| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 5, Number 2, June 2016, pages 54-59
Monoclonal B-Cell Lymphocytosis Among Egyptian Elderly Healthy Adults
Mohamad Ayed Rashwana, d, Doaa Abdallah Aladlea, Mohamad Ali Awada, Emad El din Hassan Azmyb, Amany Hassan Mansoura, Nermeen Ahmed Niazyc
aHematology Unit, Clinical Pathology Department, Faculty of Medicine, Mansoura University, Egypt
bClinical Hematology Unit, Mansoura Oncology Center, Faculty of Medicine, Mansoura University, Egypt
cPublic Health and Community Medicine Department, Faculty of Medicine, Mansoura University, Egypt
dCorresponding Author: Mohamad A. Rashwan, Hematology Unit, Clinical Pathology Department, Faculty of Medicine, Mansoura University, Egypt
Manuscript accepted for publication May 04, 2016
Short title: Monoclonal B-Cell Lymphocytosis
doi: http://dx.doi.org/10.14740/jh269w
| Abstract | ▴Top |
Background: Monoclonal B-cell lymphocytosis (MBL) is a benign expansion of clonal B lymphocytes that can be found in the blood of healthy adults, and it has the potential for evolution to chronic lymphocytic leukemia (CLL). MBL is commonly found among elderly population. We aimed to study the frequency among 200 healthy elderly adults who were chosen from general practice, ophthalmology, gynecology, cardiology, dermatology, and orthopedic preoperative patients. The selected individuals had normal hematologic parameters with no evident history of malignant disease.
Methods: Blood samples were collected on EDTA, the leukocytes were stained by CD5/CD19 and kappa/lambda/CD19, and then MBL cells were searched for using flow cytometry to detect CD19-positive B-cell population with abnormal kappa/lambda immunoglobulin light chain ratio (> 3 or < 0.3) or disease-specific immunophenotype (IPT), i.e., co-expression of CD5/CD19.
Results: The frequency of MBL among the studied samples was 5.5% without significant difference between males and females. The detected frequency found may be related to the sensitivity of the method used. Higher frequencies may be detected if more colors were used to detect more antigens.
Conclusion: The study suggested that MBL can be identified through routine hospital investigations among elderly subjects and that monoclonal proliferation of B lymphocytes can be detected in an increasing number depending on the progressive improvements in the flow cytometric techniques.
Keywords: Monoclonal B lymphocytosis; Elderly; Chronic lymphocytic leukemia
| Introduction | ▴Top |
The incidence of lymphoproliferative disorders (LPDs) varies by geographic regions; Egypt is one of the regions of high incidence of LPDs among the world and Africa [1].
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in the Western world. The median age of diagnosis in the USA, Europe and Australia is approximately 70 years [2].
According to Middle East Cancer Consortium (MECC), the non-Hodgkin lymphomas (NHLs) age standardized incidence rates (ASRs) in Egypt are (16.3/100,000 persons) [3].
The largest population-based study on hematopoietic cancers in Egypt observed that the CLL was the most common subtype of leukemia [4].
Monoclonal B-cell lymphocytosis (MBL) is an asymptomatic condition characterized by the circulation of small, clonal B-lymphocyte populations less than 5 × 109/L in the peripheral blood (PB), with no organomegaly or lymphadenopathy but has a specific immunophenotype (IPT) [5].
MBL clones show a rather heterogeneous IPT of monoclonal B cells, being both CD5+ and CD5-, but also with different levels of CD20 expression [6].
MBL may be classified according to the IPT of clonal cells into: 1) CLL-like phenotype in which cells have the same phenotype like that of CLL cases: they co-express CD5, CD19, CD23, with dim CD20, and surface immunoglobulin (sIg) expression at low levels. 2) Atypical CLL phenotype: CD5 and CD19 co-expression, CD20 bright expression and/or CD23 negativity, light chain restriction with moderate/bright sIg expression. 3) Non-CLL phenotype: negative CD5, expression of CD20, light chain restriction with moderate/bright sIg expression [7].
