| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 4, Number 2, June 2015, pages 181-183
Myelodysplastic Syndrome and Polymyalgia Rheumatica Associated With Pernicious Anemia
Koji Otsukaa, Takakazu Higuchib, d, Akihiro Nakajimaa, Ryosuke Koyamadab, Chisun Minc, Masato Okadac, Sadamu Okadab
aDepartment of Internal Medicine, St. Luke’s International Hospital, Tokyo, Japan
bDivision of Hematology, St. Luke’s International Hospital, Tokyo, Japan
cImmuno-Rheumatology Center, St. Luke’s International Hospital, Tokyo, Japan
dCorresponding Author: Takakazu Higuchi, Division of Hematology, St. Luke’s International Hospital, 1-9 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan
Manuscript accepted for publication April 23, 2015
Short title: MDS and PMR Associated With Pernicious Anemia
doi: http://dx.doi.org/10.14740/jh204w
| Abstract | ▴Top |
An 83-year-old man who had been treated for pernicious anemia (PA) developed myelodysplastic syndrome (MDS) and polymyalgia rheumatica (PMR). While PA patients are at an increased risk of malignant tumors, association with MDS is rarely reported. PA patients are also associated with various autoimmune conditions; however, association with PMR has not been reported. Derangement in the immune system associated with PA and subsequent MDS was assumed to have contributed to the development of PMR.
Keywords: Autoimmunity; Myelodysplastic syndrome; Pernicious anemia; Polymyalgia rheumatica
| Introduction | ▴Top |
Pernicious anemia (PA) is a kind of autoimmune disease [1, 2] and patients with PA are known to be at an increasing risk of developing malignant neoplasms [3, 4]. It is also known to be associated with various other autoimmune conditions, of which the clinical significance remains largely unclear, though [1, 5, 6]. Myelodysplastic syndrome (MDS) is similarly reported to be associated with various autoimmune diseases including polymyalgia rheumatica (PMR); however, the frequencies and underlying mechanisms of these conditions are still to be elucidated [7-12].
We herein report a case of PA who developed MDS during the course of the disease and was further complicated with PMR. The complication of these conditions has never been reported in the literature and it is assumed that a disturbance in the immune system associated with PA and subsequent MDS contributed to the development of PMR.
| Case Report | ▴Top |
An 83-year-old man was diagnosed as PA with positive anti-intrinsic factor and anti-parietal cell antibodies 6 years earlier and, following the normalization of the anemia with parenteral supplement of vitamin B12, on maintenance therapy with oral vitamin B12 by his family doctor. Nine months before the admission, right shoulder ache and stiffness developed and celecoxib was prescribed; however, aching and stiffness of the left shoulder followed 1 month later. Then, 5 months later, he started to have right knee pain on exercise and noticed that upper and lower extremities were edematous. He gradually lost appetite and lost 8 kg in 2 months. The blood test by his family doctor revealed normocytic anemia and neutropenia and he was referred and admitted to our hospital.
The patient had a history of exposure to atomic bomb in Hiroshima when he was 17 years old. He had no known allergies. There was no family member who had a history of either hematological or rheumatic disease.
On admission, he had a mild fever and was anemic. Bilateral upper and lower extremities were slightly edematous and the proximal upper arms and thighs were tender to pressure. The complete blood count revealed that white blood cell (WBC) count was 1.7 × 109/L with 46.5% neutrophils, 31.0% lymphocytes, 16.5% eosinophils, 3.5% basophils, and 2.5% monocytes, red blood cell (RBC) count was 2.26 × 1012/L with 0.99% reticulocytes, hemoglobin level was 6.4 g/dL, hematocrit level was 20.0%, and platelet count was 243 × 109/L. The erythrocyte sedimentation rate was 58 mm/h. The blood chemistry tests showed that total bilirubin level was 0.6 mg/dL, aspartate aminotransferase was 14 U/L (reference range: 9 - 34), alanine aminotransferase was 7 U/L (reference range: 3 - 36), lactate dehydrogenase was 147 U/L (reference range: 118 - 223), and creatinine kinase was 20 U/L (reference range: 57 - 218). Serum iron level was 11 µg/dL, ferritin was 655 ng/dL, vitamin B12 was 962 pg/dL, and C-reactive protein (CRP) was 10.33 mg/dL. Antinuclear antibody titer was 1:40 (homogenous type) and anti-Sm antibody was positive. The other autoimmune antibodies and markers examined were all negative. There were no physical findings, medical images, or laboratory findings indicative of any kinds of infections. The bone marrow aspiration revealed a slightly hypocellular bone marrow with dysplastic features such as megakaryocytes with hypolobulated and separated nuclei, micromegakaryocytes, neutrophils with nuclear hypolobulation and/or decreased cytoplasmic granules, and erythroblasts with multiple Howell-Jolly bodies (Fig. 1). There was no increase in the blast cells. The G-banding karyotyping revealed 46,XY,+1,der(1;7)(q10;p10) [8]/46,idem,del(20)(q11.2;q13.3) [8]. Based on these findings, a diagnosis of MDS (refractory anemia with multilineage dysplasia, RCMD) was made. In addition, based on the patient’s age, aching, stiffness, and tenderness of bilateral shoulders, body weight loss, and elevated erythrocyte sedimentation rate, he was also diagnosed as complicated with PMR.
