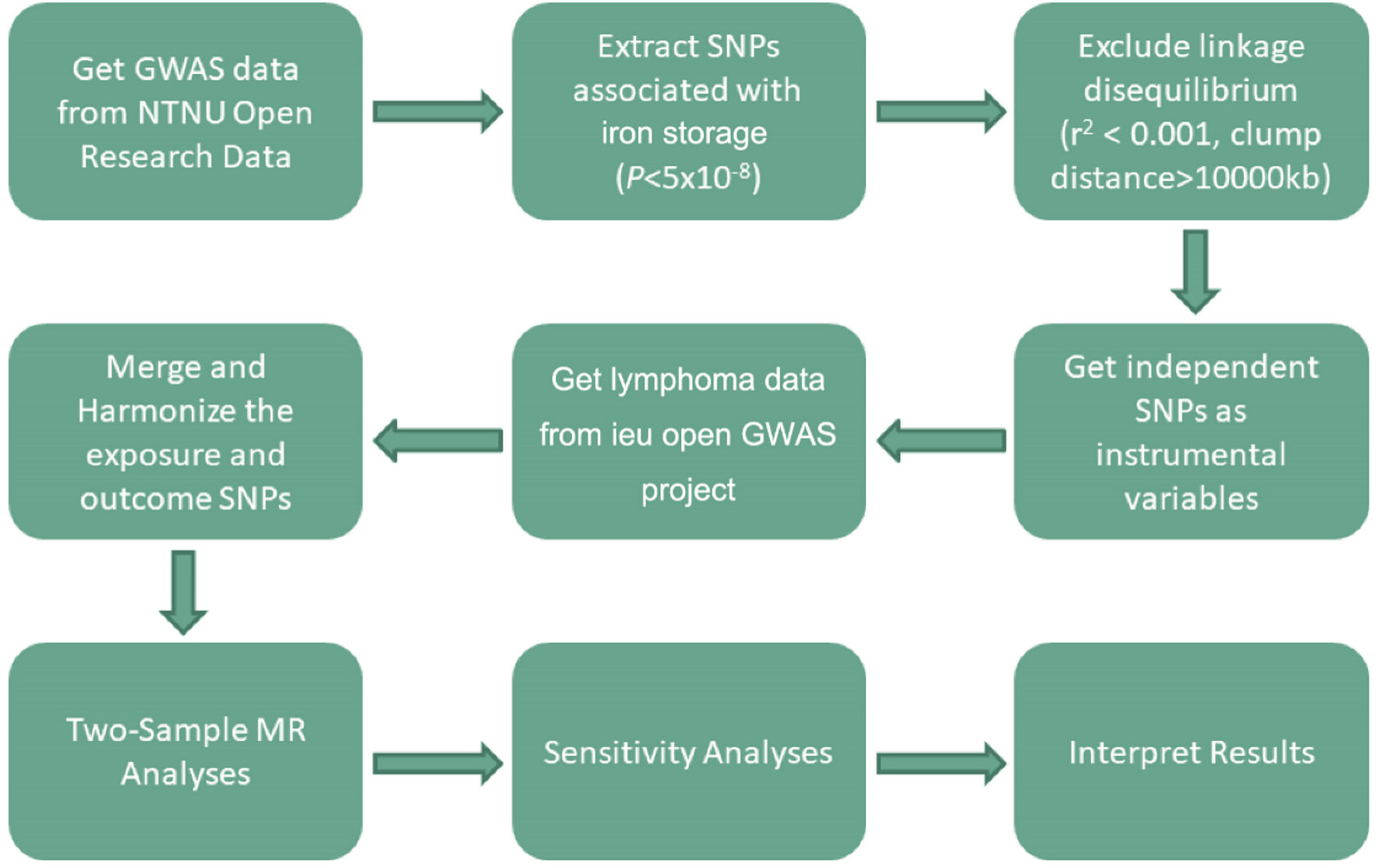 Click for large image | Figure 2. MR study flowchart. The boxes represent research steps, and the arrows indicate the flow direction. GWAS: genome-wide association study; MR: mendelian randomization; SNP: single nucleotide polymorphism. |
| Results | ▴Top |
IV selection
Following rigorous quality control, we identified 51 SNPs significantly associated with ferritin and 26 SNPs significantly associated with serum iron. To ensure independence, SNPs in LD were excluded. Importantly, none of the selected SNPs showed direct association with lymphoma risk or known confounders. An outlier, rs28929474, was identified and removed from the serum iron SNP set. Furthermore, the F-statistics for all selected SNPs exceeded 10, alleviating concerns about the bias of the IVs.