The following set of guidelines for the characterization of MBL was proposed [8]. 1) Detection of a monoclonal B-cell population in the PB with: kappa/lambda ratio > 3:1 or < 0.3:1, or more than 25% of B cells lacking or expressing low level sIg or disease-specific IPT (co-expression of CD5/CD19). 2) Exclusion criteria: organomegaly and lymphadenopathy, or associated autoimmune/infectious disease, or B-lymphocyte count > 5 × 109/L, or any other feature diagnostic of B-LPDs.
The present study aimed to detect the frequency of MBL among apparently healthy elderly adults more than 60 years old through immunophenotyping by using flow cytometry.
| Patients and Methods | ▴Top |
The study base population comprised 200 individuals (117 male and 83 female) aged 60 years or more.
They were chosen from general practice, ophthalmology, gynecology, cardiology, dermatology, orthopedic preoperative patients, or patients presenting to the emergency department.
Samples were also collected from elderly individuals at homes for elderly as they are subjected to continuous medical care.
All individuals were routinely subjected to clinical examination either in the hospital clinics or elderly houses as a part of periodic medical care; these examinations included organomegaly and lymphadenopathy.
All individuals were interviewed and the study aspects were explained in details to each individual, then a written informed consent was taken and the study was approved by the local ethics committee of the Faculty of Medicine, Mansoura University.
PB samples were obtained from the selected individuals, and then complete blood pictures were recorded.
Routine investigations were done including liver function tests, random blood glucose, renal functions tests, and screening tests for hepatitis B virus, anti-hepatitis C virus and anti-HIV antibodies.
The individuals were then selected regarding the following criteria: 1) normal leukocyte count and differential, normal platelet count, and normal hemoglobin level; 2) negative results of screening tests for hepatitis B virus (HBs Ag), hepatitis C virus antibodies and HIV antibodies; 3) individuals without lymphadenopathy and organomegaly; and 4) patients had never previously gone to a hematology, oncology, or transplantation clinic.
All samples were processed within 24 h after blood withdrawal. Washing step was performed for the blood used for kappa/lambda staining aimed to remove the cytophilic antibodies.
Flow cytometry
Leucocytes were prepared by incubation of whole blood with a four-fold excess of ammonium chloride (NH4CL 0.86% in distilled H2O) in Falcon tubes at room temperature for 15 min.
The tubes were centrifuged at 2,000 rpm for 5 min, and then the excess of ammonium chloride was discarded leaving the pellet of cells at the bottom of the tube.
Three milliliter of phosphate-buffered saline (PBS) was added to the tubes and well mixed by vortex, and then the tubes were centrifuged for 5 min at 2,000 rpm.
Excess PBS was removed, and then the pellet of the cells was mixed with 1 mL PBS, well mixed by vortex, and then counted using automated hematology analyzer.
The count was adjusted to be 1 × 106 cells suspended in 1 mL PBS and well mixed by vortex. Two hundred microns of the cell suspension were aliquoted into five tubes, and then antibodies were added to each tube.
Cells were incubated with 10 µL of the following antibody mixes: anti-CD5 fluorescein isothiocyanate (FITC)/anti-CD19 phycoerythrin (PE), anti-kappa FITC/anti-lambda PE/anti-CD19 PerCP.
Cells were incubated for 30 min in the dark at 4 °C, then washed twice with PBS suspended in 1 mL PBS and acquired immediately using an FACS Canto II flow cytometer.
For each sample at least 50,000 events were acquired, and then analyzed with the FACS DIVA software system (Becton Dickinson, USA) (Fig. 1).
