| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 13, Number 5, October 2024, pages 207-215
Siltuximab in Idiopathic Multicentric Castleman Disease: Real-World Experience
Ciprian Jitarua, b, Argyris Symeonidisc, Sorina Badelitad, Eirini Katodritoue, Andrei Colitaf, Anastasia Mpantie, Anamaria Bancosa, b, Bogdan Tigua, Petra Rotariua, b, Laura Uriana, b, Ioana Rusa, b, Delia Dimaa, b, Anca Bojana, b, Marc Damiana, b, Vasiliki Labropouloug, Mihai Stefan Muresanh, Despina Fotioui, Bogdan Feticab, Bobe Petrushevj, Angela Dascalescuk, Dimitra Dalampirae, Sanda Buruianal, Catalin Constantinescua, Mihnea Zdrengheaa, b, Meletios A. Dimopoulosi, Ciprian Tomuleasaa, b, m , Evangelos Terposi, m
aDepartment of Haematology/Medfuture Research Centre for Translational Medicine, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj Napoca, Romania
bDepartment of Haematology, Ion Chiricuta Oncology Institute, Cluj Napoca, Romania
cHaematology Division, Department of Internal Medicine, University of Patras, Patras, Greece
dDepartment of Haematology, Fundeni Clinical Institute, Bucharest, Romania
eDepartment of Haematology, Theagenion Cancer Hospital, Thessaloniki, Greece
fDepartment of Haematology, Coltea Hospital, Bucharest, Romania
gDepartment of Internal Medicine, Division of Haematology, University of Patras Medical School, Patras, Greece
hDepartment of Surgery, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj Napoca, Romania
iDepartment of Clinical Therapeutics, National and Kapodistrian University of Athens, School of Medicine, Alexandra General Hospital, Athens, Greece
jDepartment of Pathology, Regional Institute of Gastroenterology and Hepatology, Cluj Napoca, Romania
kDepartment of Hematology, Regional Institute of Oncology, Iasi, Romania
lDepartment of Hematology, Nicolae Testemitanu University of Medicine and Pharmacy, Chisinau, Moldova
mCorresponding Author: Ciprian Tomuleasa, Department of Haematology/Medfuture Research Centre for Translational Medicine, Iuliu Haitian University of Medicine and Pharmacy, Cluj Napoca, Romania; Evangelos Terpos, Department of Haematology, National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece
Manuscript submitted September 2, 2024, accepted October 4, 2024, published online October 21, 2024
Short title: Siltuximab in Patients With Castleman Disease
doi: https://doi.org/10.14740/jh1343
| Abstract | ▴Top |
Background: Castleman disease (CD) is a very rare, non-malignant lymphoproliferative disorder that can be classified as unicentric or multicentric (MCD). MCD is associated with systemic symptoms, including organ dysfunction due to cytokine dysregulation, primarily interleukin-6 (IL-6). The anti-IL-6 monoclonal antibody siltuximab is recommended as a frontline treatment for idiopathic MCD (iMCD), but real-world data on its use in routine clinical practice are limited. This study aimed to assess disease response and survival outcomes in patients with iMCD treated with siltuximab therapy in real-world settings in Greece and Romania.
Methods: This retrospective cohort study included adult patients with iMCD treated with siltuximab in clinical practice across Greece and Romania between January 2017 and December 2022. The primary endpoint was overall response rate and secondary endpoints included survival and safety outcomes. Response assessments were performed according to the Castleman Disease Collaborative Network guidelines. Patients were followed until death, loss to follow-up or study conclusion (October 2023).
Results: Forty-eight patients with iMCD were included in the study. Mean age at baseline was 65 years, with significant age differences between patients from Greece (74 years) and Romania (54 years). The majority of patients were male (68.8%) and received one prior line of therapy (75%). Patients included in the study received a median of nine cycles of siltuximab. Response data were available for 38 patients. The overall response to siltuximab was 71.1%, with 55.3% of patients achieving a complete response, and 15.8% a partial response. The estimated overall survival rate at 3 years was 74% and the median survival was 123 months. The most common adverse events (> 5%) included elevated liver enzymes, anxiety, allergic reactions and nausea/diarrhea. Serious adverse events were experienced by 16.7% of the patients.