MR analysis
MR scatter plots and random-effects IVW analysis provided robust evidence for an inverse association between both ferritin and serum iron levels and lymphoma risk. Ferritin exhibited an odds ratio (OR) of 0.777 (95% confidence interval (CI): 0.628 - 0.961, P = 0.020), indicating 22.3% reduced odds of lymphoma associated with a one standard deviation increase in ferritin levels. Similarly, serum iron demonstrated an OR of 0.776 (95% CI: 0.609 - 0.989, P = 0.040), corresponding to 22.4% decreased odds of lymphoma for a one standard deviation increase in serum iron. Complementary analyses (MR-Egger, weighted median, simple mode, and weighted mode) yielded consistent results, further supporting the observed inverse relationship (Fig. 3).
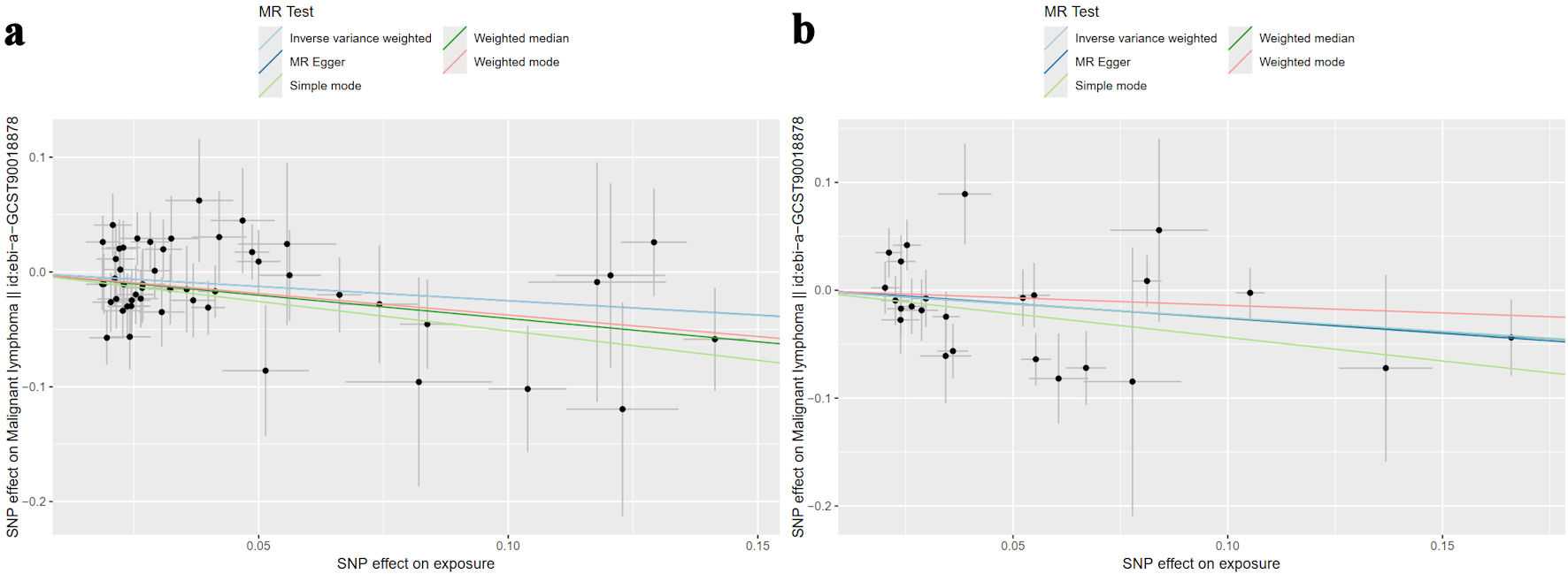 Click for large image | Figure 3. Scatter plots illustrate the causal relationship between genetically predicted iron storage and lymphoma risk using four MR methods. Each data point corresponds to an instrumental variable (IV), with its horizontal position indicating the SNP effect on iron storage - either (a) ferritin or (b) serum iron. The vertical position reflects the SNP effect on lymphoma risk. Lines connecting the SNP effects for each MR method (inverse variance-weighted (light blue), MR-Egger (dark blue), simple mode (light green), weighted median (dark green), and weighted mode (pink)) visualize the causal estimate. A negative slope of the line suggests an inverse association between iron status and lymphoma risk. Ferritin exhibited an odds ratio of 0.777 (95% CI: 0.628 - 0.961, P = 0.020), and serum iron demonstrated an odds ratio of 0.776 (95% CI: 0.609 - 0.989, P = 0.040). MR: mendelian randomization; SNP: single-nucleotide polymorphism; CI: confidence interval. |
The consequent analyses found no significant evidence of heterogeneity in the causal estimates for ferritin (P = 0.732) or serum iron (P = 0.092). Symmetrical funnel plots for both ferritin and serum iron indicated minimal publication bias (Fig. 4).