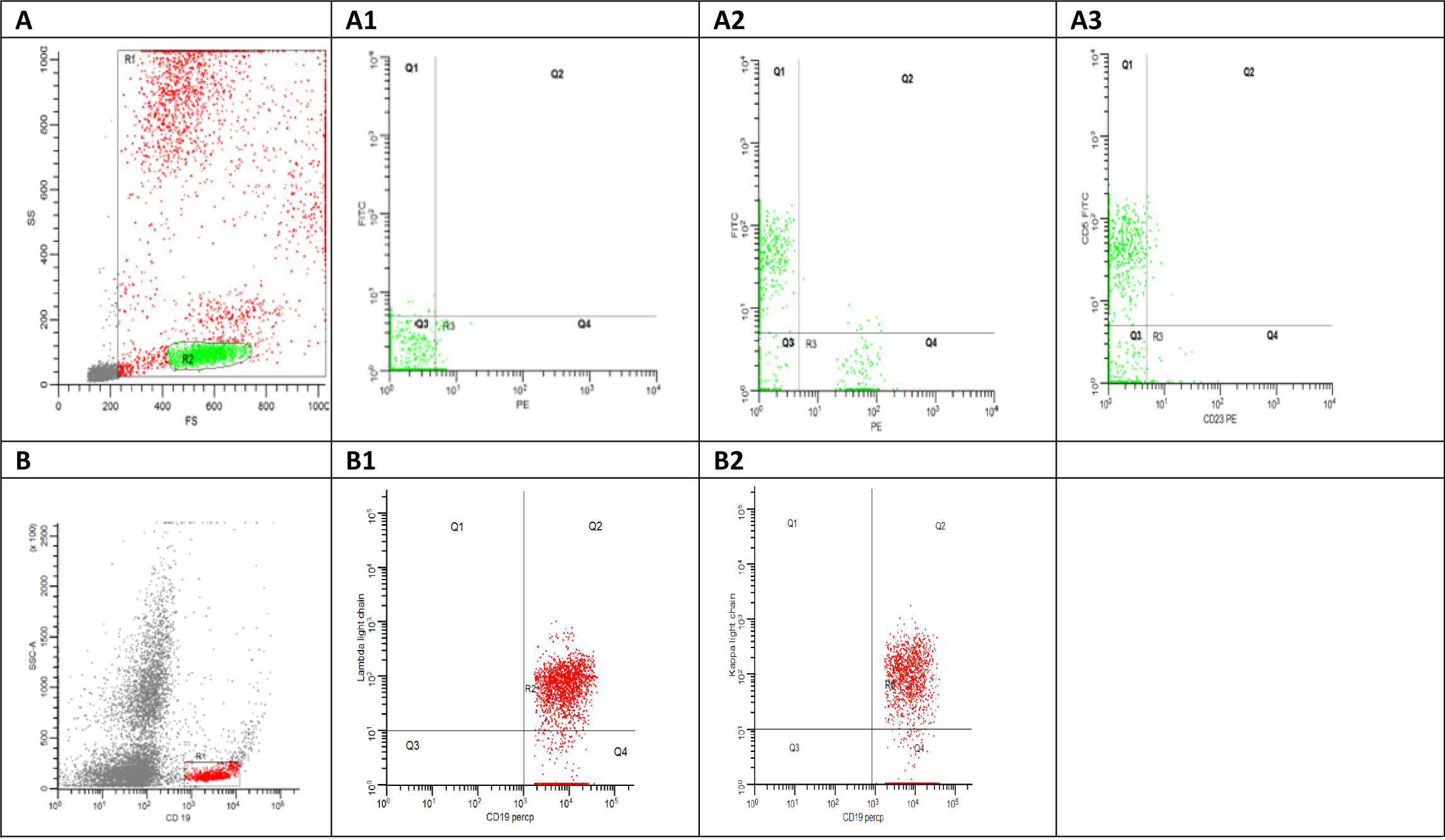 Click for large image | Figure 1. (A) Flowcytometry dot plot shows side scatter area versus forward scatter area, the red gate (R1) shows all the acquired cells excluding the debris in grey color and the green gate represent the lymphocytes gate. (A1) Flowcytometry dot plot shows the auto fluorescence at FITC and PE. (A2) Flowcytometry dot plot shows CD5 FITC versus CD19 PE. (A3) Flowcytometry dot plot shows CD5 FITC versus CD23 PE. (B) Flowcytometry dot plot shows side scatter area versus CD19 Percp. (B1) Flowcytometry dot plot shows Kappa FITC versus CD19 Percp. (B2) Flowcytometry dot plot shows lambda light chain PE versus CD19 Percp. |
Samples that met predetermined criteria of either > 40% CD19+ B cells (reference range 4-23%) or CD5 co-expression on CD19+ B cells (reference range 0-4%) were considered as MBL [9].
Data analysis
Data were entered, organized, tabulated and analyzed using the standard computer program SPSS (Statistical Package for the Social Sciences) version 20. Quantitative data were expressed as mean ± SD, while qualitative data were expressed as frequency and percent. Student’s t-test was used to measure the difference between means of two quantitative groups, while Chi-square (χ2) was used to assess the relationship between two qualitative variables, with the significant level set at 0.05.
| Results | ▴Top |
Samples from 11 subjects (5.5%) met the MBL criteria established for this study (Fig. 2). Demographic information, IPT results and clonality assessments of the 11 samples are listed in Table 1.
