| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 4, Number 4, December 2015, pages 238-241
Diffuse Intrasinusoidal Hepatic Metastasis From Occult Breast Carcinoma Presenting as Thrombotic Microangiopathy: A Case Report and Literature Review
Wei-Ying Jena, d, Yen-Lin Cheea, Lih Kin Khorb, Ming En Ngac, Limei Poona, Sanjay De Mela
aDepartment of Hematology and Oncology, National University Cancer Institute, 5 Lower Kent Ridge Road, Singapore 119074, Singapore
bDepartment of Diagnostic Imaging, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074, Singapore
cDepartment of Pathology, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074, Singapore
dCorresponding Author: Wei-Ying Jen, Department of Hematology and Oncology, National University Cancer Institute, 5 Lower Kent Ridge Road, Singapore 119074, Singapore
Manuscript accepted for publication October 13, 2015
Short title: Diffuse Intrasinusoidal Hepatic Metastasis
doi: http://dx.doi.org/10.14740/jh230w
| Abstract | ▴Top |
We report a case of a 37-year-old female presenting with microangiopathic hemolytic anemia (MAHA) and thrombocytopenia. Two years ago, she had left breast ductal carcinoma in situ (DCIS) treated with surgical resection and adjuvant radiotherapy. Radiological imaging showed numerous patchy hepatic infiltrating lesions, but no discrete mass lesion. Liver biopsy revealed diffuse intrasinusoidal hepatic metastases (DISH) from a poorly differentiated carcinoma, which stained strongly positive for cerbB2. The patient was treated for metastatic breast carcinoma with improvement in her MAHA and thrombocytopenia. DISH is a rare mode of cancer spread, and is often radiologically occult. Recognition of atypical presentations of metastatic carcinoma in patients who present with clinical features of thrombotic microangiopathy (TMA) is crucial to avoid futile and potentially dangerous interventions. In our patient, prompt liver biopsy yielded a diagnosis of metastatic liver malignancy with secondary TMA within 3 days of admission, and the patient was appropriately started on chemotherapy.
Keywords: Malignancy-associated MAHA; Diffuse intrasinusoidal hepatic metastases; Thrombotic microangiopathy
| Introduction | ▴Top |
Thrombotic microangiopathy (TMA) is a pathological term that describes vascular injury manifested by microvascular thrombosis and endothelial abnormalities. Although TMA syndromes are heterogeneous, they share similar clinico-pathological features, with microvascular thrombosis leading to clinical features of microangiopathic hemolytic anemia (MAHA) with red cell fragmentation, thrombocytopenia and end-organ injury [1]. Primary TMA syndromes include congenital and acquired deficiencies of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), also called primary thrombotic thrombocytopenic purpura (TTP), congenital and acquired complement-mediated TMA, Shiga toxin-mediated TMA (ST-HUS) and drug-mediated TMA. These primary TMA syndromes have evidence supporting a probable cause [1]. They should be distinguished from secondary TMA syndromes presenting with MAHA and thrombocytopenia, such as malignancy, disseminated intravascular coagulation (DIC), systemic infection, pregnancy-induced thrombocytopenias, severe hypertension, autoimmune disorders and hematopoietic stem cell or organ transplantation.
It is unusual for TMA to be the first presentation of systemic malignancy [2]. Separate studies have estimated the prevalence of malignancy-related TMA to be 3.5% [3], 5.6% [4] and 7.8% [5] amongst patients initially diagnosed with TTP or atypical hemolytic uremic syndrome (aHUS). Malignancy-related TMA may be a result of the neoplastic process itself [6], or secondary to chemotherapeutic agents such as gemcitabine [7] and mitomycin C [8]. Here, we report a patient with diffuse intrasinusoidal hepatic metastases (DISH) from occult breast carcinoma presenting with TMA.
| Case Report | ▴Top |
A 37-year-old woman presented to our hospital with a 1-month history of fatigue and sweating. There was no associated weight loss, fever or diarrhea. She was on no medication and did not take any herbal treatment or supplements. Two years ago, she had left breast ductal carcinoma in situ (DCIS) treated with local surgical resection and adjuvant radiotherapy, resulting in clinical remission. On examination, she was apyrexial but icteric. She had abdominal distension with ascites, and there was a 4 cm smooth and tender hepatomegaly. She had no palpable lymphadenopathy or splenomegaly; breast and neurological examinations were normal.