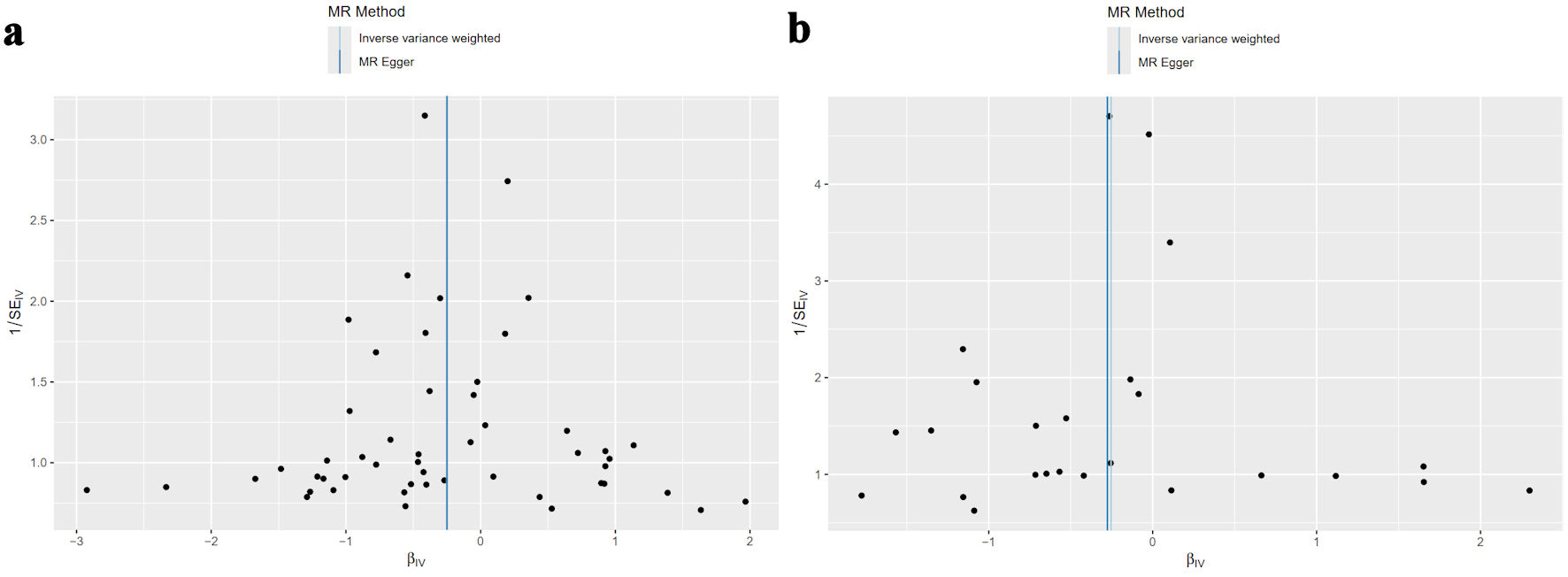 Click for large image | Figure 4. The MR-heterogeneity assay found no significant heterogeneity for ferritin (P = 0.732) or serum iron (P = 0.092). The funnel plots of the causality of iron storage and lymphoma were symmetrically distributed for (a) ferritin, and (b) serum iron. MR: mendelian randomization. |
Additionally, leave-one-out analysis confirmed that no single SNP substantially influenced the overall association between iron storage and lymphoma (Fig. 5). Finally, MR-Egger intercept analysis detected no evidence of pleiotropic effects for ferritin (P = 0.987) or serum iron (P = 0.900).