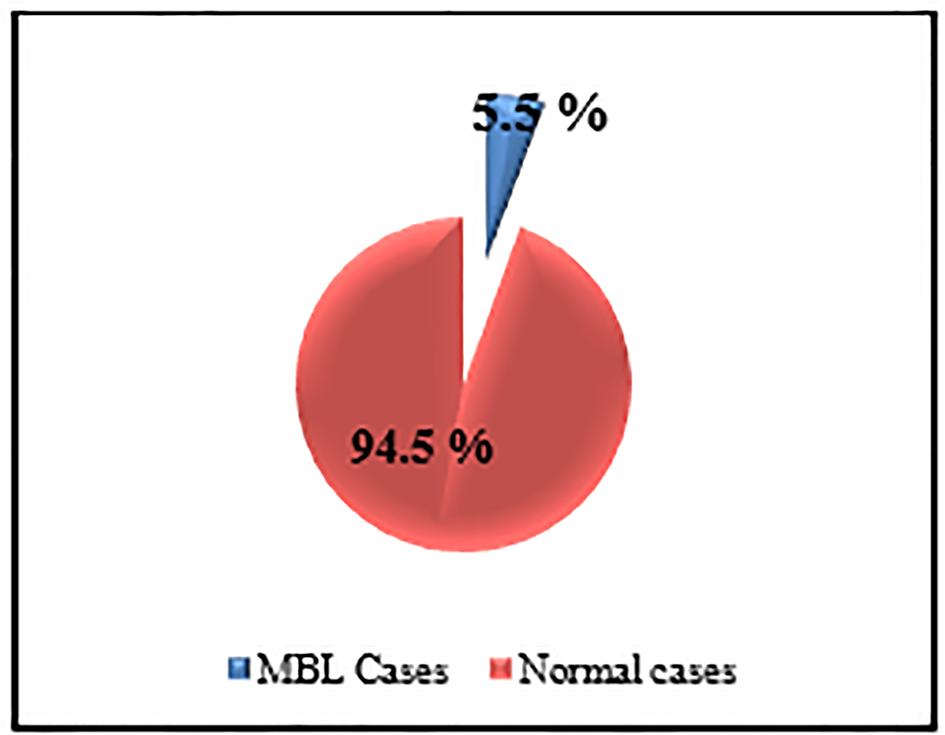 Click for large image | Figure 2. The prevalence of MBL among studied sample. |
 Click to view | Table 1. Demographics, Absolute Cell Counts, Immunophenotyping and Clonality Assessment of Individuals in MBL Study |
The mean age of MBL cases was 64.2 ± 3.45 years, six of them were males representing 5.1% (6/117) of male individuals and 3% (6/200) from the studied population and five of them were females representing 6% (5/83) of female individuals and 2.5% (5/200) from the studied population.
The percentage of the MBL individuals who were related to CLL phenotype subtype in which there were CD19+/CD5+ B cells (Fig. 3) with or without light chain restriction was 36.4% (4/11) representing 2% (4/200) of the studied sample including two males and two females, and 63.6 (7/11) of MBL individuals were related to non-CLL phenotype subtype in which there was light-chain-restricted CD19+ B cells with no CD5 expression (Fig. 4). They represent 3.5% (7/200) of the studied sample including four males and three females (Fig. 5).