Investigations showed a bicytopenia, with Hb 8.9 g/dL and platelets 52 × 109/μL. Her absolute reticulocyte count was 834,000/μL, bilirubin 144 μmol/L (direct 63 μmol/L, indirect 81 μmol/L) and lactate dehydrogenase (LDH) 8,603 U/L. Her albumin was 28 g/L, aspartate aminotransferase (AST) 307 U/L, alanine aminotransferase (ALT) 127 U/L and alkaline phosphatase (ALP) 209 U/L. She also had acute kidney injury (AKI), with a creatinine of 138 μmol/L, urea 8.8 mmol/L, sodium 132 mmol/L and potassium 3.1 mmol/L. Direct Coombs’ test was negative. A peripheral blood film showed numerous keratocytes, schistocytes, and polychromasia (Fig. 1). Her prothrombin time was 15.7 s, activated partial thromboplastin time (APTT) 32.4 s and fibrinogen 2.76 g/L. Both CA 15-3 (2,951 U) and CEA (319 U) were elevated. There was no serological evidence of autoimmune disease, Wilson’s disease, hepatitis A, B, C, E, human immunodeficiency virus (HIV) or dengue virus infection.
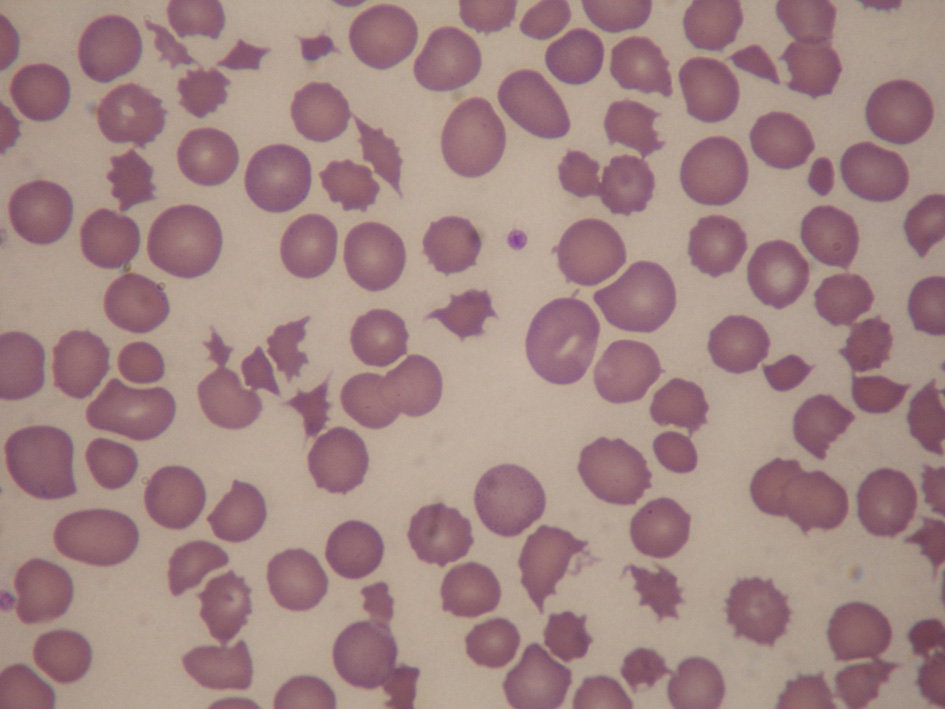 Click for large image | Figure 1. Peripheral blood film showing numerous keratocytes, schistocytes and polychromasia, in keeping with microangiopathic hemolysis. |
The 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET-CT) showed an enlarged liver with FDG uptake and edema in the intrahepatic biliary ducts along the peripheral parenchyma. However, there was no discrete lesion to suggest a neoplastic deposit. No FDG-avid lesions were seen in the chest, including the contralateral breast. She underwent magnetic resonance imaging (MRI) of the liver with magnetic resonance cholangiopancreatogram (MRCP). This showed hepatomegaly with extensive areas of signal abnormality in both lobes of the liver (Fig. 2).