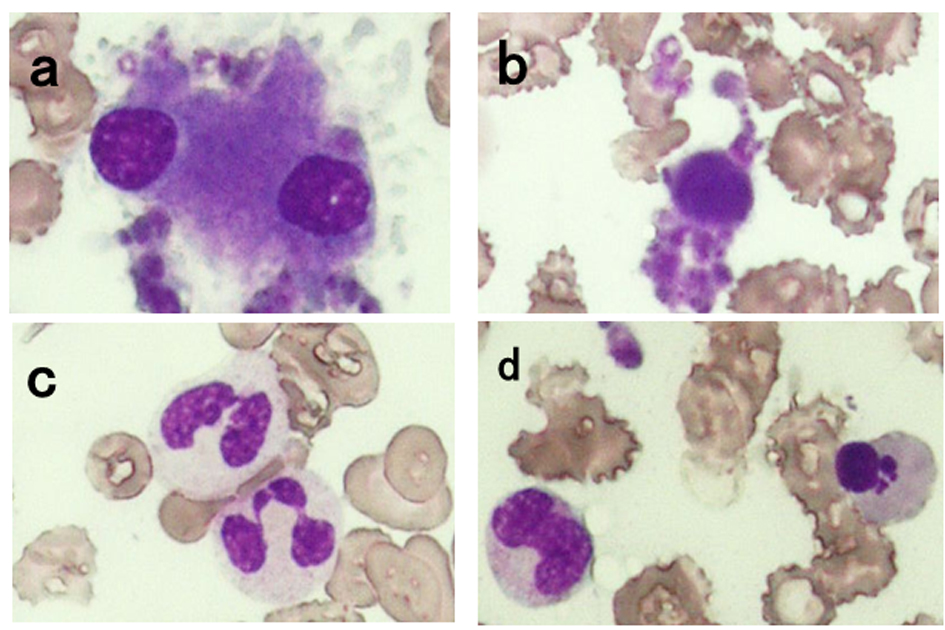 Click for large image | Figure 1. Bone marrow film examined on admission (Wright-Giemsa stain, × 400). Megakaryocytes with separated hypolobulated nuclei (a) and micromegakaryocytes (b) were observed. Neutrophils with nuclear hypolobulation and decreased cytoplasmic granules (c) and erythroblasts with multiple Howell-Jolly bodies (d) were also seen. |
Prednisolone (PSL) was started at a dose of 1 mg/kg, and the body temperature and the CRP level promptly responded, and the aching and tenderness of the extremities were relieved. However, as PSL was tapered, his symptoms get worse, which were ameliorated again with the increased dose of PSL and the addition of tacrolimus (TAC) and mizoribine (MZR) and currently he remains stable with a maintenance dose of PSL (0.15 mg/kg), TAC, and MZR 1 year after the diagnosis.
| Discussion | ▴Top |
It is well known that patients with PA are at a higher risk of developing malignant neoplasms [3, 4]. In addition to various solid cancers, especially gastric, esophageal, pancreatic, and pharyngeal cancers, the frequency of myeloid leukemia is reported to be significantly high among patients with PA [3, 4]. However, while a population-based case control study suggests an association between MDS and PA [13], MDS following PA has been rarely reported [14]. PA is an autoimmune disease in which Th1 CD4+ T lymphocytes reacting to H+/K+-ATPase in the gastric parietal cells play a role in the pathogenesis and patients with PA are at a high risk of complicating other autoimmune diseases [1, 2]; however, our literature search did not detect the association between PA and PMR. On the other hand, it is reported that various immunological abnormalities exist in patients with MDS, such as abnormal antigen presentation, reduced T-cell reaction, abnormal B-cell function, abnormal B- and T-cell interaction, reduced number and impaired function of natural killer cells, and impaired function of monocytes [7, 15, 16]. Furthermore, autoimmune diseases are reported to be complicated in about 10% of MDS patients and the association with PMR has been occasionally reported [8, 9, 11, 12]. In this regard, it is worth mentioning that, while PMR patients generally respond very favorably to steroids, those complicated with MDS tend to have a poor response to steroids and/or a steroid dependency [12] as observed in the present case.