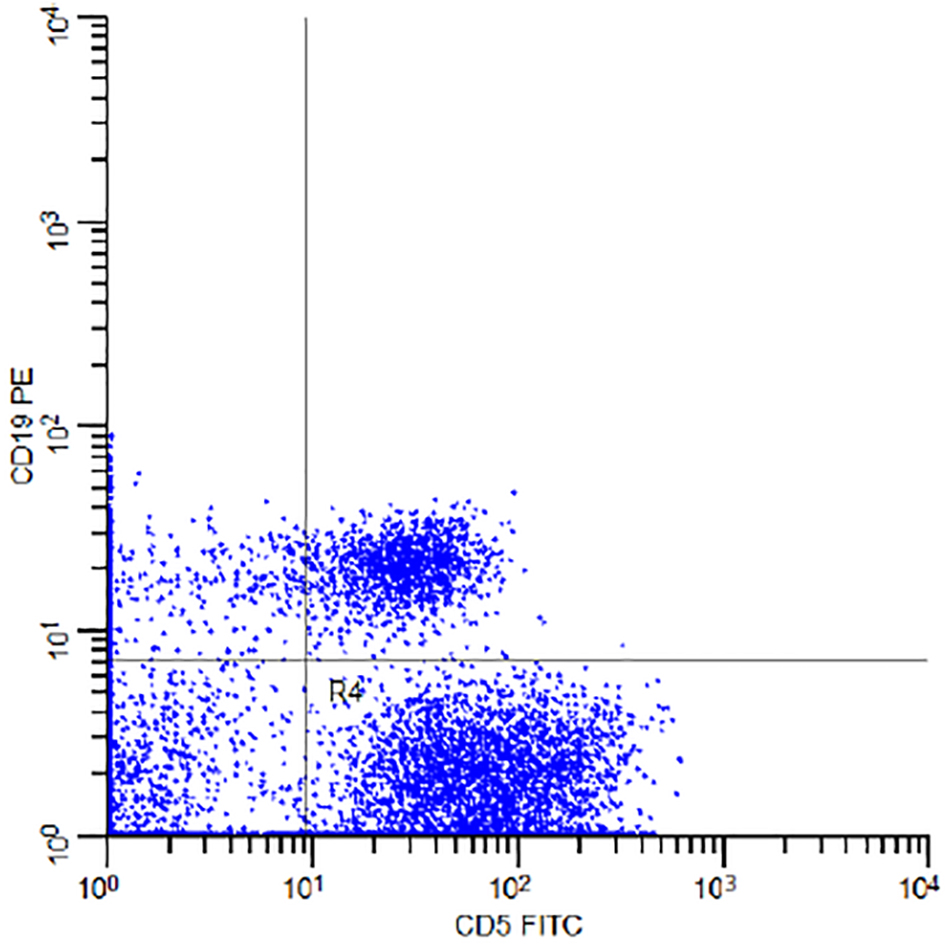 Click for large image | Figure 3. Flow cytometry dot plot of the subject number 11 in Table 1 demonstrating CD5 FITC and CD19 PE co-expression. |
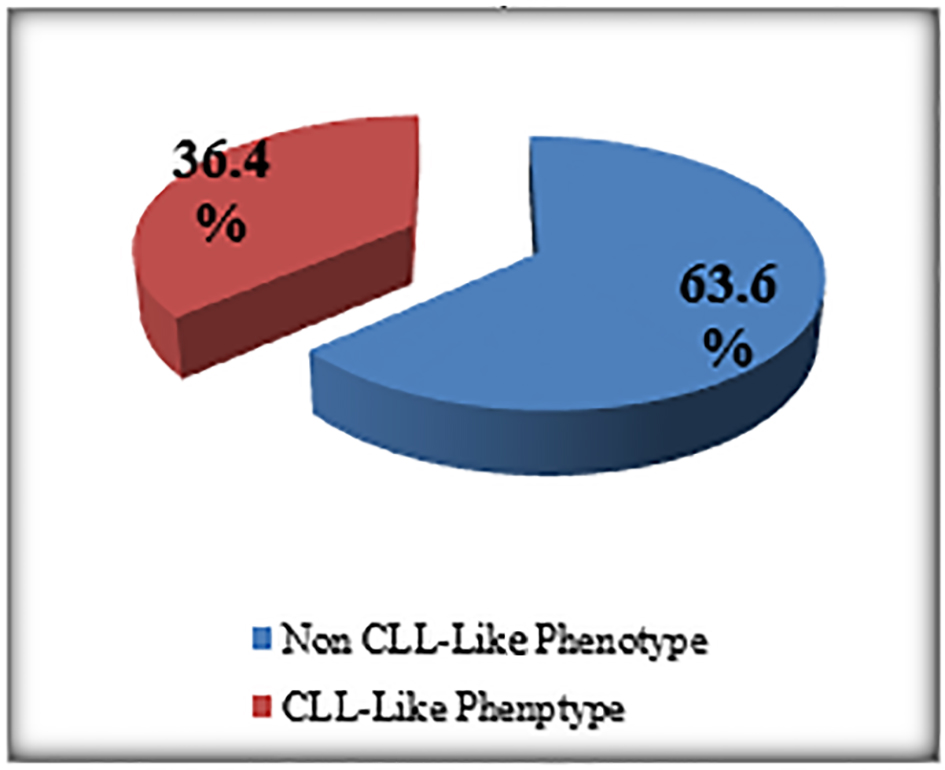 Click for large image | Figure 4. The prevalence of MBL subtypes among MBL patients. |
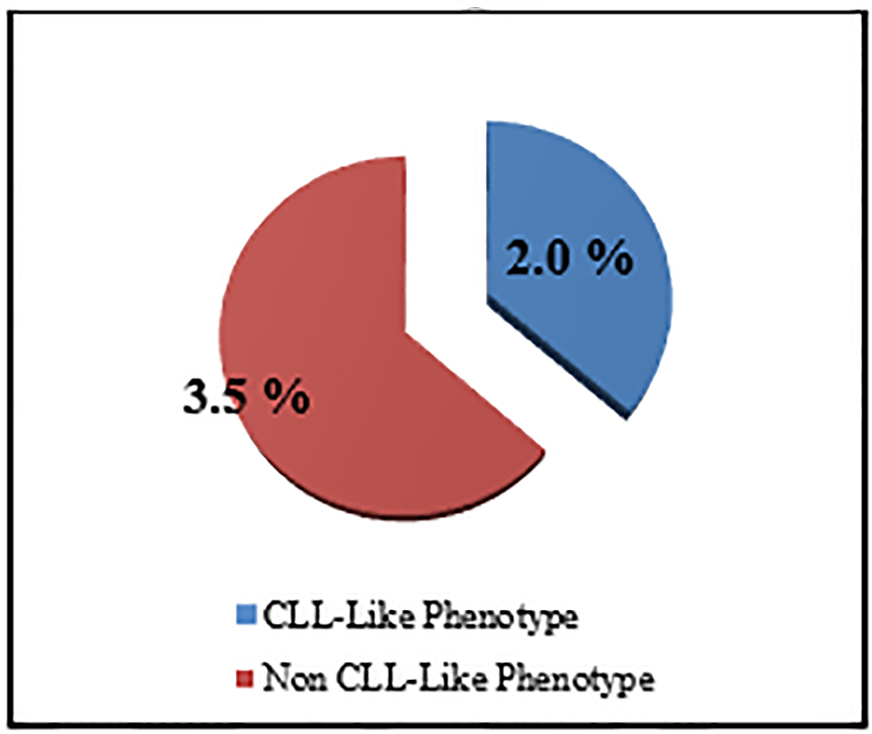 Click for large image | Figure 5. The prevalence of MBL subtypes among studied sample. |
From the 11 cases of MBL, eight cases showed light chain restriction, seven of them showed kappa light chain restriction 87.5% (7/8) and one case showed lambda light chain restriction 12.5% (1/8).
Age and gender distribution of the 200 subjects in the study demonstrated non-significant (P) value of 0.780 for gender and 0.650 for age, indicating no correlation between MBL with age and sex.
| Discussion | ▴Top |
The frequency of MBL was defined through detection of an unbalanced ratio between kappa and lambda light chain, CD5/CD19 co-expression as a sign of the presence of a monoclonal B-cell population.
Numerous technical factors were faced during light chain detection such as non-specific bindings, and also when assessing the clonality correctly in biological fluids, one must be able to detect small numbers of monoclonal B cells in the presence of polyclonal B cells. In addition, neoplastic B cells may express light chain epitopes not readily detected by all antibodies [10].
Interpretation of staining for kappa and lambda immunoglobulin light chains could be made more difficult by the presence of non-specific staining that can occur through association with Fc receptors and adherence of antibody to “sticky” cells, including damaged or dying cells.
Binding of antibodies to non-B cells can be excluded by evaluating only cells that express one or more B-lineage-associated antigens: for example, by gating on CD19 or CD20 cells, and could be minimized by incubation of cells with a blocking reagent such as immune sera prior to staining with anti-light chain antibodies [11].