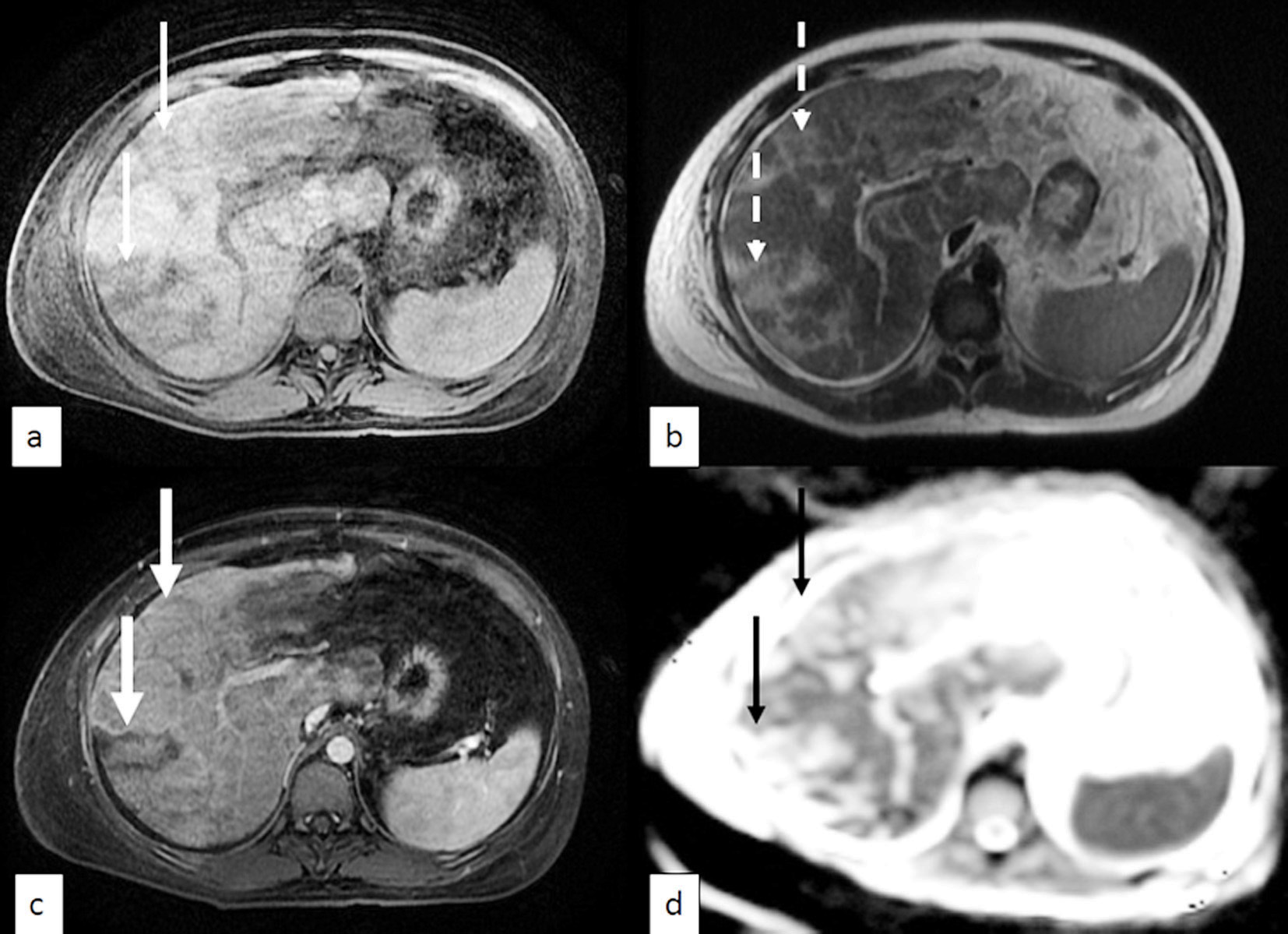 Click for large image | Figure 2. MRI of the liver. (a) T1 with fat saturation, (b) T2, (c) T1 + contrast with fat saturation, (d) apparent diffusion co-efficient (ADC). Representative images show T1 hypo-intense (thin arrows) and T2 hyper-intense (dotted arrows) ill-defined lesions distributed in a fairly linear fashion in the right hepatic lobe. The lesions do not show significant contrast enhancement (thick arrows) or restricted diffusion (black arrows). |
Despite the lack of discrete nodules on radiological imaging and the fact that DCIS is normally considered to have an excellent prognosis, malignancy-associated TMA had to be considered. A trans-jugular liver biopsy was performed. Histopathology showed intrasinusoidal nests of malignant cells (Fig. 3). These cells had features consistent with metastatic, poorly-differentiated carcinoma (AE1/3 positive, Fig. 3 inset). Hormone receptor studies were negative for estrogen and progesterone receptors, but strongly positive for cerbB2. The histology report and slides from the original DCIS lesion were not available for review and comparison with the obtained biopsy. Based on the liver biopsy features, a diagnosis of DISH was made. Malignant infiltration of the liver had caused liver dysfunction, with resultant intravascular volume depletion and renal hypoperfusion. Given her past medical history, the most likely diagnosis was metastatic breast carcinoma, and she was started on weekly paclitaxel and 3-weekly trastuzumab. Her liver and renal function improved, and her symptoms began to resolve over the next 2 -3 weeks. Her full blood count (FBC) improved, and peripheral blood film fragmentation resolved after chemotherapy.
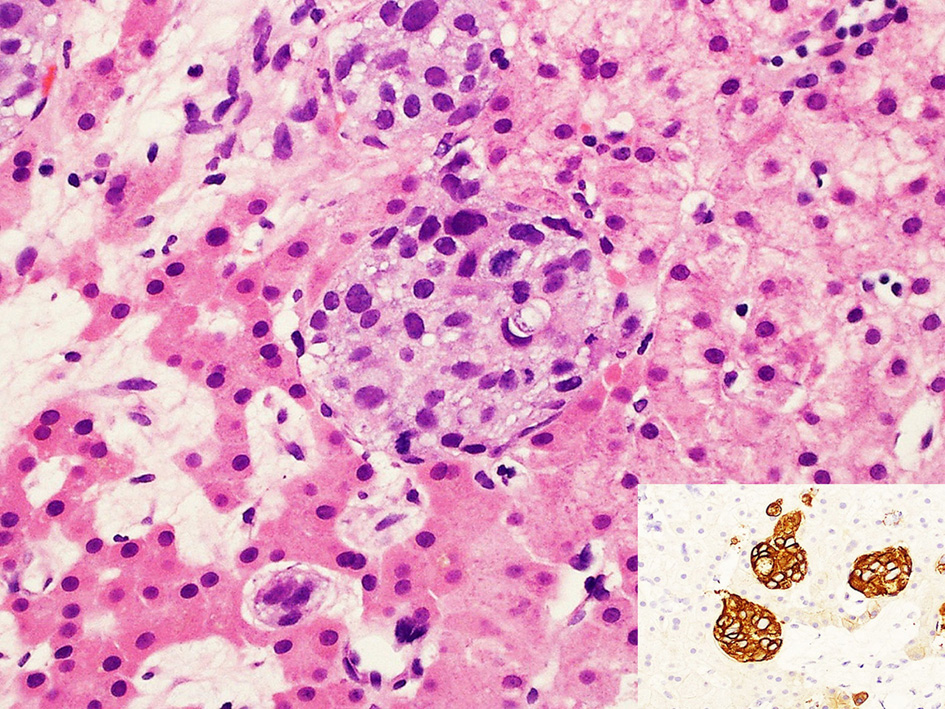 Click for large image | Figure 3. DISH. High power magnification showing well-defined rounded nests of intrasinusoidal tumor cells with enlarged nuclei, moderately abundant cytoplasm and an occasional mitotic figure (arrow). The adjacent hepatic parenchyma on the left shows atrophy of the liver cords indicative of ischemic change (hematoxylin and eosin, × 400). Inset: immunohistochemical staining with AE1/3, a cytokeratin stain, confirming the epithelial nature of the intrasinusoidal malignant cells (AE1/3 stain, × 200). |