Conclusions: Our results suggest that siltuximab-based therapy is effective in treating iMCD in real-world settings in Greece and Romania. To our knowledge, this study represents the largest real-world analysis of siltuximab in European patients with iMCD so far.
Keywords: Idiopathic multicentric Castleman disease; Siltuximab; Anti-interleukin-6; Real-world evidence
| Introduction | ▴Top |
Castleman disease (CD) is an extremely rare, non-malignant, lymphoproliferative disorder [1, 2]. Its estimated worldwide incidence is 5 - 16 per million people a year, depending on region [3], although accurate incidence rates in low- and middle-income countries are lacking. Until 2016, CD was poorly diagnosed and classified due to the lack of specific evidence-based diagnostic criteria [2].
CD can be unicentric (UCD) or multicentric (MCD), with the latter representing about 50% of diagnosed CD cases [4]. UCD has almost no symptoms, whereas MCD is characterized by systemic symptoms, enlarged lymph nodes, systemic inflammation, and organ dysfunction due to dysregulated secretion of cytokines (e.g., interleukin-6 (IL-6)) [5]. While the pathogenesis of UCD is thought to be driven by clonal expansion of lymph node stromal cells [2, 5], the etiology of MCD is less well understood. Infection with human herpes virus-8 (HHV-8) is considered to be the cause of 50% of MCD cases, and the remaining cases are either idiopathic MCD (iMCD) due to negative HHV-8 status and no other identified cause, or associated with polyneuropathy, organomegaly, endocrinopathy, M proteins, and skin changes (POEMS) [5]. IL-6 is known to play an important role in the pathogenesis of iMCD; many patients with iMCD overexpress IL-6 and respond to IL-6 inhibitors [2]. However, not all patients with iMCD have increased IL-6 levels and/or respond to anti-IL-6 therapy, suggesting the involvement of other cytokines [2].
The anti-IL-6 monoclonal antibody siltuximab is approved in over 40 countries for the treatment of iMCD [2] and is recommended by the Castleman Disease Collaborative Network (CDCN) as frontline therapy for iMCD, with or without adjunctive steroids, depending on severity [6]. Second-line treatment recommended by the CDCN includes rituximab plus steroids with or without an immunomodulatory agent in non-severe cases, or combination chemotherapy in severe cases of iMCD [6].
Due to the rarity of iMCD, it is important to report real-world data for patients treated with siltuximab in regions where modern therapies are available. Here, we report disease response and survival outcomes of patients with iMCD following therapy with siltuximab in a retrospective, real-world study conducted in Greece and Romania.
| Materials and Methods | ▴Top |
Study design and patient population
This retrospective cohort study included adult patients diagnosed with iMCD in Greek and Romanian centers between January 2017 and December 2022 and treated with siltuximab. The primary endpoint was overall response rate and secondary endpoints included survival and safety.
Diagnosis of iMCD was confirmed by expert pathologists who cross-checked all features of CD. Response to therapy was assessed in accordance with CDCN consensus guidelines. Treatment failure was defined as a newly appearing disease-related grade > 3 symptom according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE (version 4.0)), Eastern Cooperative Oncology Group score elevation of > 1 point, persistence of NCI-CTC-AE grade > 2 symptoms for ≥ 3 weeks, or radiological progression.
Patients were followed longitudinally until death, loss to follow-up, or end of study period (October 2023), whichever occurred first.
The study was conducted in accordance with the Declaration of Helsinki and approved by the respective Institutional Review Boards of the participating centers.
Study variables
Patient medical charts were checked for complete medical history, whole-body computed tomography (CT) or initial positron emission tomography, with MCD being confirmed when two or more lymph nodes were detected. Presenting symptoms were evaluated, with an emphasis on those related to IL-6 inflammatory response, such as prolonged fever, fatigue, weight loss and night sweats, in accordance with the NCI-CTCAE.
Laboratory examinations included a complete blood count with a differential count, human immunodeficiency virus and HHV-8 infections, using real-time polymerase chain reaction assessment.