Interleukin (IL)-6 and IL-1β, and transforming growth factor-β play major roles in the pathogenesis of PMR [17, 18]. While it is not known at present how and to what extent the immunological derangement associated with PA and MDS contributed to the development of PMR, we think that both PA and subsequent MDS did contribute either directly or indirectly to the development of PMR in the present case and that this first reported case of PA who subsequently developed MDS and PMR provides an incentive to explore the significance and mechanism of the deranged immune system in these conditions.
| References | ▴Top |
- Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441-1448.
doi pubmed - Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev. 2014;13(4-5):565-568.
doi pubmed - Brinton LA, Gridley G, Hrubec Z, Hoover R, Fraumeni JF, Jr. Cancer risk following pernicious anaemia. Br J Cancer. 1989;59(5):810-813.
doi pubmed - Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, Fraumeni JF, Jr. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer. 1993;71(3):745-750.
doi - Annibale B, Lahner E, Fave GD. Diagnosis and management of pernicious anemia. Curr Gastroenterol Rep. 2011;13(6):518-524.
doi pubmed - Song IC, Lee HJ, Kim HJ, Bae SB, Lee KT, Yang YJ, Park SY, et al. A multicenter retrospective analysis of the clinical features of pernicious anemia in a Korean population. J Korean Med Sci. 2013;28(2):200-204.
doi pubmed - Saif MW, Hopkins JL, Gore SD. Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2002;43(11):2083-2092.
doi pubmed - Billstrom R, Johansson H, Johansson B, Mitelman F. Immune-mediated complications in patients with myelodysplastic syndromes--clinical and cytogenetic features. Eur J Haematol. 1995;55(1):42-48.
doi pubmed - Enright H, Jacob HS, Vercellotti G, Howe R, Belzer M, Miller W. Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol. 1995;91(2):403-408.
doi pubmed - Castro M, Conn DL, Su WP, Garton JP. Rheumatic manifestations in myelodysplastic syndromes. J Rheumatol. 1991;18(5):721-727.
pubmed - Giannouli S, Voulgarelis M, Zintzaras E, Tzioufas AG, Moutsopoulos HM. Autoimmune phenomena in myelodysplastic syndromes: a 4-yr prospective study. Rheumatology (Oxford). 2004;43(5):626-632.
doi pubmed - Mekinian A, Braun T, Decaux O, Falgarone G, Toussirot E, Raffray L, Omouri M, et al. Inflammatory arthritis in patients with myelodysplastic syndromes: a multicenter retrospective study and literature review of 68 cases. Medicine (Baltimore). 2014;93(1):1-10.
doi pubmed - Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822-828.
doi pubmed - Kondo H, Imamura T. Unique sequence of pernicious anemia, stomach cancer, and myelodysplastic syndrome. Am J Hematol. 1999;62(4):261.
doi - Hamblin TJ. Immunological abnormalities in myelodysplastic syndromes. Semin Hematol. 1996;33(2):150-162.
pubmed - Aggarwal S, van de Loosdrecht AA, Alhan C, Ossenkoppele GJ, Westers TM, Bontkes HJ. Role of immune responses in the pathogenesis of low-risk MDS and high-risk MDS: implications for immunotherapy. Br J Haematol. 2011;153(5):568-581.
doi pubmed - Martinez-Taboada VM, Alvarez L, RuizSoto M, Marin-Vidalled MJ, Lopez-Hoyos M. Giant cell arteritis and polymyalgia rheumatica: role of cytokines in the pathogenesis and implications for treatment. Cytokine. 2008;44(2):207-220.
doi pubmed - Soriano A, Landolfi R, Manna R. Polymyalgia rheumatica in 2011. Best Pract Res Clin Rheumatol. 2012;26(1):91-104.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