More than one set of kappa/lambda light chain reagents may be used, for the detection of any B-cell monoclonal population by light chain restriction [12].
In the current study, we aimed to decrease the non-specific binding expected during light chain analysis through washing the samples by PBS before antibody staining, also by pairing them with CD19 during cytofluorograph analysis.
The total number of cases that could be detected as MBL was 11 (six males and five females), from 200 elderly individuals, representing 5.5% (11/200) (Fig. 2).
From the 11 cases of MBL, seven cases showed light chain restriction, six (85%) of them showed kappa light chain restricted and one case (15%) with lambda light chain restriction.
These results may agree with that found in neoplastic and normal conditions; normally in reactive lymphoid populations there is a mixture of kappa and lambda positive cells, with cells expressing kappa light chains more than those expressing lambda light chains.
Kappa expression is also more common than lambda in lymphoid neoplasms with the exception of hairy cell leukemia and mantle cell lymphoma [13].
Nieto et al (2009) [14] found that 48 (66%) of 73 cases expressed surface kappa light chain immunoglobulin and 11 (15%) expressed lambda light chain immunoglobulin.
The cases of MBL with the characteristics of CLL phenotype cells were 2% (CD5+/CD19+) and the non-CLL phenotype in 3.5 % (CD5-/CD19+) (Fig. 5).
The third category of MBL which is atypical CLL phenotype (CD5+, CD19+, CD23+, and CD20high) and cannot be determined as CD20 was not included in the current study.
The frequency of MBL in the previous studies among elderly and other population categories showed marked variation ranging from 0.14% to 14%; this could be attributed to many factors which affect the sensitivity of the technique such as the number of colors of the flow cytometry used.
Early screening studies using two-color flow cytometry identified only a monoclonal B-cell population in 0.1-3% of the general population [9].
Subsequent studies using four- to eight-color flow cytometry showed that the prevalence of monoclonal B cells increased with age, being 2.4% among individuals younger than 75 years of age as compared with 5.7% of individuals older than 75 years [15].
Regarding laboratory methodology, the major factor that influences MBL detection is the number of B cells acquired for analysis [16].
This impact is most clearly demonstrated when acquiring 5 × 106 leukocytes and recorded the highest prevalence of MBL among all general population studies [14].
The prevalence of MBL recorded by Nieto et al [14] approached that found in the familial CLL reported by Marti et al [12].
In the current study, although three colors were used during light chain analysis (CD19 PerCP, kappa FITC, and lambda PE) and two colors during CD5 FITC/CD19 PE co-expression analysis, the prevalence was 5.5% and this may indicate that this prevalence is higher in comparison with other previous studies using 2 - 3 colors and this means that using more colors with more markers may result in higher prevalence in the studied population.
Using multiple markers and multicolor flow cytometry enables sequential gating strategies which help us to detect very small populations of MBL among CD19+ B cells.
The observations from the prevalence of MBL of different studies show that the current study prevalence overlaps with some studies but with an unexpected finding in which the overall high number of non-CLL-like clones (seven cases) is in relation to CLL phenotype.
The study of Rawstron et al [17] included 1,520 subjects who were 62 - 80 years of age and had normal blood counts and no history of cancer. CLL-phenotype MBL was detected in 78 (5.1%) and non-CLL-phenotype MBL (i.e., light chain-restricted CD19+ B cells with no CD5 expression) was identified in 27 subjects (1.8%) [17].
For overall MBL, the age-specific prevalence in a Spanish study was 5.1% for the 40 - 49 years group and 5.3% for the 50 - 59 years group. The prevalence sharply increased in the older age groups: 17.5% (60 - 69 years), 21.7% (70 - 79 years), 27.3% (80 - 89 years) and 75% (> 89 years) [14].
The results of the current study regarding the age were non-significant (P = 0.650). This may be related to lower number of patients included in the present study than the Spanish one.
The current study also showed no significant difference regarding gender (P = 0.780).
Nieto et al (2009) reported that males showed higher MBL prevalence than females but without statistical significance (P) value > 0.05 [14].
MBL among 1,926 of volunteers living near or far from waste sites in the United States was totally 0.6, regarding the gender (male/female: 0.6%/0.5%) [18].