Statistical analysis
Data are reported as mean and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. Differences in baseline characteristics were tested by Pearson’s Chi-squared test, Wilcoxon rank sum exact test or Fisher’s exact test. Differences in patient outcomes were tested by Fisher’s exact test. Survival analysis was performed using the Kaplan-Meier method. P-values of < 0.05 were considered statistically significant and confidence intervals (CIs) were set to 95%. All analyses were performed using the R software (version 4.2.2 R).
| Results | ▴Top |
Patient characteristics
Overall, 48 patients with iMCD were included. All patients received siltuximab treatment at 11 mg/kg as a 1-h intravenous infusion administered every 3 weeks until treatment failure. Baseline characteristics of patients with iMCD are presented in Table 1. Overall, the mean (± SD) age of patients was 65 (± 19.0) years, with the mean age higher in Greece (74 (± 18.0) years) than in Romania (54 (± 14.0) years) (P < 0.001). There was a relative predominance of male patients (68.8%). The cohort had received a median of 1.5 prior lines of therapy, with 75% of patients receiving one prior line.
 Click to view | Table 1. Baseline Characteristics and Details of Treatment |
B symptoms were present in most patients at baseline: overall, 39.6% of patients had fever, 39.6% experienced night sweats, 62.5% reported weight loss and 68.8% asthenia, probably linked to the high presence of anemia (56.3%). Most patients had lung involvement (33.0%) as internal organ dissemination; other affected organs included the gastrointestinal tract (20.8%), kidney (8.3%) and thyroid gland (6.2%). All patients received supportive care, including treatment of constitutional symptoms with antipruritics, antipyretics, pain medicines and antihistamines. Therapeutic strategies prior to siltuximab treatment included observation, surgical resection, steroid pulse therapy, combination therapy with cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine (oncovin) and prednisolone (CHOP) with or without rituximab, combination therapy with cyclophosphamide, vincristine, and prednisone (CVP) with or without rituximab, and radiotherapy.
Disease response and survival
Patients received a median of nine cycles of siltuximab. Response data were available for 38 patients. Overall response to siltuximab was 71.1%, with 21 out of 38 (55.3%) patients achieving complete response and six (15.8%) patients’ partial response. No response or stable disease was observed in six (15.8%) patients, while progressive disease was observed in five (13.2%) patients (Table 2). At a median follow-up of 3.9 years, 28 out of 48 (58.3%) patients were alive, 23 (82.1%) of whom were progression-free; 19 (39.6%) patients had died, 10 (52.6%) of whom had died due to reasons unrelated to CD; and one patient (2.1%) was lost from follow-up. Overall, three out of 19 deaths (15.7%) were due to adverse events (cardiopulmonary events). The estimated overall survival rate at 3 years was 74% and the median survival was 123 months (95% CI: 52 - NA) (Fig. 1). The median progression-free survival (PFS) for patients with siltuximab was not reached. Patients from the Greek cohort had a higher incidence of disease progression and mortality rate at the end of the follow-up than the Romanian cohort (Table 2). In addition, four patients from this cohort reported transformation to aggressive lymphoma during treatment with siltuximab.