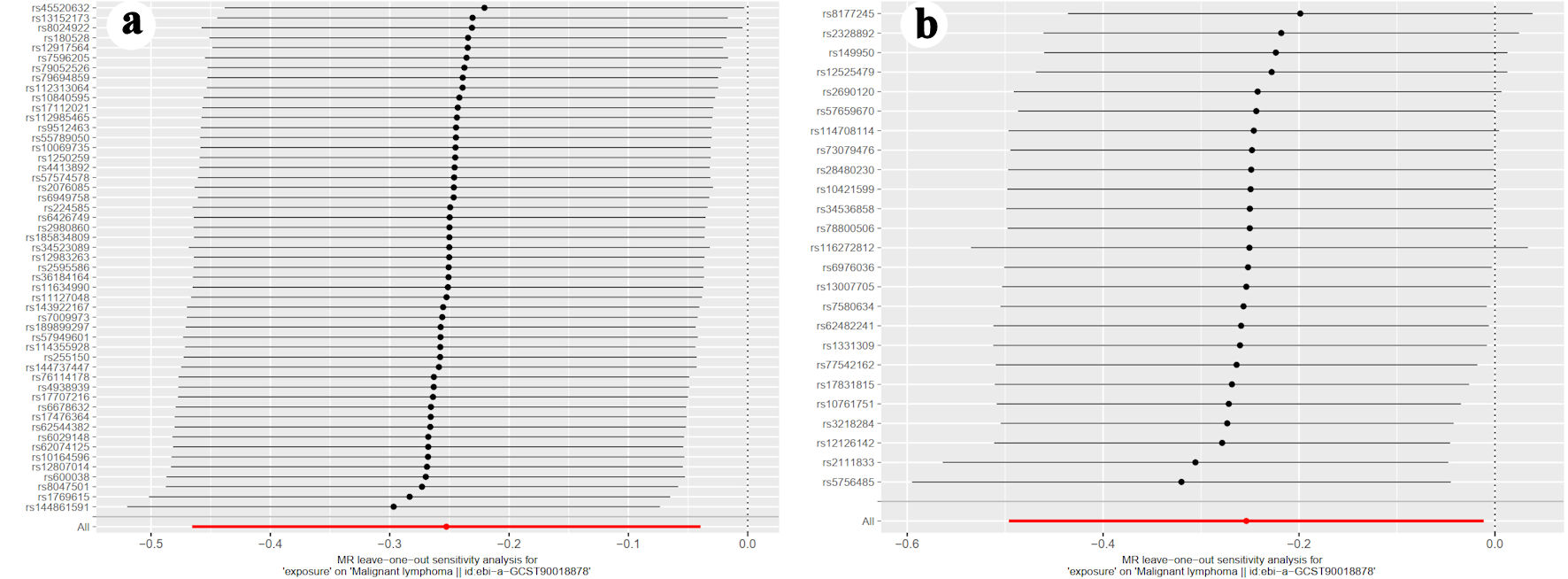 Click for large image | Figure 5. The leave-one-out graph indicated that removal of any SNPs had no fundamental effect on the results, suggesting that the MR results were reliable for (a) ferritin, and (b) serum iron. MR-Egger intercept analysis detected no evidence of pleiotropic effects for ferritin (P = 0.987) or serum iron (P = 0.900). SNP: single-nucleotide polymorphism; MR: mendelian randomization. |
| Discussion | ▴Top |
Ferritin, a ubiquitous protein, serves as a cellular iron storage depot, safely sequestering and releasing iron as needed. In contrast, serum iron represents the circulating fraction readily available for critical functions such as red blood cell production. Collectively, ferritin and serum iron constitute the majority of the body’s iron reserves [14].
Several studies report elevated ferritin levels in lymphoma patients compared to healthy controls. The high level of ferritin is often associated with disease severity and poorer prognosis [15-17]. This elevation likely stems from increased ferritin synthesis in response to inflammation and immune activation, coupled with cell necrosis and subsequent ferritin release into serum [15-17]. Consequently, elevated ferritin primarily serves as a biomarker of active malignancy rather than a causative factor [16, 17]. This phenomenon is analogous to elevated alanine aminotransferase levels in viral hepatitis. Meanwhile, iron deficiency and anemia are prevalent among lymphoma patients [18]. These conditions can manifest as anemia of chronic disease or iron-deficiency anemia, characterized by microcytic and hypochromic erythrocytes [18-20]. Notably, elevated serum ferritin can coexist with iron-deficiency anemia in some cases [18]. Consequently, when investigating iron storage as a causal risk for lymphoma, the observation of elevated ferritin and anemia represents reverse causation, complicating the research efforts.