MBL among outpatients from three clinics outside Turin, referred for routine blood tests with normal blood cell counts and had no history or suspicion of malignancy in Italy, was totally 6.4, regarding the gender (male/female: 7.4%/5.6%) [15].
Conclusion
Monoclonal proliferation of B lymphocytes can be detected in an increasing number of otherwise healthy individuals, depending on the progressive improvements in the flow cytometric techniques. The MBL patients should be followed up to detect any progression to overt CLL.
Acknowledgments
The authors would like to thank Yaser A. Yasin, professor of Public Health and Community Medicine Department, Faculty of Medicine, AL Azhar University, Egypt for his great and valuable assistance in statistical data analysis.
Competing Interests
The authors declare that they have no competing interests.
Grant Support
None.
| References | ▴Top |
- Globocan.iarc.fr, (2015). Globocan 2012 - Home. [online] Available at: http://globocan.iarc.fr/Default.aspx [Accessed 22 May 2015].
- Mulligan SP, Tam CS. Chronic lymphocytic leukemia: diagnosis and clinical staging, in Advances in the Treatment of B-cell Chronic Lymphocytic Leukemia., Keating MJ and Tam CS, Editors. Future Medicine. 2012; 6-15.
doi - Soliman A, Boffetta P. Lymphoma and Leukemia. In: Freedman L, Edwards B, Ries L, Young J, ed., Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER, 1st ed. Bethesda: NIH Pub; 2004.
- Herzog CM, Dey S, Hablas A, Khaled HM, Seifeldin IA, Ramadan M, El-Hamzawy H, et al. Geographic distribution of hematopoietic cancers in the Nile delta of Egypt. Ann Oncol. 2012;23(10):2748-2755.
doi pubmed - Parikh SA, Kay NE, Shanafelt TD. Monoclonal B-cell lymphocytosis: update on diagnosis, clinical outcome, and counseling. Clin Adv Hematol Oncol. 2013;11(11):720-729.
pubmed - Hasserjian RP. Chronic Lymphocytic Leukemia, Small Lymphocytic Lymphoma, and Monoclonal B-Cell Lymphocytosis. Surg Pathol Clin. 2010;3(4):907-931.
doi pubmed - D'Arena G, Musto P. Monoclonal B-cell lymphocytosis. Transl Med UniSa. 2014;8:75-79.
pubmed - Marti G, Abbasi F, Raveche E, Rawstron AC, Ghia P, Aurran T, Caporaso N, et al. Overview of monoclonal B-cell lymphocytosis. Br J Haematol. 2007;139(5):701-708.
doi pubmed - Rachel JM, Zucker ML, Fox CM, Plapp FV, Menitove JE, Abbasi F, Marti GE. Monoclonal B-cell lymphocytosis in blood donors. Br J Haematol. 2007;139(5):832-836.
doi pubmed - Fukushima PI, Nguyen PK, O'Grady P, Stetler-Stevenson M. Flow cytometric analysis of kappa and lambda light chain expression in evaluation of specimens for B-cell neoplasia. Cytometry. 1996;26(4):243-252.
doi - Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111(8):3941-3967.
doi pubmed - Marti GE, Carter P, Abbasi F, Washington GC, Jain N, Zenger VE, Ishibe N, et al. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2003;52(1):1-12.
doi pubmed - Rimsza LM, Day WA, McGinn S, Pedata A, Natkunam Y, Warnke R, Cook JR, et al. Kappa and lambda light chain mRNA in situ hybridization compared to flow cytometry and immunohistochemistry in B cell lymphomas. Diagn Pathol. 2014;9:144.
doi pubmed - Nieto WG, Almeida J, Romero A, Teodosio C, Lopez A, Henriques AF, Sanchez ML, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33-37.
doi pubmed - Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, Caligaris-Cappio F. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337-2342.
doi pubmed - Champion PD. A computer simulation for exploring the detection of monoclonal B-cell lymphocytosis by flow cytometry. Cytometry B Clin Cytom. 2010;78(Suppl 1):S110-114.
doi pubmed - Rawstron AC, Bennett FL, O'Connor SJ, Kwok M, Fenton JA, Plummer M, de Tute R, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575-583.
doi pubmed - Shim YK, Vogt RF, Middleton D, Abbasi F, Slade B, Lee KY, Marti GE. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry B Clin Cytom. 2007;72(5):344-353.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