 Click to view | Table 2. Patient Outcomes |
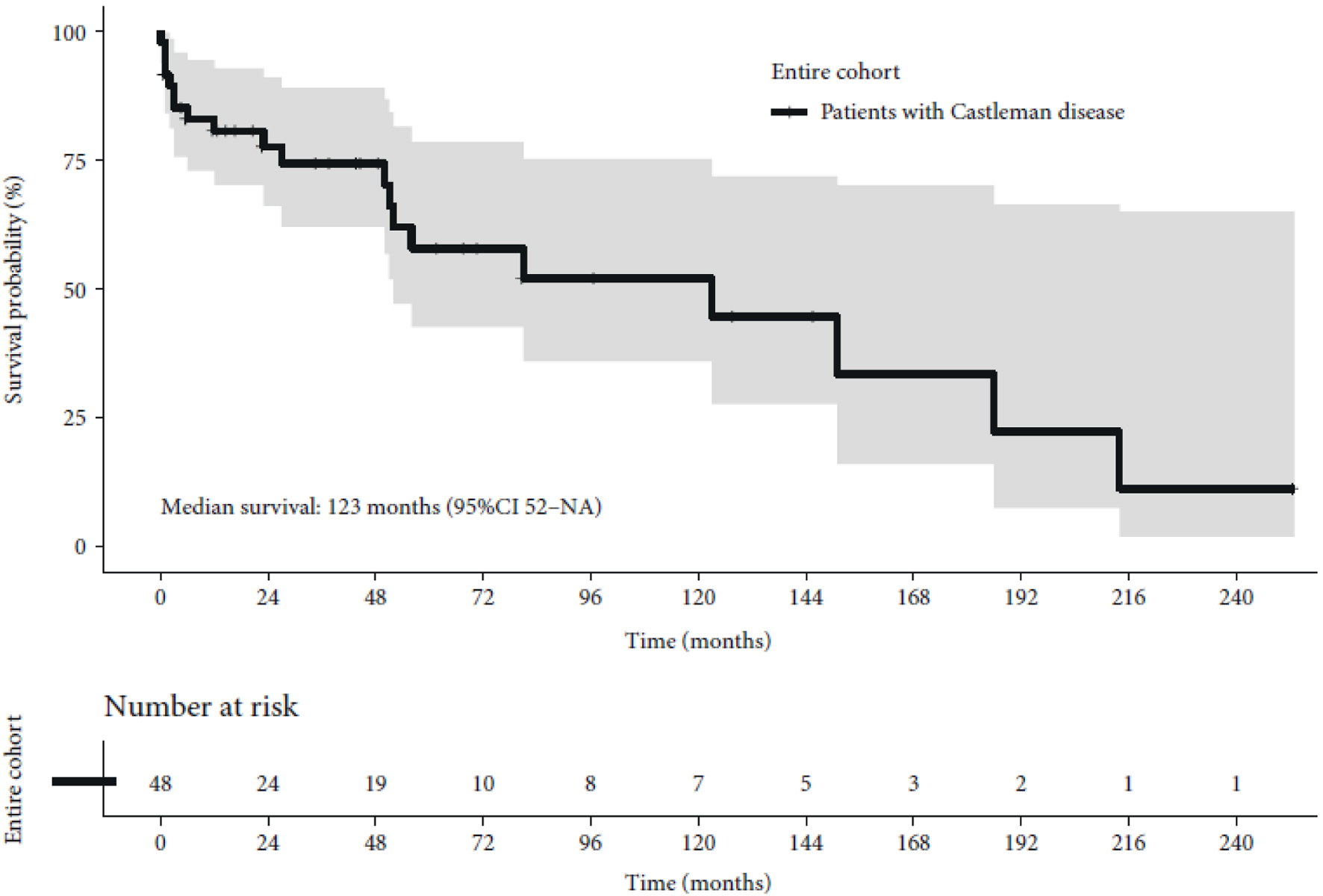 Click for large image | Figure 1. Kaplan-Meier curve of overall survival. CI: confidence interval; NA: not available. |
Safety
Adverse events included raised alanine aminotransferase, aspartate aminotransferase or bilirubin level (10.4% (n = 5)), anxiety (10.4% (n = 5)), allergic reactions (6.3% (n = 3)), nausea/diarrhea (6.3% (n = 3)), anemia (4.2% (n = 2)), thrombocytopenia (4.2% (n = 2)), hypertension (4.2% (n = 2)), bleeding (2.1% (n = 1)) and atrial fibrillation (2.1% (n = 1)) (Table 3). Overall, eight (16.7%) patients experienced serious adverse events.