| Discussion | ▴Top |
This case report is unusual in two respects. Firstly, DISH is a rare mode of cancer spread, and is often radiologically occult. The diagnosis is usually not evident until histopathological examination, frequently at post-mortem [9-11]. Secondly, the association between DISH and TMA is also rare with only one previously reported case in the literature.
The most common mode of presentation of liver metastasis is with the deposition of multiple nodular lesions, which are detectable radiologically as discrete mass lesions. DISH is a rare form of metastatic disease (less than 20 case reports in published literature), which may cause fulminant liver failure through infiltration and obstruction of hepatic sinusoids, without radiologically-apparent evidence of metastatic disease [11, 12]. The diffuse nature of the disease may allow extensive spread before becoming clinically manifest. Radiological findings are often non-specific, but may include heterogeneous liver parenchyma with capsular retractions. The PET-CT and MRI findings in our patient showed radiologically non-specific features consistent with cases reported in the literature [11, 13, 14].
DISH in association with TMA is exceedingly rare. One other case series by Allison et al reported a patient who presented with a TTP-like syndrome, with metastatic breast carcinoma infiltrating the hepatic sinusoids only becoming apparent at autopsy [14]. The authors noted the presence of widespread intravascular tumor emboli to the lungs, gastric mucosae and adrenal cortex, without concurrent parenchymal metastases. TMA may have resulted in this case, as in ours, from shear stress created by numerous metastasizing carcinoma cells. Alternatively, crowding of hepatic sinusoids in itself, or endothelial damage from malignant deposition, could result in shearing forces on circulating erythrocytes and MAHA with thrombocytopenia.