Iron homeostasis is crucial for maintaining optimal immune function. Both iron deficiency and overload can impair immunity [5, 6, 21]. Given the established link between lymphoma susceptibility and immunodeficiency [2, 4], it is plausible that abnormal iron storage contributes to lymphoma risk. While indirect evidence suggests a potential association between red meat intake and lymphoma risk [22-24], to date, no prospective clinical trial or case-cohort study has definitively explored the causal relationship between iron storage levels and lymphoma development. MR analysis offers a compelling alternative to address the challenges of reverse causation and confounding inherent in observational studies. By leveraging genetic variants as IVs, MR can provide a more robust assessment of the causal link between iron homeostasis and lymphoma risk. To our knowledge, this is the first MR study investigating this association. Given the random assignment of genetic variants at conception, MR effectively mitigates the influence of confounding factors, allowing for more definitive causal inferences when adequately powered and employing carefully selected SNPs.
Our findings suggest that iron deficiency increases susceptibility to lymphoma. We hypothesize that this association may be mediated by iron deficiency’s detrimental impact on immune function. Decades of research have established iron as a critical element for normal immune system development [10, 25]. Iron deficiency compromises immune response capacity, particularly affecting immune cell proliferation [7, 8, 19, 26]. The global burden of iron deficiency, especially in developing countries, is substantial, with children disproportionately affected [25]. Beyond anemia, iron deficiency is associated with increased infection risk due to impaired immune function, as well as cognitive, physical, and developmental deficits [25, 27]. Experimental evidence indicates that iron regulates T-lymphocyte function, and deficiency impairs cell-mediated immunity, potentially delaying its normal development [26].
The main limitations of this study include causal inference, generalizability, complexity of iron metabolism, some unmeasured confounders, SNP selection, etc. Therefore, in order to strengthen our findings and establish a clearer causal link, we propose conducting prospective cohort studies focusing on familial clustering lymphoma. By monitoring ferritin and iron levels, tracking long-term iron supplementation, and assessing lymphoma incidence, these studies can provide more compelling evidence regarding iron’s potential role in lymphoma prevention.
Conclusions
This MR study suggests that individuals with genes linked to higher iron levels have a lower risk of developing lymphoma, but further research is necessary before making any clinical recommendations.
Acknowledgments
The exposure and outcome data were downloaded from the public available datasets, NTNU open research data (https://doi.org/10.18710/S9TJEL) and the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/). The author thanks all investigators for sharing these data.
Financial Disclosure
The author reported there is no funding associated with the work featured in this article.
Conflict of Interest
None to declare.
Informed Consent
As this study exclusively utilized publicly available summary-level data and involved no patient interaction, informed consent is unnecessary.
Author Contributions
As the sole author, Boyuan Wu’s contributions encompass the entire research process, including conceptualization, study design, data acquisition, analysis, interpretation of results, and manuscript preparation.
Data Availability
This study analyzed publicly available datasets that can be accessed via NTNU open research data (https://doi.org/10.18710/S9TJEL) and the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/).
| References | ▴Top |
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care. 2016;43(4):661-675.
doi pubmed - Chen J, Zhong Y, Zeng X, Huang C, Lan B. Diagnostic test of dynamic computed tomography in early gastrointestinal lymphoma and precancerous lesions. Transl Cancer Res. 2022;11(6):1689-1696.
doi pubmed pmc - Jian Y, Ding G, Yang D, Du J. B-cell lymphoblastic lymphoma-associated renal damage: A case report and literature review. Exp Ther Med. 2023;25(2):85.
doi pubmed pmc - Alves AMA, Torres US, Velloni FG, Ribeiro BJ, Tiferes DA, D'Ippolito G. The many faces of primary and secondary hepatic lymphoma: imaging manifestations and diagnostic approach. Radiol Bras. 2019;52(5):325-330.
doi pubmed pmc - Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front Mol Biosci. 2019;6:116.
doi pubmed pmc - Katsarou A, Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med. 2020;75:100866.