 Click to view | Table 3. Adverse Events in the Overall Study Population (N = 48) |
| Discussion | ▴Top |
Prognosis and survival rates for patients with MCD were very poor before siltuximab-based therapies became available in Greece and Romania, with prior treatments being based on interferon alpha or chemotherapy; only one-third of patients survived more than 3 years [7, 8]. Siltuximab was originally evaluated in a randomized, double-blind, placebo-controlled phase 2 study in patients with iMCD who also received best supportive care [7]. Durable disease and symptom responses occurred in 34.0% of patients in the siltuximab group compared with 0% in the placebo group (P = 0.0012) [7]. At a later follow-up, PFS was significantly longer for patients treated with siltuximab versus placebo (P = 0.0001), with median PFS not reached for siltuximab versus 14.5 months for placebo [8]. The 2-year estimates for PFS were 91% versus 37% for siltuximab and placebo, respectively [8]. In our real-world cohort of patients with iMCD, treatment with siltuximab resulted in an estimated 3-year overall survival rate of 74%. Siltuximab therapy generally has a more acceptable safety profile than classic chemotherapy, which is associated with severe hematological, gastrointestinal, and renal adverse events [9-11]. In our study, 16% of patients treated with siltuximab experienced severe adverse events and 6.3% of patients died due to adverse events. Still, the toxicity following siltuximab-based therapy is much lower than other therapeutic alternatives that include chemotherapy-based regimens and/or immunomodulatory agents [11].
MCD is very rare and real-world data for patients with iMCD treated with siltuximab are currently scarce, with only two publications related to European patients (from Italy [12] and Poland [13]) published so far. In the Italian study, nine patients with iMCD received siltuximab treatment for a median of 285 days (range, 104 - 1,113 days) [12]. The overall response and complete response rates were both 33.3% (3/9), with response durations of 20 - 37 months at the time of analysis [12]. In the Polish study, 11 patients with iMCD received siltuximab treatment for a median of 16 months (range, 3 - 65 months) [13]. The overall response rate was 72.7% (8/11), with two patients achieving complete response and six achieving partial response [13]. Our findings are broadly consistent with these reports [12, 13], and we believe that our analysis represents the largest real-world study of European patients with iMCD treated with siltuximab conducted to date. While the previous study in Italy included nine patients and the Polish study 11 patients, we now report data for 48 patients treated with siltuximab in routine clinical practice in Romania and Greece. We noticed some differences in baseline characteristics between our cohorts and those previously reported. The Greek cohort in the current study shows poor prognosis, older age, and more complications of malignancy compared with patients of the previously published Polish study as suggested by the presented signs and symptoms, organ manifestations and number of previous therapies at baseline [13]. These differences might be related to regional characteristics. However, it should be noted that the rate of complete response was higher in the current study (over 50% in both cohorts) than in the Italian and Polish cohorts (18% and 33%, respectively) [12, 13]. Compared to the Italian and Polish studies, our study reported a higher rate of serious adverse events: these were reported for over 16% of patients in our study, but for no patients in the previous reports as all adverse events were either grade 1 or 2 [12, 13]. Furthermore, the rate of death due to adverse events was higher in the current study than in the Italian and the Polish studies, but this difference seems to be driven by the Greek cohort and might be related to the higher mean age of this group compared to both the Romanian cohort and the Italian and Polish populations [12, 13]. The difference in age between the two cohorts in the current study is likely related to the newly approved National Plan for Cancer Management, introduced by the Romanian government, in which large screening programmes for all cancers have been implemented [14].
Overall, the safety data of the current study are broadly in line with the ones reported in the pivotal phase II trial of siltuximab, with only anxiety, bleeding and atrial fibrillation reported as newly identified events and similar rates of serious adverse events (16.7% vs. 23%) [15].
Despite limitations such as the relatively small sample size, the retrospective nature of the study, and the potential inclusion of bias and confounding factors, this study provides valuable insights into the effectiveness and safety/tolerability of siltuximab when used in clinical practice. Further data are required to optimize therapy and improve therapeutic outcomes in patients with iMCD.
Acknowledgments
Editorial support for the preparation of this manuscript was provided by mXm Medical Communications funded by Recordati Rare Diseases. The content of the article represents the views of the authors and has not been influenced by third-party sponsorship.
Financial Disclosure
Editorial assistance for the preparation of the manuscript was funded by Recordati Rare Diseases. CT is funded by an international grant from the European Hematology Association (EHA-SWG Immunotherapy Project 2024 - CAR NK cells for tumor-associated macrophage immunomodulation - a new era of immunotherapy), as well as by a bilateral collaboration grant between Romania and Moldova (PN-IV-P8-8.3-ROMD-2023-0036), and by a national grant of the Romanian Research Ministry (PNRR/2023/C9/MCID/I8) entitled “Creating a Research Group of Excellence to develop cell and immune therapy technology to target the tumor microenvironment” (project code: CF 106/31.07.2024, contract number: 760278/26.03.2024).