The distinction between malignancy-related TMA and primary TTP as a cause of MAHA and thrombocytopenia had significant implications for the management of our patient. Treatment for primary TTP is plasma exchange, whereas that of malignancy-related TMA is directed at the underlying malignancy [4]. Plasma exchange would not be predicted to be effective in malignancy-related TMA [15], and exposes the patient to unnecessary procedure-related morbidity and mortality, including death due to sepsis from catheter-related bloodstream infections and complications of catheter insertion, such as hemorrhage, pneumothorax and catheter-related thrombosis [16]. This is because, unlike primary TTP, the pathogenesis of malignancy-related TMA is not due to deficiency of the von Willebrand factor (vWF)-cleaving protease, ADAMSTS13 [17].
However, distinguishing between malignancy-related MAHA and other forms of TMA can be difficult during the acute presentation, especially in situations where urgent treatment with plasma exchange is required. Although diagnostic confirmation of primary TTP can be made with demonstration of low ADAMSTS13 enzyme levels, this assay is not routinely available at a rapid enough turnover to enable decision-making in the first 24 - 48 h. A retrospective analysis of a French TMA registry has found that patients with malignancy-related TMA are more likely to be older, have a history of malignancy and have a longer prodrome consisting of severe weight loss, bone pain, asthenia and/or dyspnea. Neurological involvement was less common in malignancy-related TMA, and renal involvement tended to be less severe. Biochemically, malignancy-related TMA tended to be associated with DIC (lower median fibrinogen levels and persistently raised D-dimers), in contrast with other forms of TMA [6]. However, none of these clinical or laboratory features are specific, and diagnosis of malignancy-associated TMA always requires histological confirmation.