doi pubmed - Nairz M, Weiss G. Iron in infection and immunity. Mol Aspects Med. 2020;75:100864.
doi pubmed - Jiang Y, Li C, Wu Q, An P, Huang L, Wang J, Chen C, et al. Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat Commun. 2019;10(1):2935.
doi pubmed pmc - Wang Z, Yin W, Zhu L, Li J, Yao Y, Chen F, Sun M, et al. Iron drives T helper cell pathogenicity by promoting RNA-binding protein PCBP1-mediated proinflammatory cytokine production. Immunity. 2018;49(1):80-92.e87.
doi pubmed - Ahluwalia N, Sun J, Krause D, Mastro A, Handte G. Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr. 2004;79(3):516-521.
doi pubmed - Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, Bainter W, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48(1):74-78.
doi pubmed pmc - Donertas HM, Fabian DK, Valenzuela MF, Partridge L, Thornton JM. Common genetic associations between age-related diseases. Nat Aging. 2021;1(4):400-412.
doi pubmed pmc - Moksnes MR, Graham SE, Wu KH, Hansen AF, Gagliano Taliun SA, Zhou W, Thorstensen K, et al. Genome-wide meta-analysis of iron status biomarkers and the effect of iron on all-cause mortality in HUNT. Commun Biol. 2022;5(1):591.
doi pubmed pmc - Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060.
doi pubmed pmc - Das S, Kashyap A, Chopra N, Aggarwal KC, Misra A, Singh A. Ferritin as an indicator of disease activity in Hodgkin lymphoma in pediatric patients. Am J Blood Res. 2022;12(1):11-16.
pubmed pmc - Liu X, Liang Y, Shen Z, Wei L, Yang J, Piao Y, Sang W, et al. A novel prognostic model based on ferritin and nomogram-revised risk index could better stratify patients with extranodal natural killer/T-cell lymphoma. Cancer Med. 2023;12(9):10660-10671.
doi pubmed pmc - Shen Z, Zhang S, Zhang M, Hu L, Sun Q, He C, Yan D, et al. The addition of ferritin enhanced the prognostic value of international prognostic index in diffuse large B-cell lymphoma. Front Oncol. 2021;11:823079.
doi pubmed pmc - Yasmeen T, Ali J, Khan K, Siddiqui N. Frequency and causes of anemia in Lymphoma patients. Pak J Med Sci. 2019;35(1):61-65.
doi pubmed pmc - Ghosh J, Singh RK, Saxena R, Gupta R, Vivekanandan S, Sreenivas V, Raina V, et al. Prevalence and aetiology of anaemia in lymphoid malignancies. Natl Med J India. 2013;26(2):79-81.
pubmed - Zubrikhina GN, Blindar VN, Matveeva, II, Demina EA. [The characteristics of anemic syndrome in patients with prevalent phases of Hodgkin lymphoma before treatment]. Klin Lab Diagn. 2015;60(2):28-32.
pubmed - Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, Pierson Y, et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563(7732):564-568.
doi pubmed pmc - Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, Colt JS, et al. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: results from a NCI-SEER case-control study. Carcinogenesis. 2006;27(2):293-297.
doi pubmed - Daniel CR, Sinha R, Park Y, Graubard BI, Hollenbeck AR, Morton LM, Cross AJ. Meat intake is not associated with risk of non-Hodgkin lymphoma in a large prospective cohort of U.S. men and women. J Nutr. 2012;142(6):1074-1080.
doi pubmed pmc - Solimini AG, Lombardi AM, Palazzo C, De Giusti M. Meat intake and non-Hodgkin lymphoma: a meta-analysis of observational studies. Cancer Causes Control. 2016;27(5):595-606.
doi pubmed - Armitage AE, Moretti D. The importance of iron status for young children in low- and middle-income countries: a narrative review. Pharmaceuticals (Basel). 2019;12(2):59.
doi pubmed pmc - Gao X, Song Y, Wu J, Lu S, Min X, Liu L, Hu L, et al. Iron-dependent epigenetic modulation promotes pathogenic T cell differentiation in lupus. J Clin Invest. 2022;132(9):e152345.
doi pubmed pmc - Onabanjo OO, Jerling JC, Covic N, Van Graan A, Taljaard C, Mamabolo RL. Association between iron status and white blood cell counts in African schoolchildren of the North-West Province, South Africa. J Epidemiol Glob Health. 2012;2(3):103-110.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