Conflict of Interest
None of the authors has any conflict of interest to declare.
Informed Consent
Informed consent was obtained from all patients involved in the study.
Author Contributions
CJ, AS, SB, EK, AC, AM, AB, BT, PR, LU, IR, DD, AB, MD, VL, MSM, DF, BF, BP, AD, DD, SB, CC, MZ, MAD, CT, and ET contributed to the patient management. CJ gathered all of the clinical data. ET and CT coordinated the project.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
CD: Castleman disease; CDCN: Castleman Disease Collaborative Network; CI: confidence interval; HHV-8: human herpes virus-8; IL-6: interleukin-6; iMCD: idiopathic multicentric Castleman disease; MCD: multicentric Castleman disease; NA: not applicable; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; UCD: unicentric Castleman disease; PFS: progression-free survival; POEMS: polyneuropathy, organomegaly, endocrinopathy, M proteins, and skin changes; SD: standard deviation
| References | ▴Top |
- Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822-830.
doi pubmed - Carbone A, Borok M, Damania B, Gloghini A, Polizzotto MN, Jayanthan RK, Fajgenbaum DC, et al. Castleman disease. Nat Rev Dis Primers. 2021;7(1):84.
doi pubmed - Simpson D. Epidemiology of Castleman disease. Hematol Oncol Clin North Am. 2018;32(1):1-10.
doi pubmed - Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, Menke DM, et al. The clinical spectrum of Castleman's disease. Am J Hematol. 2012;87(11):997-1002.
doi pubmed - Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353-1364.
doi pubmed - van Rhee F, Voorhees P, Dispenzieri A, Fossa A, Srkalovic G, Ide M, Munshi N, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115-2124.
doi pubmed - Danaila C, Mihailovici MS. [Castleman's disease. Discussion related to case report]. Rev Med Chir Soc Med Nat Iasi. 2000;104(3):133-141.
pubmed - Pavlidis NA, Briassoulis E, Klouvas G, Bai M. Is interferon-a an active agent in Castleman's disease? Ann Oncol. 1992;3(1):85-86.
doi pubmed - Fajgenbaum DC, Pierson SK, Kanhai K, Bagg A, Alapat D, Lim MS, Lechowicz MJ, et al. The disease course of Castleman disease patients with fatal outcomes in the ACCELERATE registry. Br J Haematol. 2022;198(2):307-316.
doi pubmed - Yu L, Tu M, Cortes J, Xu-Monette ZY, Miranda RN, Zhang J, Orlowski RZ, et al. Clinical and pathological characteristics of HIV- and HHV-8-negative Castleman disease. Blood. 2017;129(12):1658-1668.
doi pubmed - Rehman MEU, Chattaraj A, Neupane K, Rafae A, Saeed S, Basit J, Ibrahim A, et al. Efficacy and safety of regimens used for the treatment of multicentric Castleman disease: A systematic review. Eur J Haematol. 2022;109(4):309-320.
doi pubmed - Tonialini L, Bonfichi M, Ferrero S, Malipiero G, Nozza A, Argnani L, Zinzani PL. Siltuximab in relapsed/refractory multicentric Castleman disease: Experience of the Italian NPP program. Hematol Oncol. 2018;36(4):689-692.
doi pubmed - Ostrowska B, Szymczyk A, Olszewska-Szopa M, Romejko-Jarosinska J, Domanska-Czyz K, Dabrowska-Iwanicka A, Tomczak W, et al. Efficacy of siltuximab in the treatment of idiopathic multicentric castleman disease, the first Polish, real-world experience with long-term observation. Leuk Lymphoma. 2021;62(12):3031-3034.
doi pubmed - Scintee SG, Vladescu C. The National Plan for Beating Cancer is officially approved. Available at: https://eurohealthobservatory.who.int/monitors/health-systems-monitor/analyses/hspm/romania-2016/the-national-plan-for-beating-cancer-is-officially-approved. Accessed October 2024.
- van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fossa A, Simpson D, et al. Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966-974.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