Given the rarity of both DISH and its association with TMA, a high index of suspicion was required to make a diagnosis of malignancy-related TMA, particularly given its radiologically occult findings. In our case, prompt liver biopsy yielded a diagnosis of metastatic liver malignancy within 3 days of admission, and the patient was appropriately started on chemotherapy.
Conclusion
Malignancy-associated TMA is well described, but its association with DISH is unusual. The diagnosis of malignancy in our patient is critical, because primary TTP is treated with plasma exchange while malignancy-related TMA is managed by treating the underlying cancer. Our case highlights the importance of a high index of suspicion in excluding a diagnosis of malignancy-associated TMA, especially when the presentation of metastatic disease is atypical.
| References | ▴Top |
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666.
doi pubmed - George JN. Systemic malignancies as a cause of unexpected microangiopathic hemolytic anemia and thrombocytopenia. Oncology (Williston Park). 2011;25(10):908-914.
- Vesely SK, George JN, Lammle B, Studt JD, Alberio L, El-Harake MA, Raskob GE. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102(1):60-68.
doi pubmed - Elliott MA, Letendre L, Gastineau DA, Winters JL, Pruthi RK, Heit JA. Cancer-associated microangiopathic hemolytic anemia with thrombocytopenia: an important diagnostic consideration. Eur J Haematol. 2010;85(1):43-50.
doi - Levandovsky M, Harvey D, Lara P, Wun T. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS): a 24-year clinical experience with 178 patients. J Hematol Oncol. 2008;1:23.
doi pubmed - Oberic L, Buffet M, Schwarzinger M, Veyradier A, Clabault K, Malot S, Schleinitz N, et al. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14(8):769-779.
doi pubmed - Richmond J, Gilbar P, Abro E. Gemcitabine-induced thrombotic microangiopathy. Intern Med J. 2013;43(11):1240-1242.
doi pubmed - Gordon LI, Kwaan HC. Thrombotic microangiopathy manifesting as thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in the cancer patient. Semin Thromb Hemost. 1999;25(2):217-221.
doi pubmed - Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JF, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89(2):284-290.
doi pubmed - McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72(2):133-139.
doi pubmed - Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5(7):405-408.
doi pubmed - Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15(11):1235-1240.
doi pubmed - Alberti N, Bechade D, Dupuis F, Crombe A, Neuville A, Debled M, Palussiere J, et al. Hepar lobatum carcinomatosum associated with liver metastases from breast cancer: report of five cases. Diagn Interv Imaging. 2015;96(1):73-78.
doi pubmed - Allison KH, Fligner CL, Parks WT. Radiographically occult, diffuse intrasinusoidal hepatic metastases from primary breast carcinomas: a clinicopathologic study of 3 autopsy cases. Arch Pathol Lab Med. 2004;128(12):1418-1423.
pubmed - Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91(4):195-205.
doi pubmed - Nguyen L, Terrell DR, Duvall D, Vesely SK, George JN. Complications of plasma exchange in patients treated for thrombotic thrombocytopenic purpura. IV. An additional study of 43 consecutive patients, 2005 to 2008. Transfusion. 2009;49(2):392-394.
doi pubmed - Fontana S, Gerritsen HE, Kremer Hovinga J, Furlan M, Lammle B. Microangiopathic haemolytic anaemia in metastasizing malignant tumours is not associated with a severe deficiency of the von Willebrand factor-cleaving protease. Br J Haematol. 2001;113(1):100-102.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


